Spectrofluorometric and Molecular Docking Studies on the Binding of Curcumenol and Curcumenone to Human Serum Albumin
Abstract
:1. Introduction
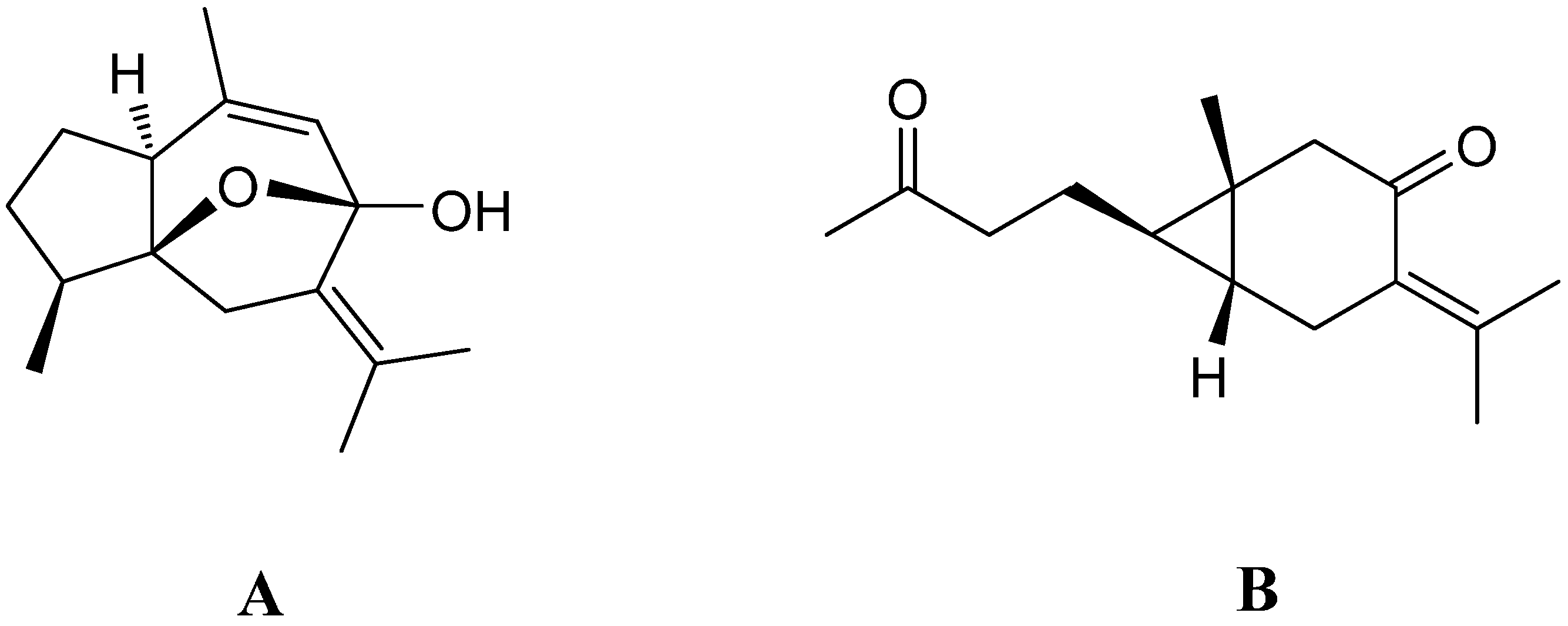
2. Results and Discussion
2.1. Quenching Mechanism
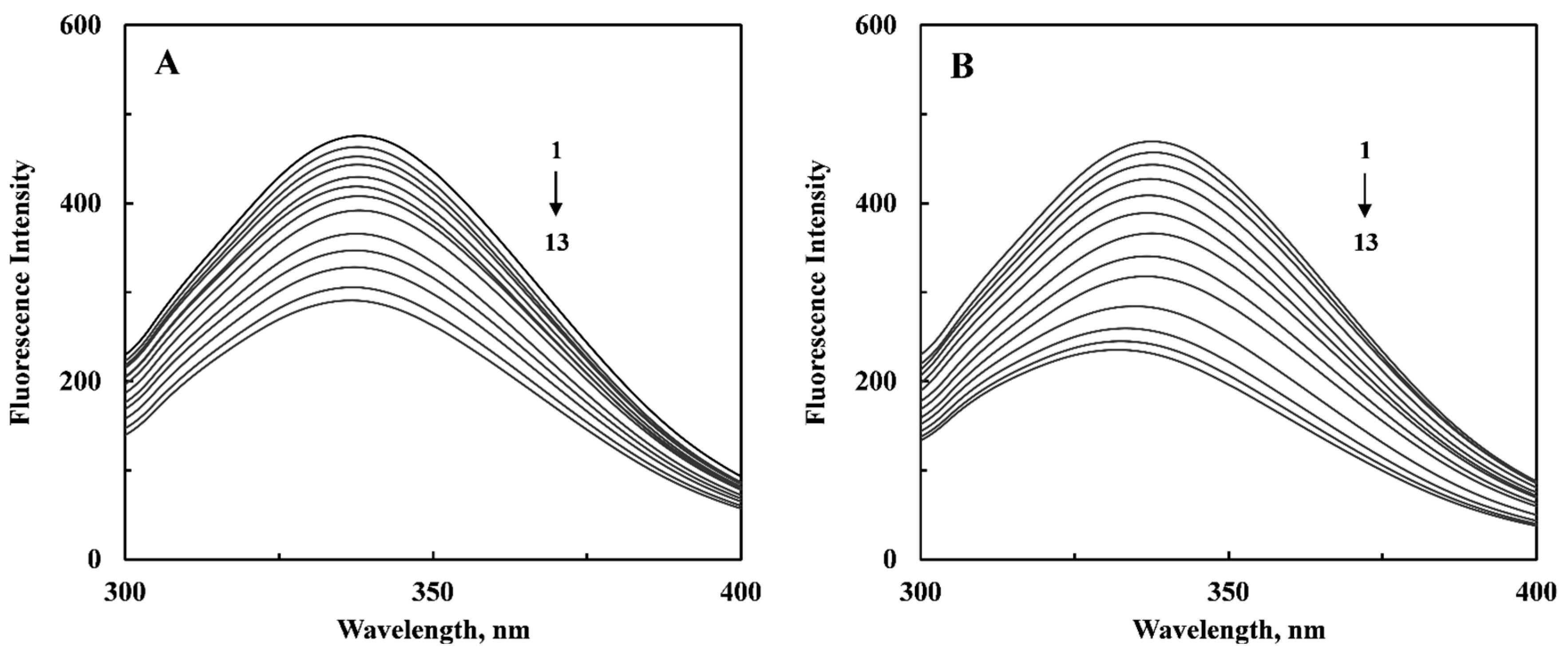
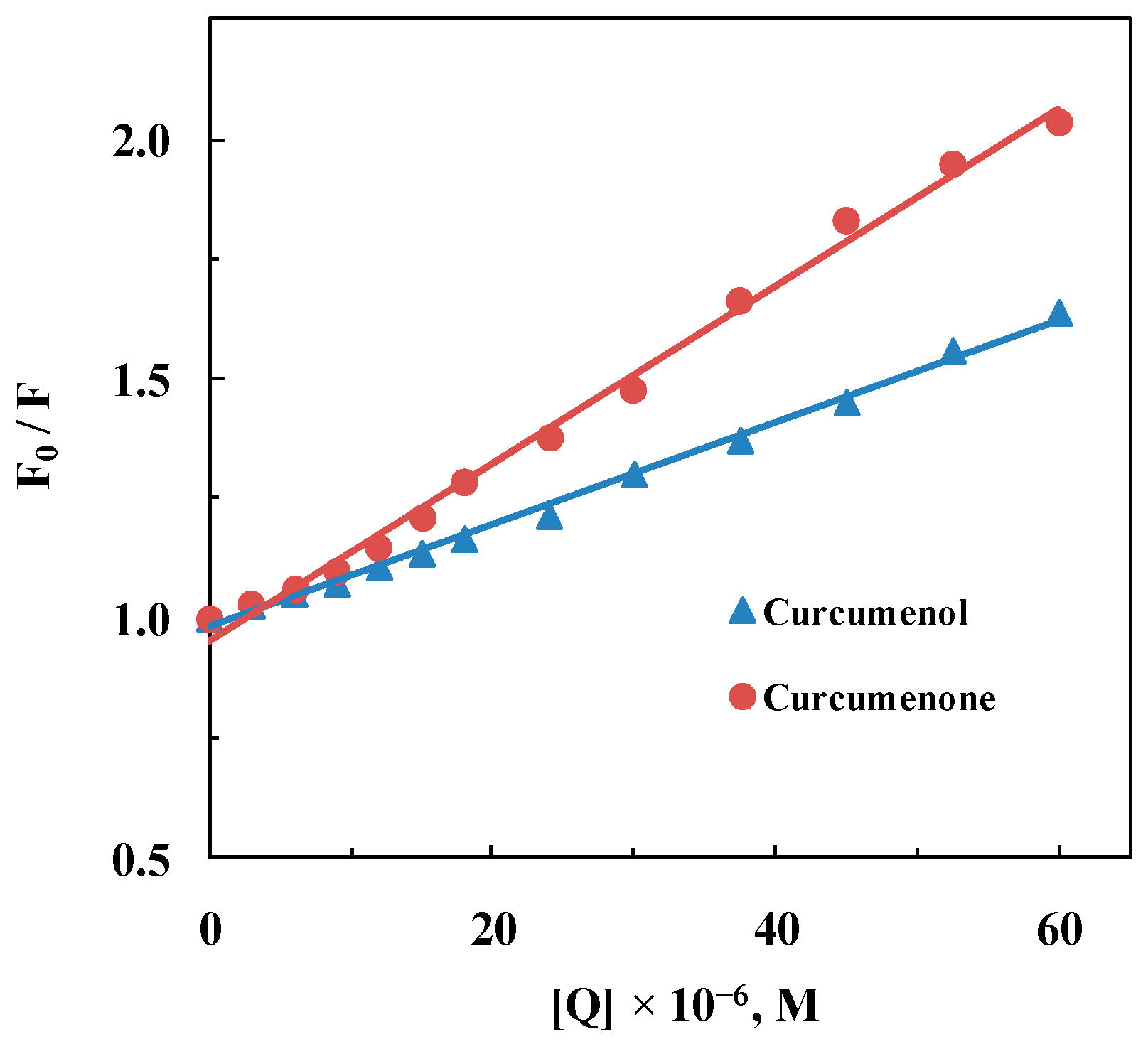
| Parameter | Curcumenol | Curcumenone |
|---|---|---|
| KSV, M−1 | 1.07 × 104 | 1.85 × 104 |
| kq, M−1·s−1 | 1.67 × 1012 | 2.90 × 1012 |
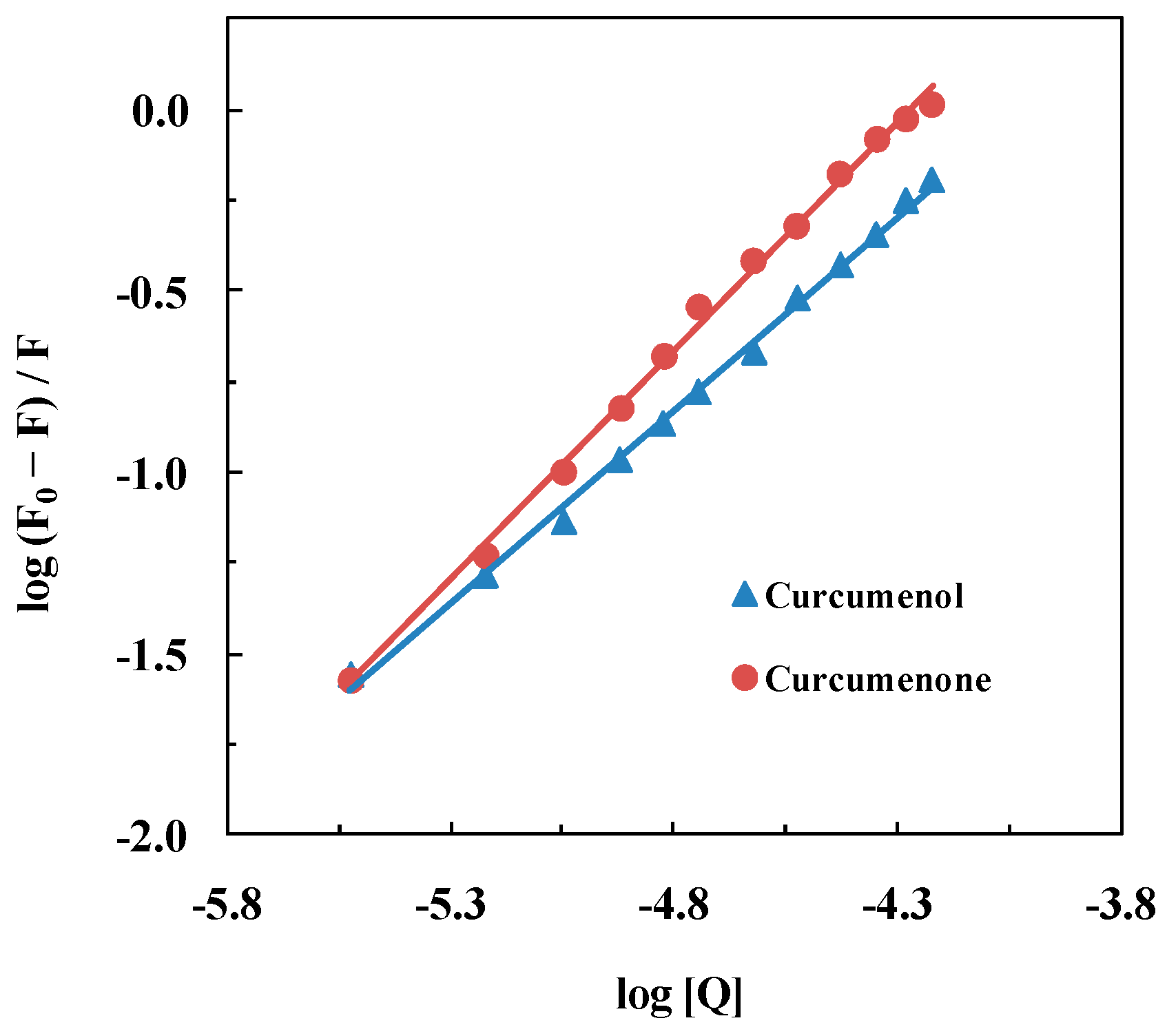
| Kb, M−1 | 1.97 × 104 | 2.46 × 105 |
| n | 1.07 | 1.26 |
2.2. Binding Parameters
2.3. Molecular Docking
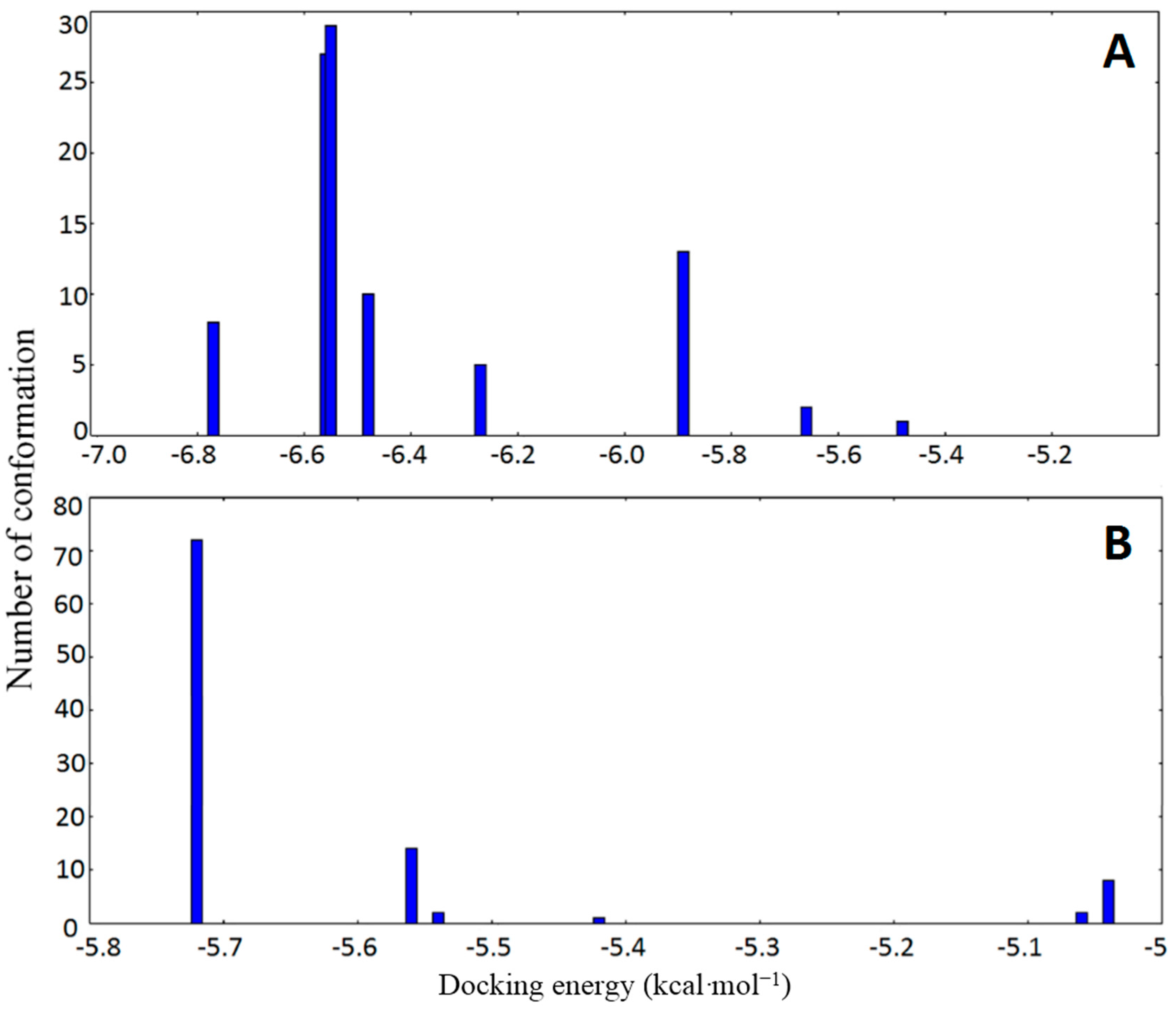
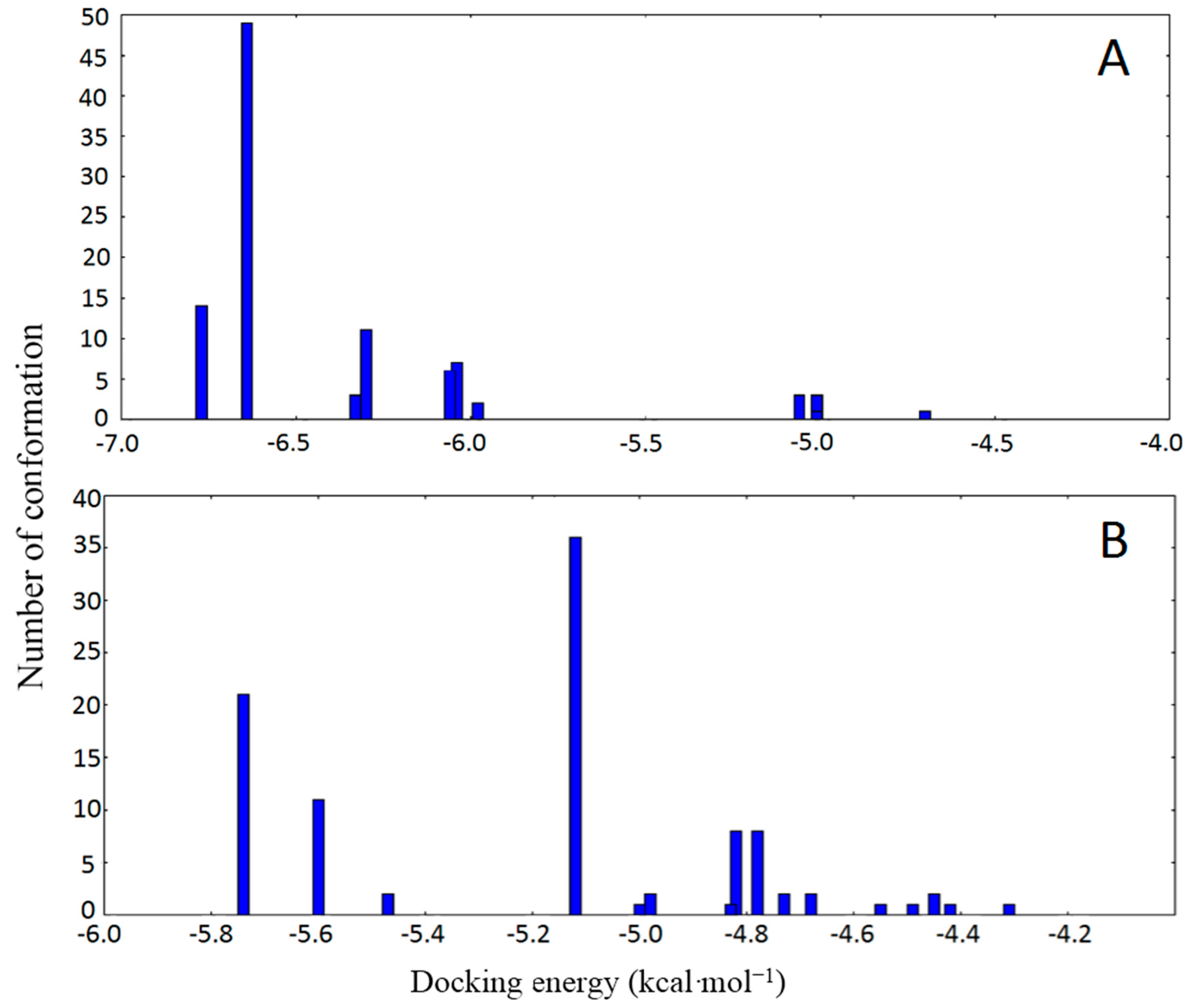
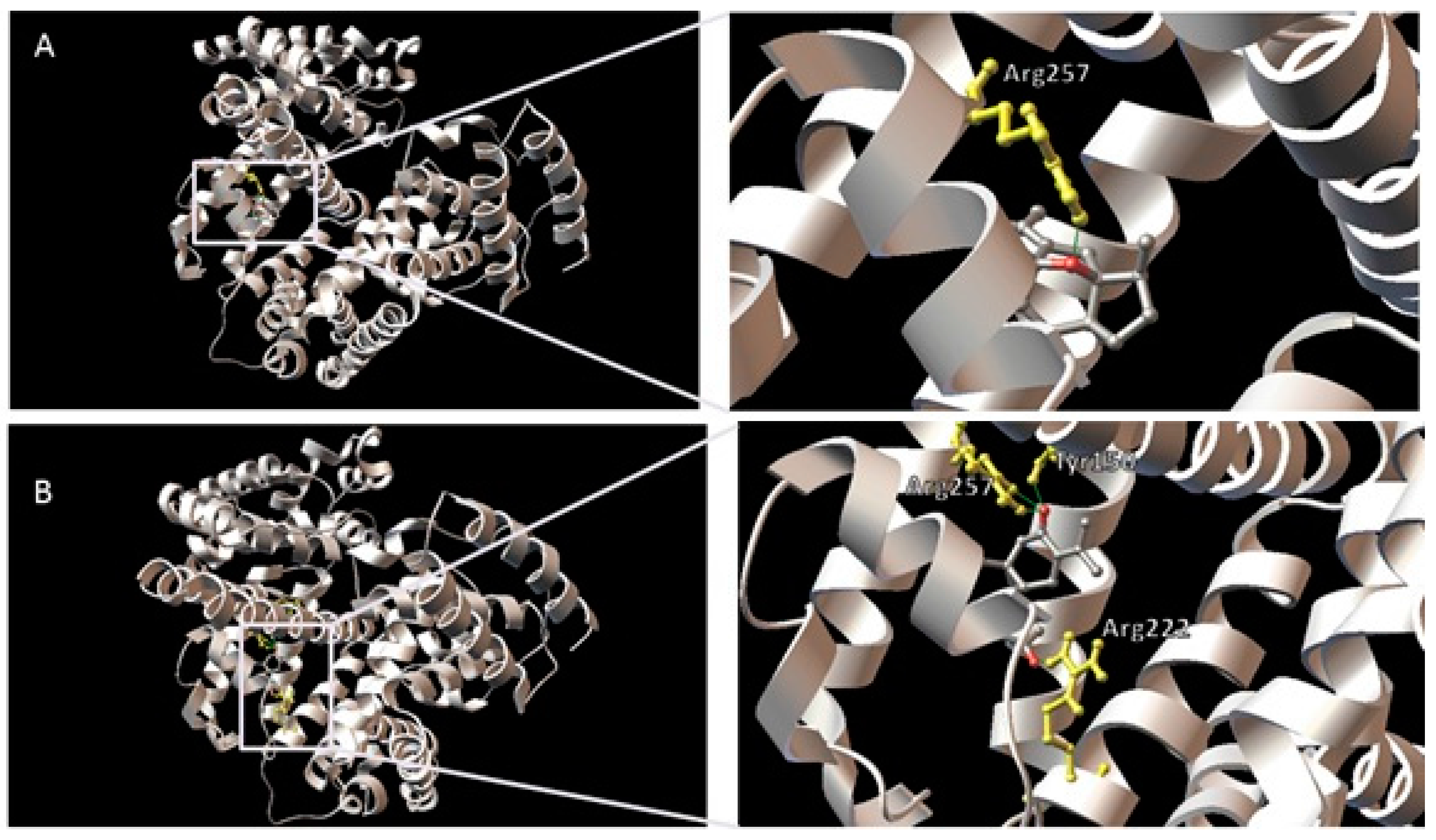
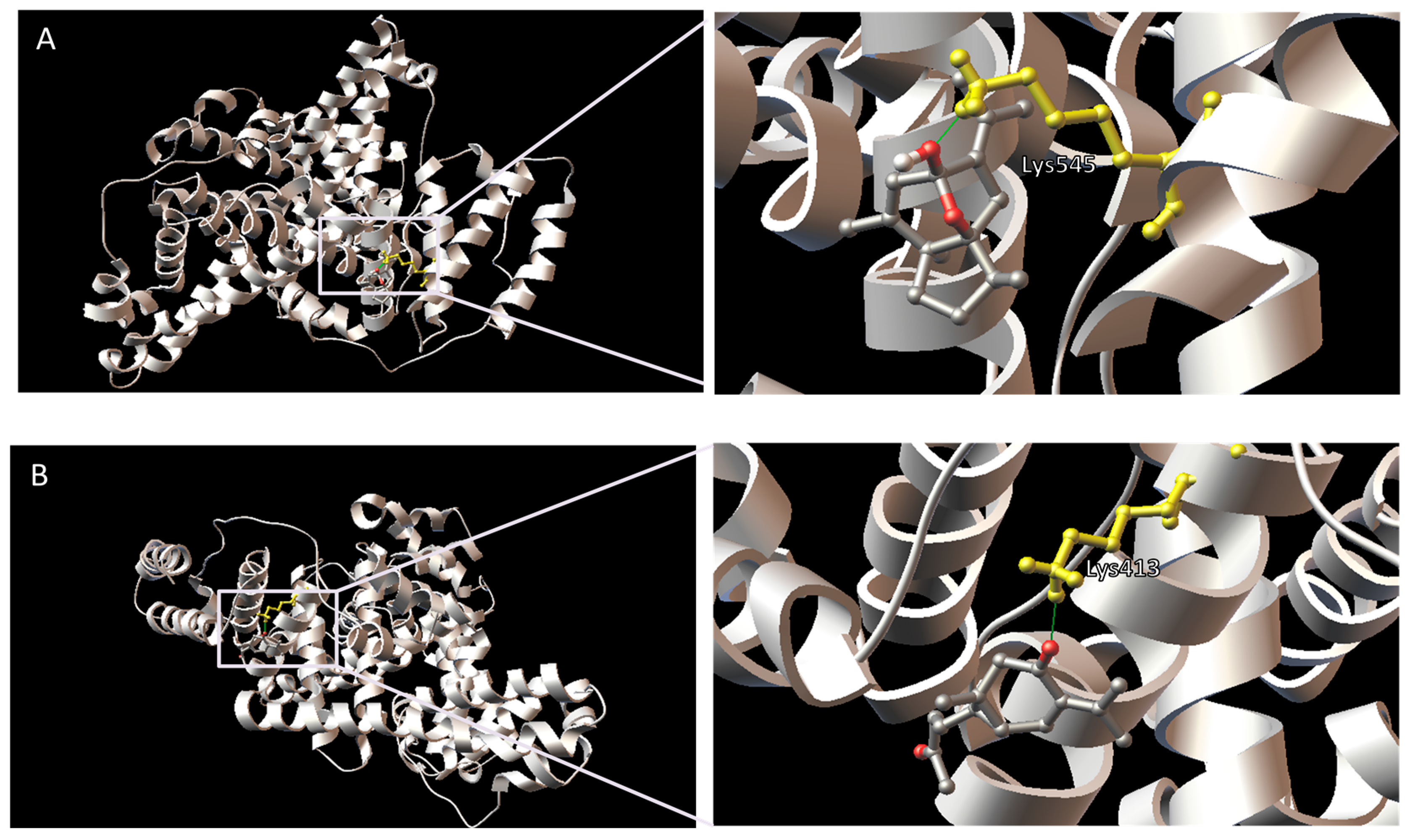
| Compound | HSA Binding Site | Protein Atom | Ligand Atom | Distance (Å) |
|---|---|---|---|---|
| Curcumenol | Site I | Arg257:HH22 | O (ethereal) | 1.907 |
| Site II | Lys545:HZ3 | O (hydroxyl) | 1.684 | |
| Curcumenone | Site I | Tyr150:HH | O (ketone) | 1.907 |
| Arg222:HH11 | O (acetyl) | 1.930 | ||
| Arg257:HE | O (ketone) | 1.969 | ||
| Arg257:HH22 | O (ketone) | 2.236 | ||
| Site II | Lys413:HZ3 | O (ketone) | 2.025 |
3. Experimental Section
3.1. Materials
3.2. Isolation and Purification of Curcumenol and Curcumenone
3.3. Preparation of Protein and Ligand Solutions
3.4. Ligand Binding Studies
3.5. Analysis of the Binding Data
3.6. Molecular Modelling
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lobo, R.; Prabhu, K.S.; Shirwaikar, A.; Shirwaikar, A. Curcuma zedoaria Rosc. (white turmeric): A review of its chemical, pharmacological and ethnomedicinal properties. J. Pharm. Pharmacol. 2009, 61, 13–21. [Google Scholar]
- Etoh, H.; Kondoh, T.; Yoshioka, N.; Sugiyama, K.; Ishikawa, H.; Tanaka, H. 9-Oxo-neoprocurcumenol from Curcuma aromatica (Zingiberaceae) as an attachment inhibitor against the blue mussel, Mytilus edulis galloprovincialis. Biosci. Biotechnol. Biochem. 2003, 67, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Fujii, E.; Endo, M.; Wada, K.; Tokunaga, Y.; Shiba, N.; Hohsho, H.; Shibuya, H.; Muraki, T. Antiinflammatory potency of dehydrocurdione, a zedoary-derived sesquiterpene. Inflamm. Res. 1998, 47, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Makabe, H.; Maru, N.; Kuwabara, A.; Kamo, T.; Hirota, M. Anti-inflammatory sesquiterpenes from Curcuma zedoaria. Nat. Prod. Res. 2006, 20, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Abraham, G.; Manju, V.S.; Mathew, M.; Vimala, B.; Sundaresan, S.; Nambisan, B. Antimicrobial activity of Curcuma zedoaria and Curcuma malabarica tubers. J. Ethnopharmacol. 2005, 99, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Syu, W.J.; Shen, C.C.; Don, M.J.; Ou, J.C.; Lee, G.H.; Sun, C.M. Cytotoxicity of curcuminoids and some novel compounds from Curcuma zedoaria. J. Nat. Prod. 1998, 61, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.; Ibrahim, H.; Khaw, S.; Saw, L. Gingers of Peninsular Malaysia and Singapore; Natural History Publications (Borneo): Kota Kinabalu, Malaysia, 1999. [Google Scholar]
- De Fátima Navarro, D.; de Souza, M.M.; Neto, R.A.; Golin, V.; Niero, R.; Yunes, R.A.; Delle Monache, F.; Cechinel Filho, V. Phytochemical analysis and analgesic properties of Curcuma zedoaria grown in Brazil. Phytomedicine 2002, 9, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, O.A.A.; Rahman, S.N.S.A.; Awang, K.; Wahab, N.A.; Looi, C.Y.; Thomas, N.F.; Malek, S.N.A. Cytotoxic constituents from the rhizomes of Curcuma zedoaria. Sci. World J. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Sukari, M.A.H.; Wah, T.S.; Saad, S.M.; Rashid, N.Y.; Rahmani, M.; Lajis, N.H.; Hin, T.Y. Bioactive sesquiterpenes from Curcuma ochrorhiza and Curcuma heyneana. Nat. Prod. Res. 2010, 24, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Ninomiya, K.; Morikawa, T.; Yoshikawa, M. Inhibitory effect and action mechanism of sesquiterpenes from Zedoariae Rhizoma on d-galactosamine/lipopolysaccharide-induced liver injury. Bioorg. Med. Chem. Lett. 1998, 8, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Sukari, M.A.H.; Saad, S.M.; Lajis, N.H.; Rahmani, M.; Muse, R.; Yusuf, U.K.; Riyanto, S. Chemical constituents and bioactivity of Curcuma aeruginosa Roxb. Nat. Prod. Sci. 2007, 13, 175–179. [Google Scholar]
- Matsuda, H.; Morikawa, T.; Ninomiya, K.; Yoshikawa, M. Absolute stereostructure of carabrane-type sesquiterpene and vasorelaxant-active sesquiterpenes from Zedoariae Rhizoma. Tetrahedron 2001, 57, 8443–8453. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Tamaki, T. Effects of the extracts and an active compound curcumenone isolated from Curcuma zedoaria rhizomes on alcohol-induced drunkenness in mice. Fitoterapia 2013, 84, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Peters, T. All about Albumin: Biochemistry, Genetics, and Medical Applications; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Carter, D.C.; Ho, J.X. Structure of serum albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar] [PubMed]
- Yang, F.; Zhang, Y.; Liang, H. Interactive association of drugs binding to human serum albumin. Int. J. Mol. Sci. 2014, 15, 3580–3595. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, E.; Rabbani, G.; Zaidi, N.; Singh, S.; Rehan, M.; Khan, M.M.; Rahman, S.K.; Quadri, Z.; Shadab, M.; Ashraf, M.T.; et al. Stereo-selectivity of human serum albumin to enantiomeric and isoelectronic pollutants dissected by spectroscopy, calorimetry and bioinformatics. PLoS One 2011, 6, e26186. [Google Scholar]
- Belatik, A.; Hotchandani, S.; Bariyanga, J.; Tajmir-Riahi, H.A. Binding sites of retinol and retinoic acid with serum albumins. Eur. J. Med. Chem. 2012, 48, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Varlan, A.; Hillebrand, M. Bovine and human serum albumin interactions with 3-carboxyphenoxathiin studied by fluorescence and circular dichroism spectroscopy. Molecules 2010, 15, 3905–3919. [Google Scholar] [CrossRef] [PubMed]
- Lackowicz, J.R. Principles of Fluorescence Spectroscopy; Plenum Press: New York, NY, USA, 1983; pp. 111–150. [Google Scholar]
- Freitas, P.G.; Barbosa, A.F.; Saraiva, L.A.; Camps, I.; da Silveira, N.J.F.; Veloso, M.P.; Santos, M.H.; Schneedorf, J.M. Mangiferin binding to serum albumin is non-saturable and induces conformational changes at high concentrations. J. Lumin. 2012, 132, 3027–3034. [Google Scholar] [CrossRef]
- Feroz, S.R.; Mohamad, S.B.; Bujang, N.; Malek, S.N.A.; Tayyab, S. Multispectroscopic and molecular modeling approach to investigate the interaction of flavokawain B with human serum albumin. J. Agric. Food Chem. 2012, 60, 5899–5908. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Nakamura, S.; Qu, Y.; Matsuda, H.; Pongpiriyadacha, Y.; Wu, L.; Yoshikawa, M. Structures of new sesquiterpenes from Curcuma comosa. Chem. Pharm. Bull. 2008, 56, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Firman, K.; Kinoshita, T.; Itai, A.; Sankawa, U. Terpenoids from Curcuma heyneana. Phytochemistry 1988, 27, 3887–3891. [Google Scholar] [CrossRef]
- Wallevik, K. Reversible Denaturation of human serum albumin by pH, temperature, and guanidine hydrochloride followed by optical rotation. J. Biol. Chem. 1973, 248, 2650–2655. [Google Scholar] [PubMed]
- Song, M.; Liu, S.; Yin, J.; Wang, H. Interaction of human serum album and C60 aggregates in solution. Int. J. Mol. Sci. 2011, 12, 4964–4974. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zied, O.K.; Al-Shihi, O.I.K. Characterization of subdomain iia binding site of human serum albumin in its native, unfolded, and refolded states using small molecular probes. J. Am. Chem. Soc. 2008, 130, 10793–10801. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, A.; Villa, L.; Vistoli, G. VEGA: A versatile program to convert, handle and visualize molecular structure on Windows-based PCs. J. Mol. Graph. Model. 2002, 21, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Dewar, M.J.S.; Zoebisch, E.G.; Healy, E.F.; Stewart, J.J.P. Development and use of quantum mechanical molecular models. J. Am. Chem. Soc. 1985, 107, 3902–3909. [Google Scholar]
- Goodsell, D.S.; Morris, G.M.; Olson, A.J. Automated docking of flexible ligands: Applications of autodock. J. Mol. Recognit. 1996, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdi, O.A.A.; Feroz, S.R.; Shilpi, J.A.; Anouar, E.H.; Mukarram, A.K.; Mohamad, S.B.; Tayyab, S.; Awang, K. Spectrofluorometric and Molecular Docking Studies on the Binding of Curcumenol and Curcumenone to Human Serum Albumin. Int. J. Mol. Sci. 2015, 16, 5180-5193. https://doi.org/10.3390/ijms16035180
Hamdi OAA, Feroz SR, Shilpi JA, Anouar EH, Mukarram AK, Mohamad SB, Tayyab S, Awang K. Spectrofluorometric and Molecular Docking Studies on the Binding of Curcumenol and Curcumenone to Human Serum Albumin. International Journal of Molecular Sciences. 2015; 16(3):5180-5193. https://doi.org/10.3390/ijms16035180
Chicago/Turabian StyleHamdi, Omer Abdalla Ahmed, Shevin Rizal Feroz, Jamil A. Shilpi, El Hassane Anouar, Abdul Kadir Mukarram, Saharuddin B. Mohamad, Saad Tayyab, and Khalijah Awang. 2015. "Spectrofluorometric and Molecular Docking Studies on the Binding of Curcumenol and Curcumenone to Human Serum Albumin" International Journal of Molecular Sciences 16, no. 3: 5180-5193. https://doi.org/10.3390/ijms16035180






