Synthesis of a Temperature-Sensitive Matrine-Imprinted Polymer and Its Potential Application for the Selective Extraction of Matrine from Radix Sophorae Tonkinensis
Abstract
:1. Introduction
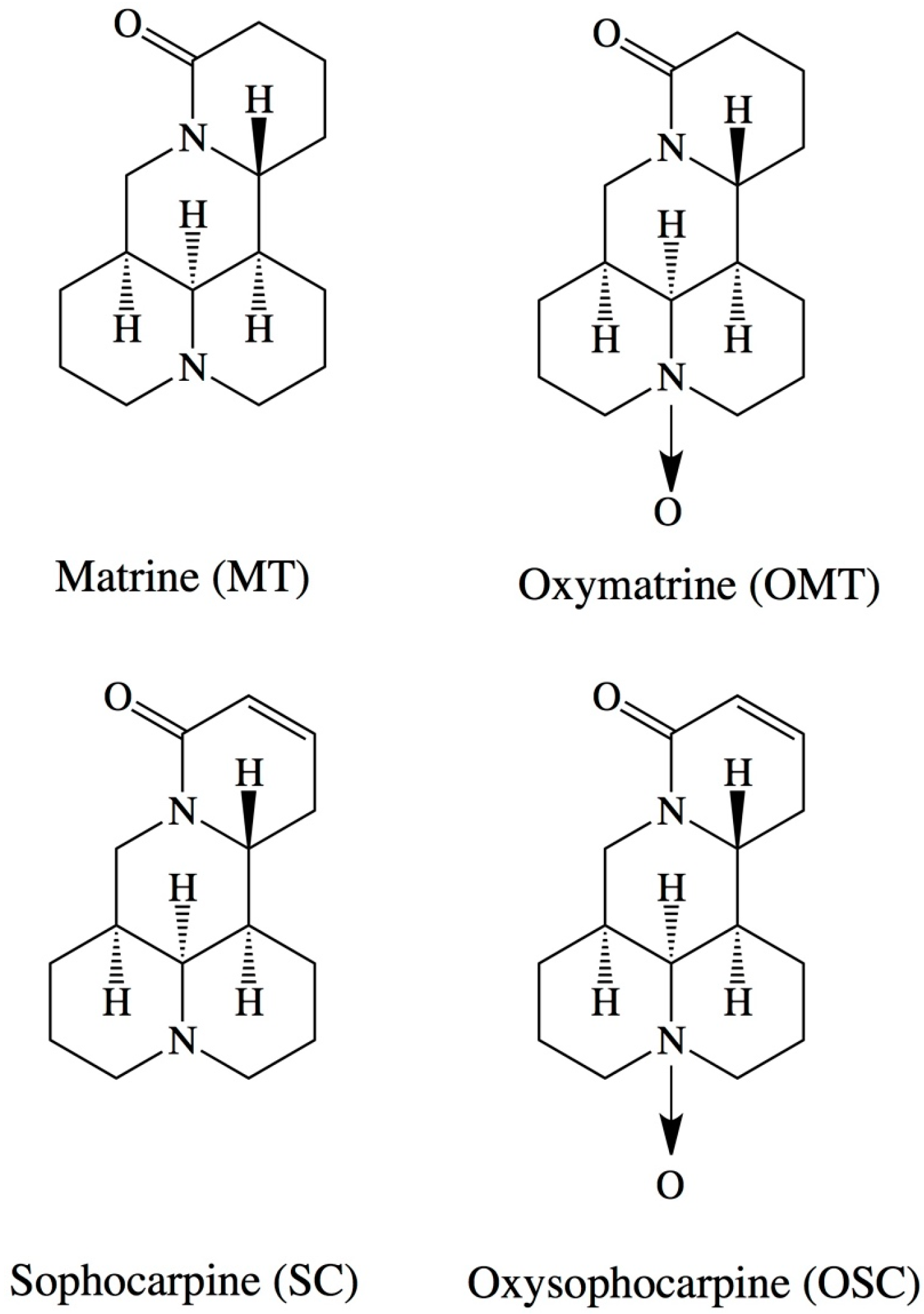
2. Results and Discussion
2.1. Polymer Synthesis and Characterization
| Composition (mmol) | MIP1 | NIP1 | MIP2 | NIP2 | MIP3 | NIP3 | MIP4 | NIP4 |
|---|---|---|---|---|---|---|---|---|
| Matrine (MT) | 1.5 | 0 | 1.5 | 0 | 1.5 | 0 | 1.5 | 0 |
| Methacrylic acid (MAA) | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Ethylene glycol dimethacrylate (EDMA) | 9.6 | 9.6 | 14.4 | 14.4 | 19.2 | 19.2 | 28.8 | 28.8 |
| N-isopropyl acrylamide (NIPA) | 19.2 | 19.2 | 14.4 | 14.4 | 9.6 | 9.6 | 0 | 0 |
| 2,2′-Azobis(2-isobutyronitrile) (AIBN) | 1.9 | 1.9 | 1.9 | 1.9 | 1.9 | 1.9 | 1.9 | 1.9 |
| Polymer | Particle Size a (μm) | Pore Diameter (nm) | Pore Volume (μL/g) | BET Surface Area (m2/g) |
|---|---|---|---|---|
| MIP1 | 5.52 ± 0.03 | 21.19 ± 0.32 | 2.73 ± 0.18 | 7.42 ± 0.32 |
| NIP1 | 6.01 ± 0.04 | 19.48 ± 0.29 | 1.91 ± 0.11 | 6.35 ± 0.28 |
| MIP2 | 5.62 ± 0.03 | 18.25 ± 0.24 | 1.53 ± 0.09 | 6.28 ± 0.31 |
| NIP2 | 6.33 ± 0.05 | 17.89 ± 0.23 | 1.65 ± 0.10 | 5.70 ± 0.22 |
| MIP3 | 7.72 ± 0.06 | 13.32 ± 0.27 | 1.27 ± 0.08 | 4.41 ± 0.19 |
| NIP3 | 9.37 ± 0.08 | 11.89 ± 0.17 | 1.19 ± 0.09 | 2.92 ± 0.12 |
| MIP4 | 8.23 ± 0.08 | 16.11 ± 0.19 | 1.02 ± 0.07 | 3.62 ± 0.14 |
| NIP4 | 12.78 ± 0.14 | 14.45 ± 0.23 | 0.76 ± 0.04 | 2.18 ± 0.11 |
2.2. Binding Experiments
| Temperature (°C) | MIP2 | NIP2 | ||||||
|---|---|---|---|---|---|---|---|---|
| High-Affinity Site | Low-Affinity Site | High-Affinity Site | Low-Affinity Site | |||||
| Kd ± SE a (μM) | Qmax ± SE (μmol/g) | Kd ± SE (μM) | Qmax ± SE (μmol/g) | Kd ± SE (μM) | Qmax ± SE (μmol/g) | Kd ± SE (μM) | Qmax ± SE (μmol/g) | |
| 50 | 507 ± 63.5 | 197 ± 3.92 | 4210 ± 192 | 422 ± 66.3 | – | – | 1720 ± 277 | 225 ± 15.8 |
| 25 | – | – | 1620 ± 227 | 204 ± 24.5 | – | – | 1910 ± 223 | 202 ± 16.4 |
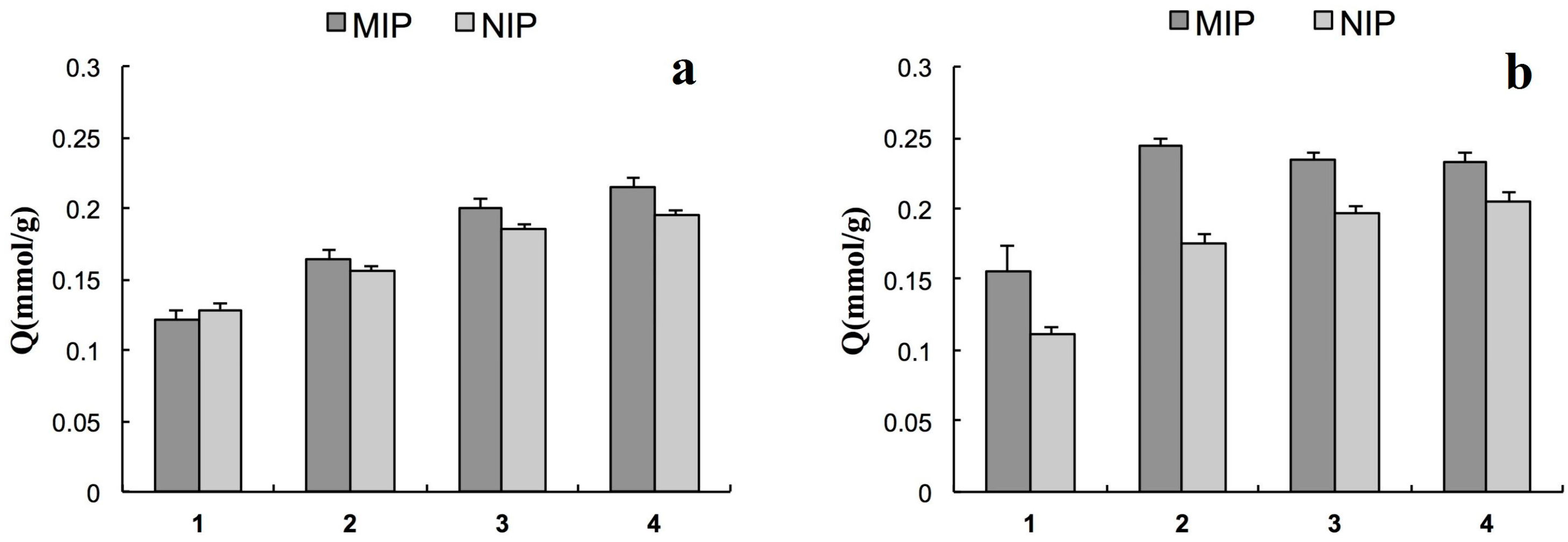
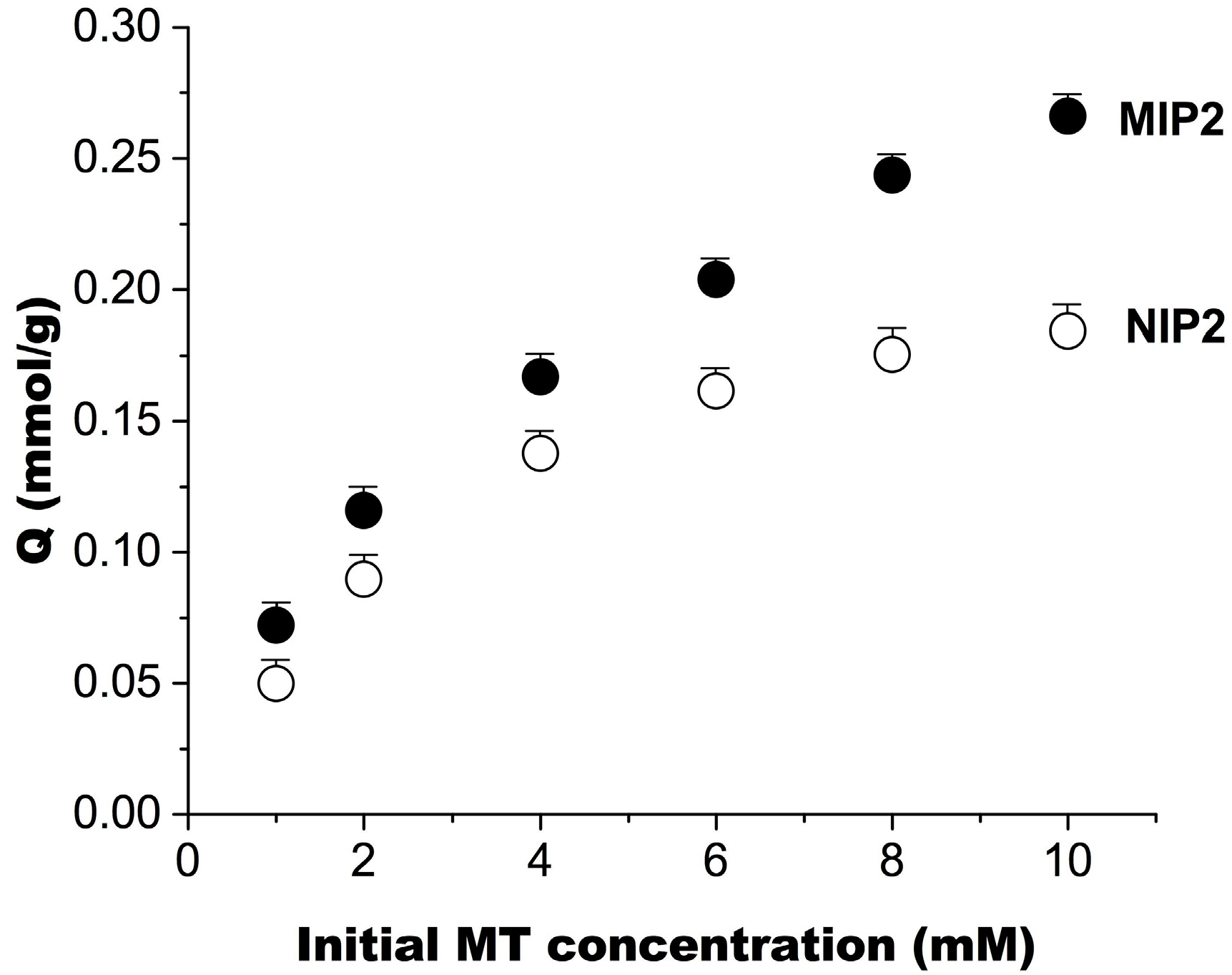
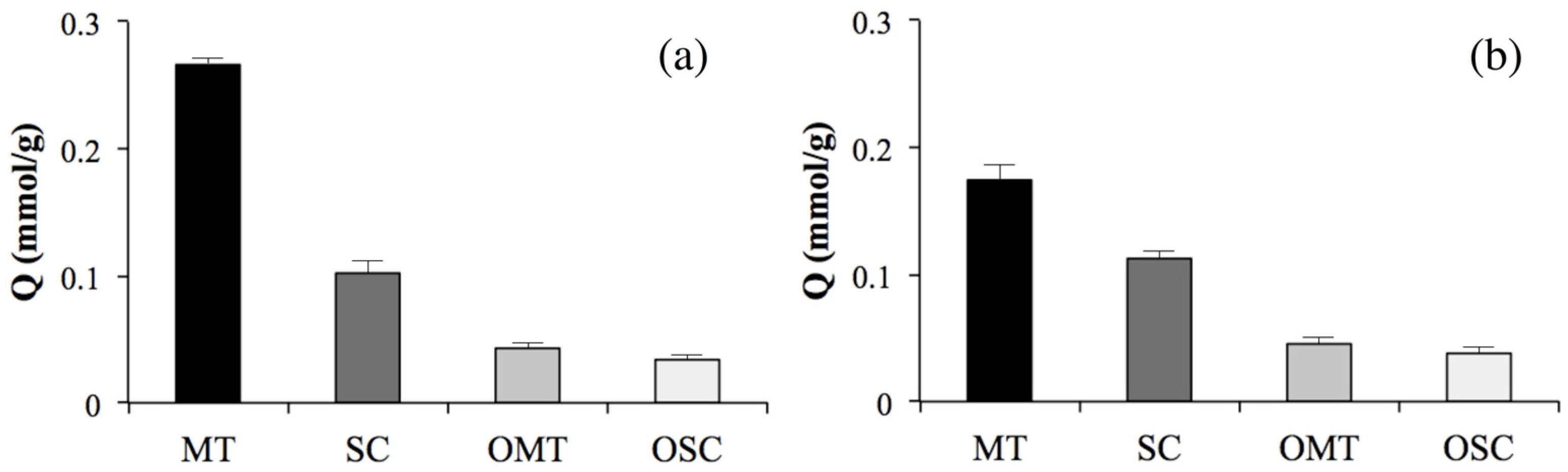
2.3. Molecular Recognition Properties of MIP2
2.4. Temperature Effect on Recognition Ability
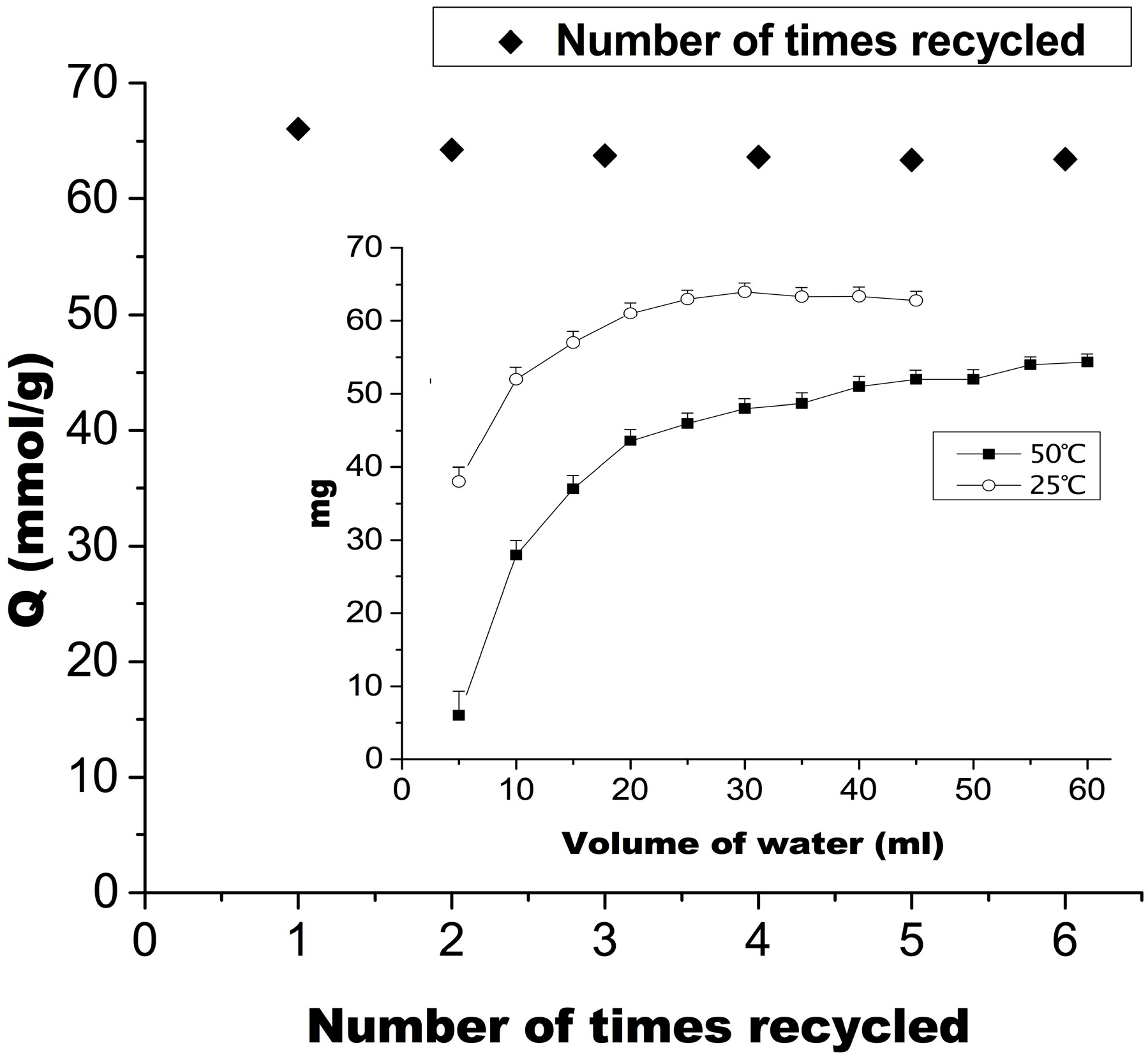
2.5. Recycling Times and Elution Process of MIP2

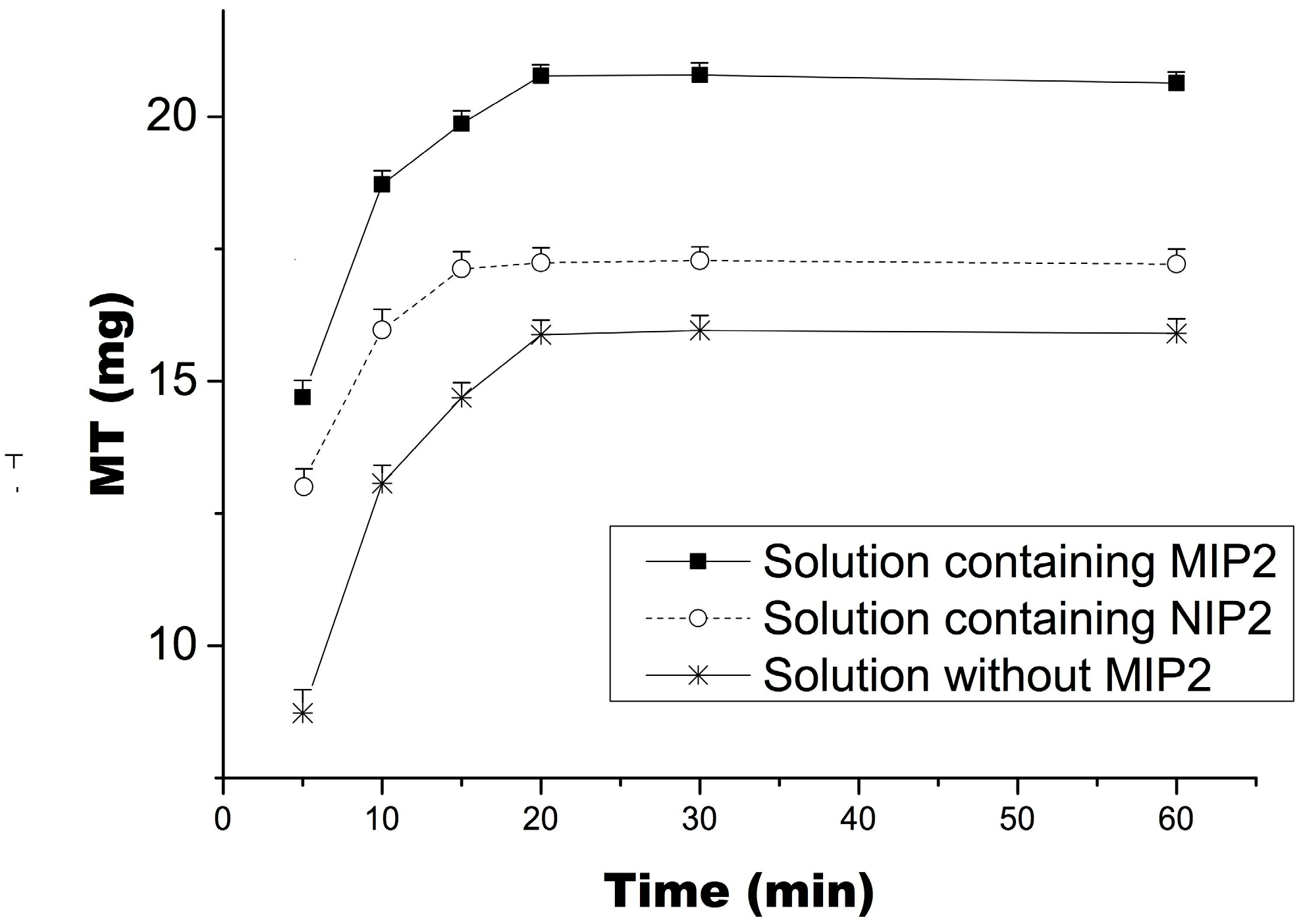
2.6. Selective Extraction of Matrine from Radix Sophorae Tonkinensis by MIP2 Coupled with Ultrasonic Agitation and Desorption

3. Experimental Section
3.1. Chemicals and Materials
3.2. Instrumentation
3.3. Polymer Synthesis
3.4. Characterization Methods
3.5. Binding Experiments
3.6. Determination of the Thermoresponsive Polymers’ Binding Characteristics
3.7. Recycling Times and Elution Process of MIP2
3.8. Selective MT Extraction from Radix Sophorae Tonkinensis by MIP2 Coupled with Ultrasonic Agitation and Desorption
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haginaka, J. Monodispersed, molecularly imprinted polymers as affinity-based chromatography media. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 866, 3–13. [Google Scholar] [CrossRef]
- Turiel, E.; Martin-Esteban, A. Molecularly imprinted polymers for sample preparation: A review. Anal. Chim. Acta 2010, 668, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef] [PubMed]
- Rachkov, A.; Minoura, N. Towards molecularly imprinted polymers selective to peptides and proteins. The epitope approach. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2001, 1544, 255–266. [Google Scholar] [CrossRef]
- Slinchenko, O.; Rachkov, A.; Miyachi, H.; Ogiso, M.; Minoura, N. Imprinted polymer layer for recognizing double-stranded DNA. Biosens. Bioelectron. 2004, 20, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.E.; Park, K.; Peppas, N.A. Molecular imprinting within hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, M.; Nagai, K. Shrinking kinetics of poly (N-isopropylacrylamide) gels T-jumped across their volume phase transition temperatures. Macromolecules 1999, 32, 7461–7468. [Google Scholar] [CrossRef]
- Xin, H.B.; Liu, S.F. Effects of matrine on myocardial contraction and arrhythmia in isolated heart atria. Acta Pharmacol. Sin. 1987, 8, 501–505. [Google Scholar]
- Hu, Z.L.; Zhang, J.P.; Yu, X.B.; Lin, W.; Qian, D.H.; Wan, M.B. Effect of matrine on lipopolysaccharides/d-galactosamine-induced hepatitis and tumor necrosis factor release from macrophages in vitro. Zhongguo Yao Li Xue Bao 1996, 17, 351–353. [Google Scholar] [PubMed]
- Lai, J.-P.; He, X.-W.; Jiang, Y.; Chen, F. Preparative separation and determination of matrine from the Chinese medicinal plant Sophora flavescens Ait by molecularly imprinted solid-phase extraction. Anal. Bioanal. Chem. 2003, 375, 264–269. [Google Scholar] [PubMed]
- Funaya, N.; Haginaka, J. Matrine- and oxymatrine-imprinted monodisperse polymers prepared by precipitation polymerization and their applications for the selective extraction of matrine-type alkaloids from Sophora flavescens Aiton. J. Chromatogr. A 2012, 1248, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Fang, Q.; Feng, B.; Sun, S.; Du, W.; Amut, E.; Xiao, A.; Chang, C. Matrine-imprinted monolithic stationary phase for extraction and purification of matrine from Sophorae flavescentis Ait. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2011, 879, 894–900. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, L.; Song, C.; Zhang, X. Molecularly imprinted solid-phase extraction of matrine from radix Sophorae tonkinensis. Analyst 2011, 136, 3016–3022. [Google Scholar] [CrossRef] [PubMed]
- Vlatakis, G.; Andersson, L.I.; Müller, R.; Mosbach, K. Drug assay using antibody mimics made by molecular imprinting. Nature 1993, 361, 645–647. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Wang, L.; Liu, X.; Yang, H.; Ren, F.; Gan, L.; Jiang, W. Synthesis of a Temperature-Sensitive Matrine-Imprinted Polymer and Its Potential Application for the Selective Extraction of Matrine from Radix Sophorae Tonkinensis. Int. J. Mol. Sci. 2015, 16, 3441-3451. https://doi.org/10.3390/ijms16023441
Jiang M, Wang L, Liu X, Yang H, Ren F, Gan L, Jiang W. Synthesis of a Temperature-Sensitive Matrine-Imprinted Polymer and Its Potential Application for the Selective Extraction of Matrine from Radix Sophorae Tonkinensis. International Journal of Molecular Sciences. 2015; 16(2):3441-3451. https://doi.org/10.3390/ijms16023441
Chicago/Turabian StyleJiang, Minjie, Lisheng Wang, Xu Liu, Hua Yang, Fan Ren, Lizhen Gan, and Weizhe Jiang. 2015. "Synthesis of a Temperature-Sensitive Matrine-Imprinted Polymer and Its Potential Application for the Selective Extraction of Matrine from Radix Sophorae Tonkinensis" International Journal of Molecular Sciences 16, no. 2: 3441-3451. https://doi.org/10.3390/ijms16023441





