Poly-γ-Glutamic Acid Induces Apoptosis via Reduction of COX-2 Expression in TPA-Induced HT-29 Human Colorectal Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Treatment with Poly-γ-Glutamic Acid (PGA) Has an Anti-Proliferative Effect on HT-29
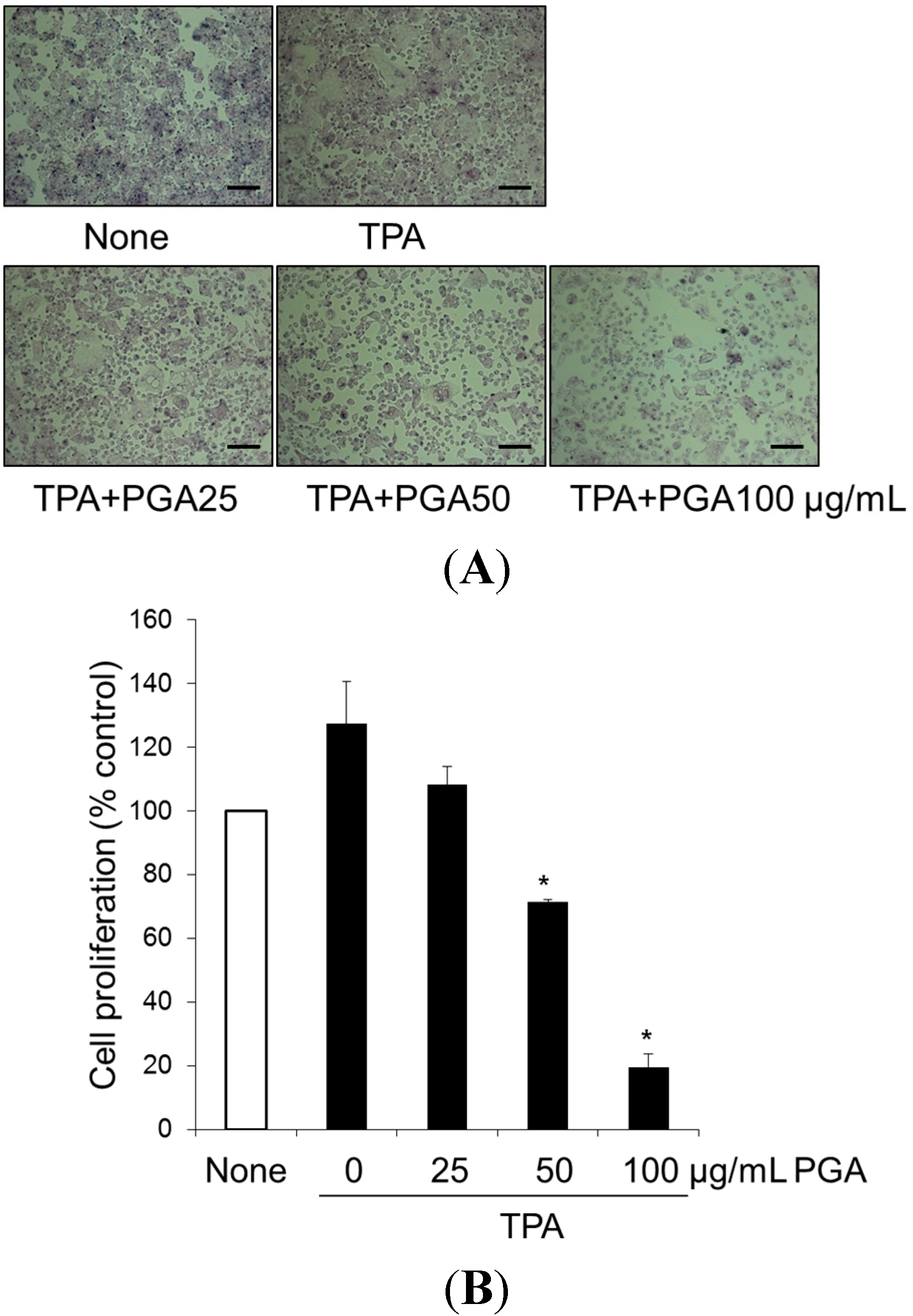
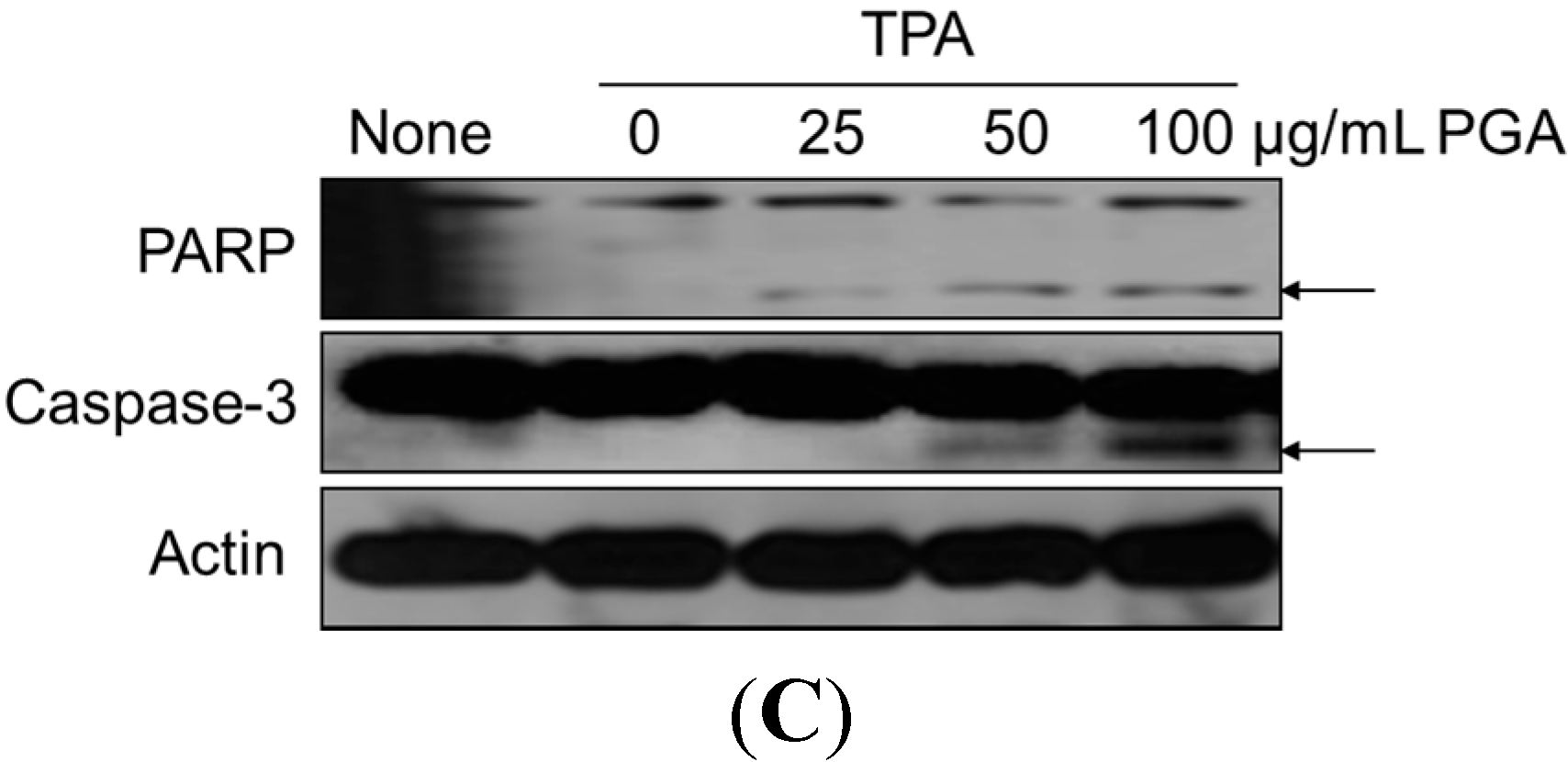
2.2. PGA Induces Apoptosis in HT-29 Cells

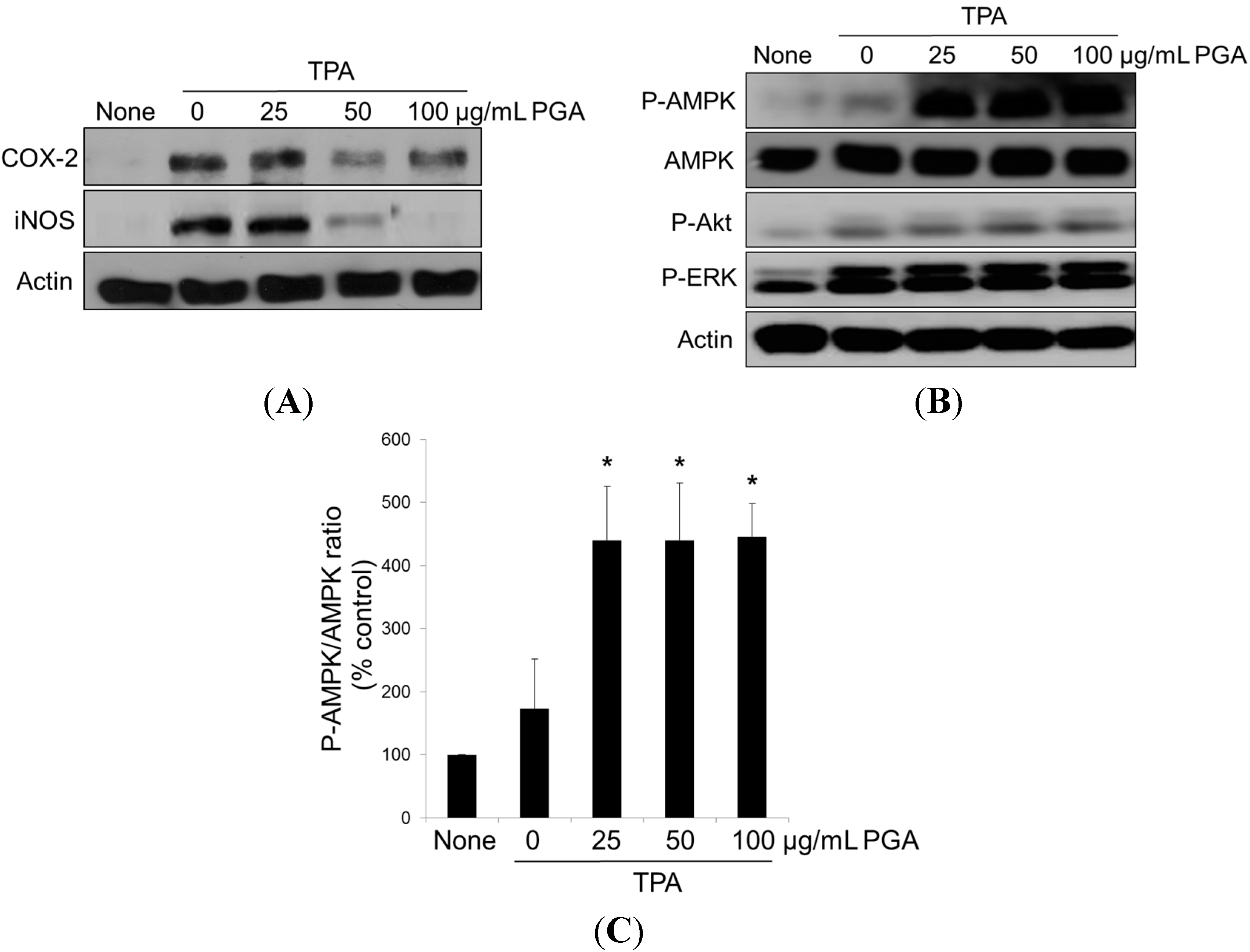
2.3. PGA Reduces TPA-Dependent COX-2 and Inducible Nitric Oxide Synthase (iNOS) Expression and Promotes AMPK Phosphorylation in HT-29 Cells
3. Discussion
4. Material and Method
4.1. Cell Culture Conditions and Reagents
4.2. Cell Proliferation Assay
4.3. Annexin V Assay
4.4. TUNEL Assay
4.5. Western Blot Analysis
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taylor, P.R.; Graeenwald, P. Nutritional interventions in cancer prevention. J. Clin. Oncol. 2005, 23, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Pourhoseingholi, M.A. Increased burden of colorectal cancer in Asia. World J. Gastrointest. Oncol. 2012, 4, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Asensi, M.; Ortega, A.; Mena, S.; Feddi, F.; Estrela, J.M. Natural polyphenols in cancer therapy. Crit. Rev. Clin. Lab. Sci. 2011, 48, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Grosch, S.; Tegeder, I.; Niederberger, E.; Brautigam, L.; Geisslinger, G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001, 15, 2742–2744. [Google Scholar] [PubMed]
- Hwang, J.T.; Kim, Y.M.; Surh, Y.J.; Baik, H.W.; Lee, S.K.; Ha, J.; Park, O.J. Selenium regulates cyclooxygenase-2 and extracellular signal-regulated kinase signaling pathways by activating AMP-activated protein kinase in colon cancer cells. Cancer Res. 2006, 66, 10057–10063. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Ha, J.; Park, I.J.; Lee, S.K.; Baik, H.W.; Kim, Y.M.; Park, O.J. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007, 247, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; DuBois, R.N. COX-2: A molecular target for colorectal cancer prevention. J. Clin. Oncol. 2005, 23, 2840–2855. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Selvarajan, K.; Hasan, M.R.; Chan, A.P.; Jin, C.; Kim, J.; Chan, S.K.; Le, N.D.; Kim, Y.B.; Tai, I.T. Inhibition of COX-2 in colon cancer modulates tumor growth and MDR-1 expression to enhance tumor regression in therapy-refractory cancers in vivo. Neoplasia 2012, 14, 624–633. [Google Scholar] [PubMed]
- Nagata, D.; Mogi, M.; Walsh, K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J. Biol. Chem. 2003, 278, 31000–31006. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.Z.; Dias, M.M.; Ropelle, E.R.; Osorio-Costa, F.; Rossato, F.A.; Vercesi, A.E.; Saad, M.J.; Carvalheira, J.B. Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth. Clin. Cancer Res. 2011, 17, 3993–4005. [Google Scholar] [CrossRef] [PubMed]
- Rattan, R.; Giri, S.; Singh, A.K.; Singh, I. 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J. Biol. Chem. 2005, 280, 39582–39593. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; He, Y.Y. Targeting the AMP-activated protein kinase for cancer prevention and therapy. Front. Oncol. 2013, 3, 175. [Google Scholar] [PubMed]
- Hwang, J.T.; Kwon, D.Y.; Yoon, S.H. AMP-activated protein kinase: A potential target for the diseases prevention by natural occurring polyphenols. New Biotechnol. 2009, 26, 17–22. [Google Scholar] [CrossRef]
- Shih, I.L.; Van, Y.T. The production of poly-(γ-glutamic acid) from microorganisms and its various applications. Bioresour. Technol. 2001, 79, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Lee, T.Y.; Bae, H.C.; Hahm, J.H.; Kim, Y.H.; Park, C.; Kang, T.H.; Kim, C.J.; Sung, M.H.; Poo, H. Oral administration of high molecular mass poly-γ-glutamate induces NK cell-mediated antitumor immunity. J. Immunol. 2007, 179, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Davaatseren, M.; Hwang, J.T.; Park, J.H.; Kim, M.S.; Wang, S.; Sung, M.J. Poly-γ-glutamic acid attenuates angiogenesis and inflammation in experimental colitis. Mediat. Inflamm. 2013, 2013, 982383. [Google Scholar] [CrossRef]
- Kim, S.; Oh, S.J.; Lee, J.; Han, J.; Jeon, M.; Jung, T.; Lee, S.K.; Bae, S.Y.; Kim, J.; Gil, W.H.; et al. Berberine suppresses TPA-induced fibronectin expression through the inhibition of VEGF secretion in breast cancer cells. Cell. Physiol. Biochem. 2013, 32, 1541–1550. [Google Scholar]
- Nam, K.S.; Shon, Y.H. Chemopreventive effects of polysaccharides extract from Asterina pectinifera on HT-29 human colon adenocarcinoma cells. BMB Rep. 2009, 31, 277–280. [Google Scholar] [CrossRef]
- Scheckel, K.A.; Degner, S.C.; Romagnolo, D.F. Rosmarinic acid antagonizes activator protein-1-dependent activation of cyclooxygenase-2 expression in human cancer and nonmalignant cell lines. J. Nutr. 2008, 138, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Kasdagly, M.; Radhakrishnan, S.; Reddivari, L.; Veeramachaneni, D.N.; Vanamala, J. Colon carcinogenesis: Influence of Western diet-induced obesity and targeting stem cells using dietary bioactive compounds. Nutrition 2014, 30, 1242–1256. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Rommel, C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Cho, H.J.; Yu, R.; Lee, K.W.; Chun, H.S.; Park Yoon, J.H. Mechanisms underlying apoptosis-inducing effects of kaempferol in HT-29 human colon cancer cells. Int. J. Mol. Sci. 2014, 15, 2722–2737. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.W.; Suh, Y.J. Synergistic apoptotic effect of celecoxib and luteolin on breast cancer cells. Oncol. Rep. 2013, 29, 819–825. [Google Scholar] [PubMed]
- Yi Lau, G.T.; Leung, L.K. The dietary flavonoid apigenin blocks phorbol 12-myristate 13-acetate-induced COX-2 transcriptional activity in breast cell lines. Food Chem. Toxicol. 2010, 48, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Ha, J.; Park, O.J. Combination of 5-fluorouracil and genistein induces apoptosis synergistically in chemo-resistant cancer cells through the modulation of AMPK and COX-2 signaling pathways. Biochem. Biophys. Res. Commun. 2005, 332, 433–440. [Google Scholar] [CrossRef]
- Chen, L.G.; Hung, L.Y.; Tsai, K.W.; Pan, Y.S.; Tsai, Y.D.; Li, Y.Z.; Liu, Y.W. Wogonin, a bioactive flavonoid in herbal tea, inhibits inflammatory cyclooxygenase-2 gene expression in human lung epithelial cancer cells. Mol. Nutr. Food. Res. 2008, 52, 1349–1357. [Google Scholar] [CrossRef]
- Park, I.J.; Lee, Y.K.; Hwang, J.T.; Kwon, D.Y.; Ha, J.; Park, O.J. Green tea catechin controls apoptosis in colon cancer cells by attenuation of H2O2-stimulated COX-2 expression via the AMPK signaling pathway at low-dose H2O2. Ann. N. Y. Acad. Sci. 2009, 1171, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Park, S.Y.; Kim, Y.M.; Park, O.J. Regulatory effect of the AMPK-COX-2 signaling pathway in curcumin-induced apoptosis in HT-29 colon cancer cells. Ann. N. Y. Acad. Sci. 2009, 1171, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Sun, M.; Tang, H.Y.; Simone, T.M.; Wu, Y.H.; Grandis, J.R.; Cao, H.J.; Davis, P.J.; Davis, F.B. Resveratrol causes COX-2- and p53-dependent apoptosis in head and neck squamous cell cancer cells. J. Cell Biochem. 2008, 104, 2131–2142. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hua, B.; Saud, S.M.; Lin, H.; Hou, W.; Matter, M.S.; Jia, L.; Colburn, N.H.; Young, M.R. Berberine regulates AMP-activated protein kinase signaling pathways and inhibits colon tumorigenesis in mice. Mol. Carcinog. 2014. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, E.J.; Hur, H.J.; Park, J.H.; Sung, M.J.; Kwon, D.Y.; Hwang, J.T. Citrus junos Tanaka peel extract attenuates experimental colitis and inhibits tumour growth in a mouse xenograft model. J. Funct. Foods 2014, 8, 301–308. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, E.J.; Sung, M.J.; Park, J.H.; Yang, H.J.; Kim, M.S.; Hur, H.J.; Hwang, J.-T. Poly-γ-Glutamic Acid Induces Apoptosis via Reduction of COX-2 Expression in TPA-Induced HT-29 Human Colorectal Cancer Cells. Int. J. Mol. Sci. 2015, 16, 7577-7586. https://doi.org/10.3390/ijms16047577
Shin EJ, Sung MJ, Park JH, Yang HJ, Kim MS, Hur HJ, Hwang J-T. Poly-γ-Glutamic Acid Induces Apoptosis via Reduction of COX-2 Expression in TPA-Induced HT-29 Human Colorectal Cancer Cells. International Journal of Molecular Sciences. 2015; 16(4):7577-7586. https://doi.org/10.3390/ijms16047577
Chicago/Turabian StyleShin, Eun Ju, Mi Jeong Sung, Jae Ho Park, Hye Jeong Yang, Myung Sunny Kim, Haeng Jeon Hur, and Jin-Taek Hwang. 2015. "Poly-γ-Glutamic Acid Induces Apoptosis via Reduction of COX-2 Expression in TPA-Induced HT-29 Human Colorectal Cancer Cells" International Journal of Molecular Sciences 16, no. 4: 7577-7586. https://doi.org/10.3390/ijms16047577




