Genetic Variant in Interleukin-18 Is Associated with Idiopathic Recurrent Miscarriage in Chinese Han Population
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. The Clinical Characteristics of Study Participants
| Characteristic | Cases | Controls | p Value |
|---|---|---|---|
| Mean age (years) | 31.40 ± 5.08 | 31.97 ± 4.39 | 0.061 |
| Smoking (%) | 36 (7.44) | 45 (9.61) | 0.246 |
| Mean BMI (kg/m2) | 20.89 ± 3.00 | 21.22 ± 2.89 | 0.079 |
| Menarche (years) | 13.11 ± 1.16 | 13.30 ± 1.43 | 0.025 |
| Irregular menstrual history (%) | 63 (13.02) | 38 (8.12) | 0.015 |
| Number of pregnancies | 4.89 ± 1.19 | 2.98 ± 1.27 | <0.0001 |
| Abortion | 3.82 ± 0.731 | 0 ± 0 | <0.0001 |
2.1.2. IL-18 Genotype Distribution
| SNP | Genotype | Case (n = 484) | Control (n = 468) | Additive p Value | Dominant p Value | Recessive p Value |
|---|---|---|---|---|---|---|
| rs360717 | GG | 349 (0.721) | 332 (0.709) | 0.800 | 0.690 | 0.547 |
| AG | 129 (0.267) | 128 (0.274) | ||||
| AA | 6 (0.012) | 8 (0.017) | ||||
| rs187238 | GG | 338 (0.758) | 357 (0.763) | 1.05 × 10−4 | 0.025 | 2.43 × 10−5 |
| GC | 108 (0.223) | 102 (0.218) | ||||
| CC | 38 (0.079) | 9 (0.019) | ||||
| rs1946518 | AA | 181 (0.374) | 178 (0.380) | 0.905 | 0.839 | 0.656 |
| AC | 216 (0.446) | 211 (0.451) | ||||
| CC | 87 (0.180) | 79 (0.169) |
| Model | p Value | Wald Value | OR | 95% CI |
|---|---|---|---|---|
| Additive | 0.021 | 5.345 | 4.343 | 1.250–15.009 |
| Dominant | 0.298 | 1.084 | 1.245 | 0.824–1.881 |
| Recessive | 0.015 | 5.909 | 4.343 | 1.329–14.190 |
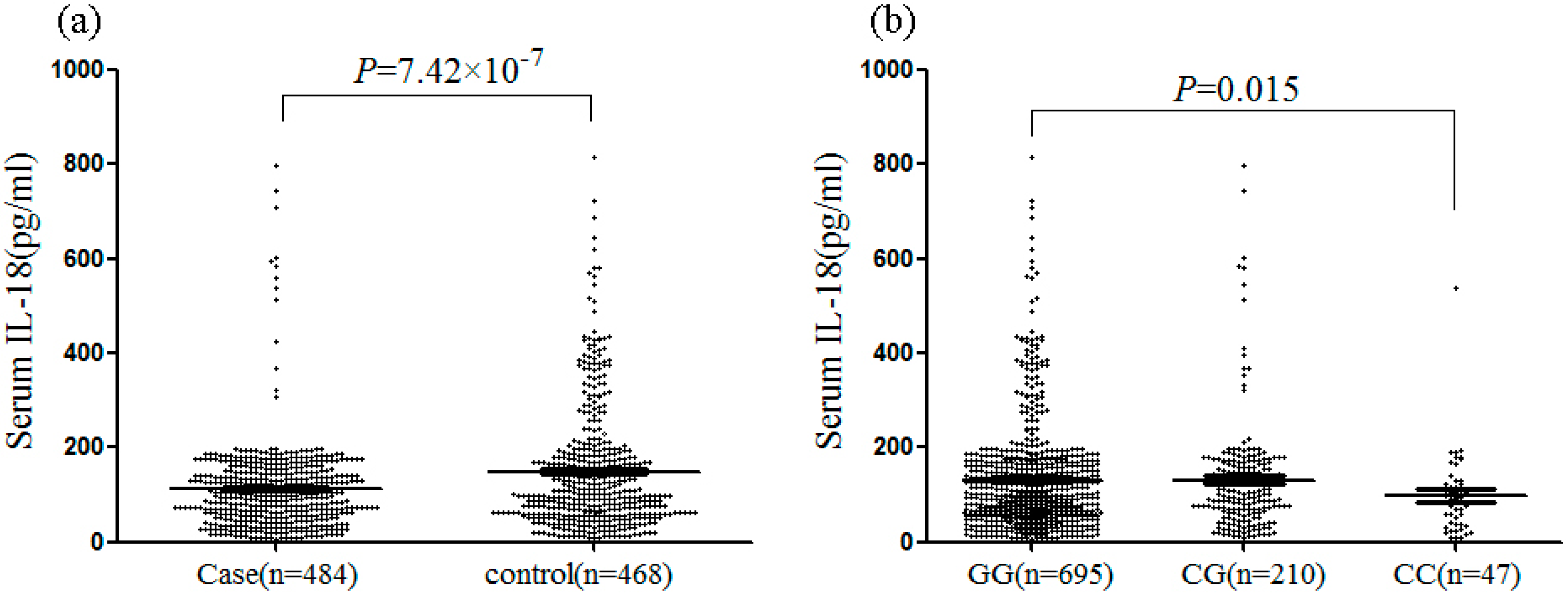
2.1.3. Mean Serum IL-18 Levels
2.2. Discussion
3. Experimental Section
3.1. Patients and Controls
3.2. IL-18 Genotyping
3.3. Serum IL-18 Measurements
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Branch, D.W.; Gibson, M.; Silver, R.M. Clinical practice. Recurrent miscarriage. N. Engl. J. Med. 2010, 363, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Sierra, S.; Stephenson, M. Genetics of recurrent pregnancy loss. Semin. Reprod. Med. 2006, 24, 17–24. [Google Scholar] [CrossRef] [PubMed]
- George, L.; Granath, F.; Johansson, A.L.; Olander, B.; Cnattingius, S. Risks of repeated miscarriage. Paediatr. Perinat. Epidemiol. 2006, 20, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Rull, K.; Nagirnaja, L.; Laan, M. Genetics of recurrent miscarriage: Challenges, current knowledge, future directions. Front. Genet. 2012, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Laird, S.M.; Tuckerman, E.M.; Cork, B.A.; Linjawi, S.; Blakemore, A.I.; Li, T.C. A review of immune cells and molecules in women with recurrent miscarriage. Hum. Reprod. Updat. 2003, 9, 163–174. [Google Scholar] [CrossRef]
- Wilson, R.; Moor, J.; Jenkins, C.; Miller, H.; Walker, J.J.; McLean, M.A.; Norman, J.; McInnes, I.B. Abnormal first trimester serum interleukin 18 levels are associated with a poor outcome in women with a history of recurrent miscarriage. Am. J. Reprod. Immunol. 2004, 51, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Hefler, L.A.; Tempfer, C.B.; Bashford, M.T.; Unfried, G.; Zeillinger, R.; Schneeberger, C.; Koelbl, H.; Nagele, F.; Huber, J.C. Polymorphisms of the angiotensinogen gene, the endothelial nitric oxide synthase gene, and the interleukin-1β gene promoter in women with idiopathic recurrent miscarriage. Mol. Hum. Reprod. 2002, 8, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, V.; Papale, A.; Galli, C.L.; Marinovich, M.; Corsini, E. Role of ROS and HMGB1 in contact allergen-induced IL-18 production in human keratinocytes. J. Investig. Dermatol. 2014, 134, 2719–2727. [Google Scholar] [CrossRef] [PubMed]
- Weidlich, S.; Bulau, A.M.; Schwerd, T.; Althans, J.; Kappler, R.; Koletzko, S.; Mayr, D.; Bufler, P. Intestinal expression of the anti-inflammatory interleukin-1 homologue IL-37 in pediatric inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2014, 59, e18–e26. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, N.; Sukhova, G.K.; Libby, P.; Reynolds, R.S.; Young, J.L.; Schonbeck, U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: Implications for atherogenesis. J. Exp. Med. 2002, 195, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Blom, L.; Poulsen, L.K. IL-1 family members IL-18 and IL-33 upregulate the inflammatory potential of differentiated human Th1 and Th2 cultures. J. Immunol. 2012, 189, 4331–4337. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tan, J.; Wang, Y.; Meldrum, K.K.; Dinarello, C.A.; Meldrum, D.R. IL-18 binding protein-expressing mesenchymal stem cells improve myocardial protection after ischemia or infarction. Proc. Natl. Acad. Sci. USA 2009, 106, 17499–17504. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Choi, J.M.; Kim, B.H.; Lee, C.M.; Cho, W.K.; Choe, G.; Kim, D.H.; Lee, C.G.; Elias, J.A. IL-18 induces emphysema and airway and vascular remodeling via IFN-γ, IL-17A, and IL-13. Am. J. Respir. Crit. Care Med. 2012, 185, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 binding protein. Front. Immunol. 2013, 4, 289. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.; Volk, M.; Medica, I.; Kapovic, M.; Meden-Vrtovec, H.; Peterlin, B. Polymorphisms in the interleukin-12/18 genes and recurrent spontaneous abortion. Am. J. Reprod. Immunol. 2007, 58, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Al-Khateeb, G.M.; Sater, M.S.; Finan, R.R.; Mustafa, F.E.; Al-Busaidi, A.S.; Al-Sulaiti, M.A.; Almawi, W.Y. Analysis of interleukin-18 promoter polymorphisms and changes in interleukin-18 serum levels underscores the involvement of interleukin-18 in recurrent spontaneous miscarriage. Fertil. Steril. 2011, 96, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, S.; Ghiam, A.F.; Mojtahedi, Z.; Dehaghani, A.S.; Amani, D.; Ghaderi, A. Interleukin-18 gene promoter polymorphisms and recurrent spontaneous abortion. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 128, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Barbaux, S.; Poirier, O.; Godefroy, T.; Kleinert, H.; Blankenberg, S.; Cambien, F.; Tiret, L. Differential haplotypic expression of the interleukin-18 gene. Eur. J. Hum. Genet. 2007, 15, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Khripko, O.P.; Sennikova, N.S.; Lopatnikova, J.A.; Khripko, J.I.; Filipenko, M.L.; Khrapov, E.A.; Gelfgat, E.L.; Yakushenko, E.V.; Kozlov, V.A.; Sennikov, S.V. Association of single nucleotide polymorphisms in the IL-18 gene with production of IL-18 protein by mononuclear cells from healthy donors. Mediat. Inflamm. 2008, 2008, 309721. [Google Scholar]
- Messaoudi, S.; Dandana, M.; Magdoud, K.; Meddeb, S.; Ben Slama, N.; Hizem, S.; Mahjoub, T. Interleukin-18 promoter polymorphisms and risk of idiopathic recurrent pregnancy loss in a Tunisian population. J. Reprod. Immunol. 2012, 93, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P. Interleukin-18: Recent advances. Curr. Opin. Hematol. 2004, 11, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Sedimbi, S.K.; Hagglof, T.; Karlsson, M.C. IL-18 in inflammatory and autoimmune disease. Cell. Mol. Life Sci. 2013, 70, 4795–4808. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Chiasson, V.L.; Bounds, K.R.; Mitchell, B.M. Regulation of the Anti-Inflammatory Cytokines Interleukin-4 and Interleukin-10 during Pregnancy. Front. Immunol. 2014, 5, 253. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Jenkins, C.; Miller, H.; McInnes, I.B.; Moore, J.; McLean, M.A.; Walker, J.J. Abnormal cytokine levels in non-pregnant women with a history of recurrent miscarriage. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, M.D.; Ross, J.W.; Stein, D.R.; White, F.J.; Desilva, U.W.; Geisert, R.D. Endometrial caspase 1 and interleukin-18 expression during the estrous cycle and peri-implantation period of porcine pregnancy and response to early exogenous estrogen administration. Reprod. Biol. Endocrinol. 2010, 8, 33. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, J.; Tong, Y.; Zhou, J.; Liu, Q.; Yang, J. Genetic Variant in Interleukin-18 Is Associated with Idiopathic Recurrent Miscarriage in Chinese Han Population. Int. J. Mol. Sci. 2015, 16, 4180-4189. https://doi.org/10.3390/ijms16024180
Yue J, Tong Y, Zhou J, Liu Q, Yang J. Genetic Variant in Interleukin-18 Is Associated with Idiopathic Recurrent Miscarriage in Chinese Han Population. International Journal of Molecular Sciences. 2015; 16(2):4180-4189. https://doi.org/10.3390/ijms16024180
Chicago/Turabian StyleYue, Jun, Yu Tong, Jing Zhou, Qingqing Liu, and Jiyun Yang. 2015. "Genetic Variant in Interleukin-18 Is Associated with Idiopathic Recurrent Miscarriage in Chinese Han Population" International Journal of Molecular Sciences 16, no. 2: 4180-4189. https://doi.org/10.3390/ijms16024180
APA StyleYue, J., Tong, Y., Zhou, J., Liu, Q., & Yang, J. (2015). Genetic Variant in Interleukin-18 Is Associated with Idiopathic Recurrent Miscarriage in Chinese Han Population. International Journal of Molecular Sciences, 16(2), 4180-4189. https://doi.org/10.3390/ijms16024180





