Budesonide-Loaded Guar Gum Microspheres for Colon Delivery: Preparation, Characterization and in Vitro/in Vivo Evaluation
Abstract
:1. Introduction
2. Results and Discussion
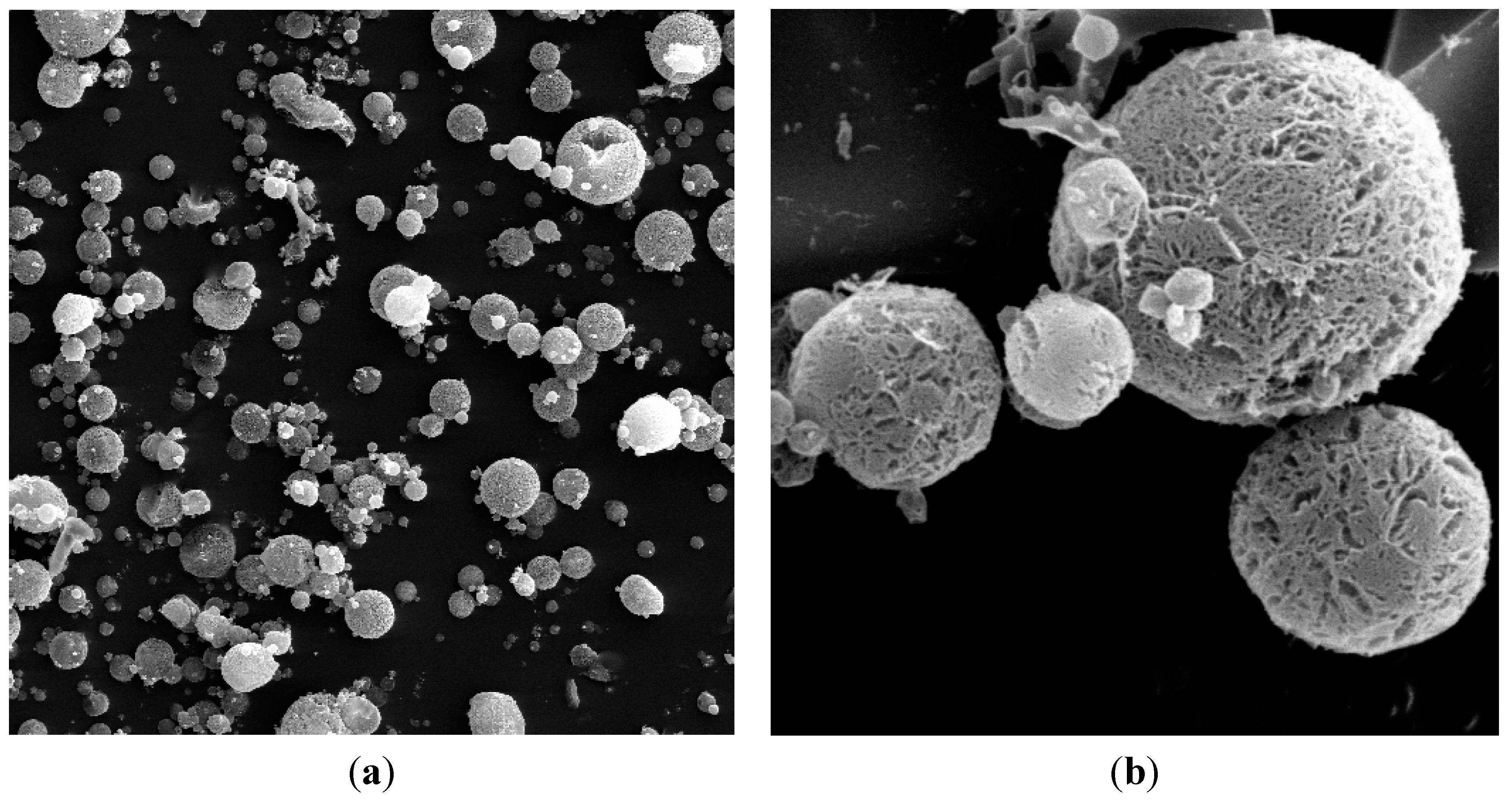
2.1. Physicochemical Characterization
2.2. In Vitro Release

2.3. Pharmacokinetic Studies
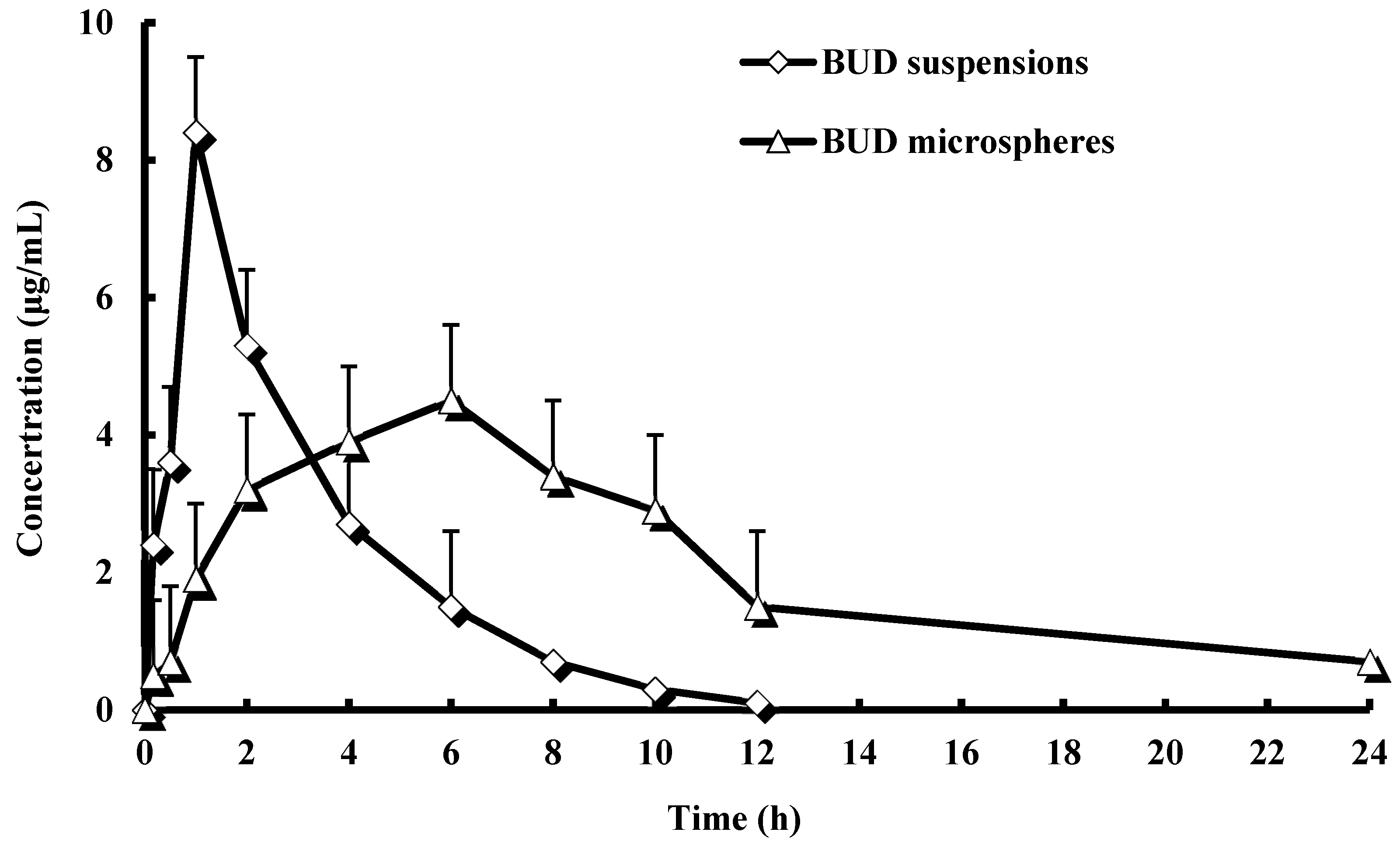
| Parameter | Formulations | |
|---|---|---|
| Suspensions | Microspheres | |
| t1/2α (h) | 0.34 ± 0.12 | 0.87 ± 0.35 * |
| t1/2β (h) | 2.16 ± 1.03 | 5.58 ± 1.72 * |
| Cmax (µg/mL) | 8.41 ± 2.12 | 4.57 ± 1.34 * |
| AUC0–t (µg·h/mL) | 27.45 ± 9.61 | 50.74 ± 15.29 * |
| AUC0–∞ (µg·h/mL) | 35.63 ± 11.42 | 67.82 ± 21.32 * |
| MRT (h) | 1.16 ± 1.08 | 4.62 ± 1.32 * |
| CL (L/h) | 3.21 ± 0.83 | 0.82 ± 0.12 * |
2.4. Evaluation of Colon Targeting
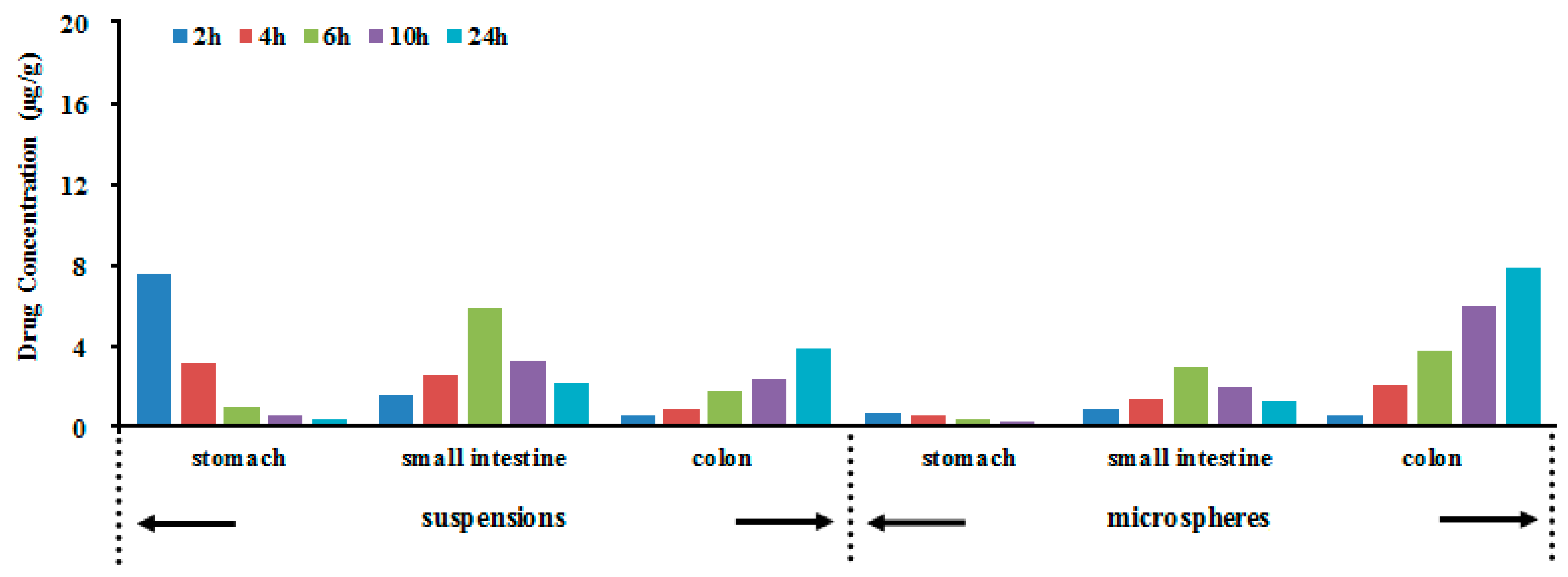
| Formulation | Stomach | Small Intestine | Colon |
|---|---|---|---|
| BUD suspensions (µg·h/g) | 23.63 ± 5.03 | 67.54 ± 19.51 | 54.81 ± 16.71 |
| BUD microspheres (µg·h/g) | 5.65 ± 1.38 | 35.26 ± 11.79 | 117.62 ± 21.82 |
| Ratio a | 0.24 | 0.52 | 2.15 * |
3. Materials
3.1. Microspheres Preparation
3.2. Physicochemical Characterization
3.2.1. Particle Size Analysis
3.2.2. Scanning Electron Microscopy
3.2.3. Drug Loading
3.3. In Vitro Drug Release
3.4. Pharmacokinetic Studies
3.5. Evaluation of Colon Targeting [17]
3.6. Analysis Method
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Varshosaz, J.; Emami, J.; Trvakoli, N.; Fassihi, A.; Minaiyan, M.; Ahmadi, F.; Dorkoosh, F. Synthesis and evaluation of dextran-budesonide conjugates as colon specific prodrugs for treatment of ulcerative colitis. Int. J. Pharm. 2009, 365, 69–76. [Google Scholar] [CrossRef] [PubMed]
- El-gendy, N.; Gorman, E.M.; Munson, E.J.; Berkland, C. Budesondie nanoparticle agglomerates as dry powder aerosols with rapid dissolution. J. Pharm. Sci. 2009, 98, 2731–2746. [Google Scholar] [CrossRef] [PubMed]
- Odze, R. Diagnostic problems and advances in inflammatory bowel disease. Mod. Pathol. 2003, 16, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Sbreu, M.T.; Cohen, R.; Tremaine, W. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006, 130, 940–987. [Google Scholar] [CrossRef] [PubMed]
- Karrouta, Y.; Neutb, C.; Wilsc, D.; Siepmann, F.; Deremaux, L.; Desreumaux, P.; Siepmann, J. Novel polymeric film coatings for colon targeting: How to adjust desired membrane properties. Int. J. Pharm. 2009, 371, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, M.K.; Jain, S.K. Pharmaceutical approaches to colon targeted drug delivery systems. J. Pharm. Pharm. Sci. 2003, 6, 33–66. [Google Scholar] [PubMed]
- Vandamme, T.F.; Lenourry, A.; Charrueau, C.; Chaumeil, J.C. The use of polysaccharides to target drugs to the colon. Carbohydr. Polym. 2002, 48, 219–231. [Google Scholar] [CrossRef]
- Chourasia, M.K.; Jain, S.K. Design and development of multi particulate system for targeted drug delivery to colon. Drug Deliv. 2004, 11, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, M.K.; Jain, S.K. Polysaccharides for colon targeted drug delivery. Drug Deliv. 2004, 11, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Gliko-Kabir, I.; Yagen, B.; Baluom, M.; Rubinstein, A. Phosphated crosslinked guar for colon-specific drug delivery. II. In vitro and in vivo evaluation in the rat. J. Control. Release 2000, 63, 129–134. [Google Scholar]
- Sihna, V.R.; Kumria, R. Microbial triggered drug delivery to colon. Eur. J. Pharm. Sci. 2003, 18, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Qing, D.; De-Ying, C.; Bai, X.; Fanli-Fang. Pectin/Ethylcellulose as film coatings for colon-specific drug delivery: Preparation and in vitro evaluation using 5-fluorouracil pellets. PDA. J. Pharm. Sci. Technol. 2007, 61, 121–130. [Google Scholar]
- Krishnaiah, Y.S.R.; Seetha Devi, A.; Nageswara Rao, L.; Bhaskar Reddy, P.R.; Karthikeyan, R.S.; Satyanarayana, V. Guar gum as a carrier for colon specific delivery; influence of metronidazole and tinidazole on in vitro release of albendazole from guar gum matrix tablets. J. Pharm. Pharm. Sci. 2001, 4, 235–243. [Google Scholar] [PubMed]
- Krishnaiah, Y.S.R.; Satyanarayana, V.; Dinesh Kumar, B.; Karthikeyan, R.S. In vitro drug release studies on guar gum based colon targeted oral delivery systems of 5-fluorouracil. Eur. J. Pharm. Sci. 2002, 16, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, B.; Shen, F.; Fu, Q. Formulation and characterization of albumin microspheres containing norcantharidate for liver tumor targeting. Drug Deliv. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Choursia, M.K.; Jain, S.K. Potential of guar gum microspheres for target specific drug release to colon. J. Drug Target. 2014, 12, 435–442. [Google Scholar] [CrossRef]
- Jin, L.; Ding, Y.; Feng, M.; Cao, Q. Preparation oral levofloxacin colon-specific microspheres delivery: In vitro and in vivo studies. Drug Deliv. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhou, H. Budesonide-Loaded Guar Gum Microspheres for Colon Delivery: Preparation, Characterization and in Vitro/in Vivo Evaluation. Int. J. Mol. Sci. 2015, 16, 2693-2704. https://doi.org/10.3390/ijms16022693
Liu Y, Zhou H. Budesonide-Loaded Guar Gum Microspheres for Colon Delivery: Preparation, Characterization and in Vitro/in Vivo Evaluation. International Journal of Molecular Sciences. 2015; 16(2):2693-2704. https://doi.org/10.3390/ijms16022693
Chicago/Turabian StyleLiu, Ye, and Hong Zhou. 2015. "Budesonide-Loaded Guar Gum Microspheres for Colon Delivery: Preparation, Characterization and in Vitro/in Vivo Evaluation" International Journal of Molecular Sciences 16, no. 2: 2693-2704. https://doi.org/10.3390/ijms16022693






