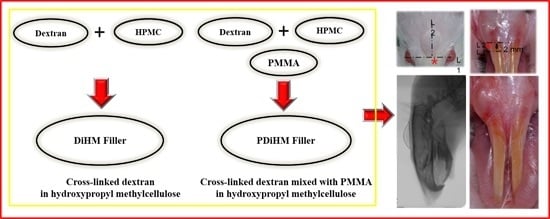
Effects of PMMA and Cross-Linked Dextran Filler for Soft Tissue Augmentation in Rats
Abstract
:1. Introduction
2. Results
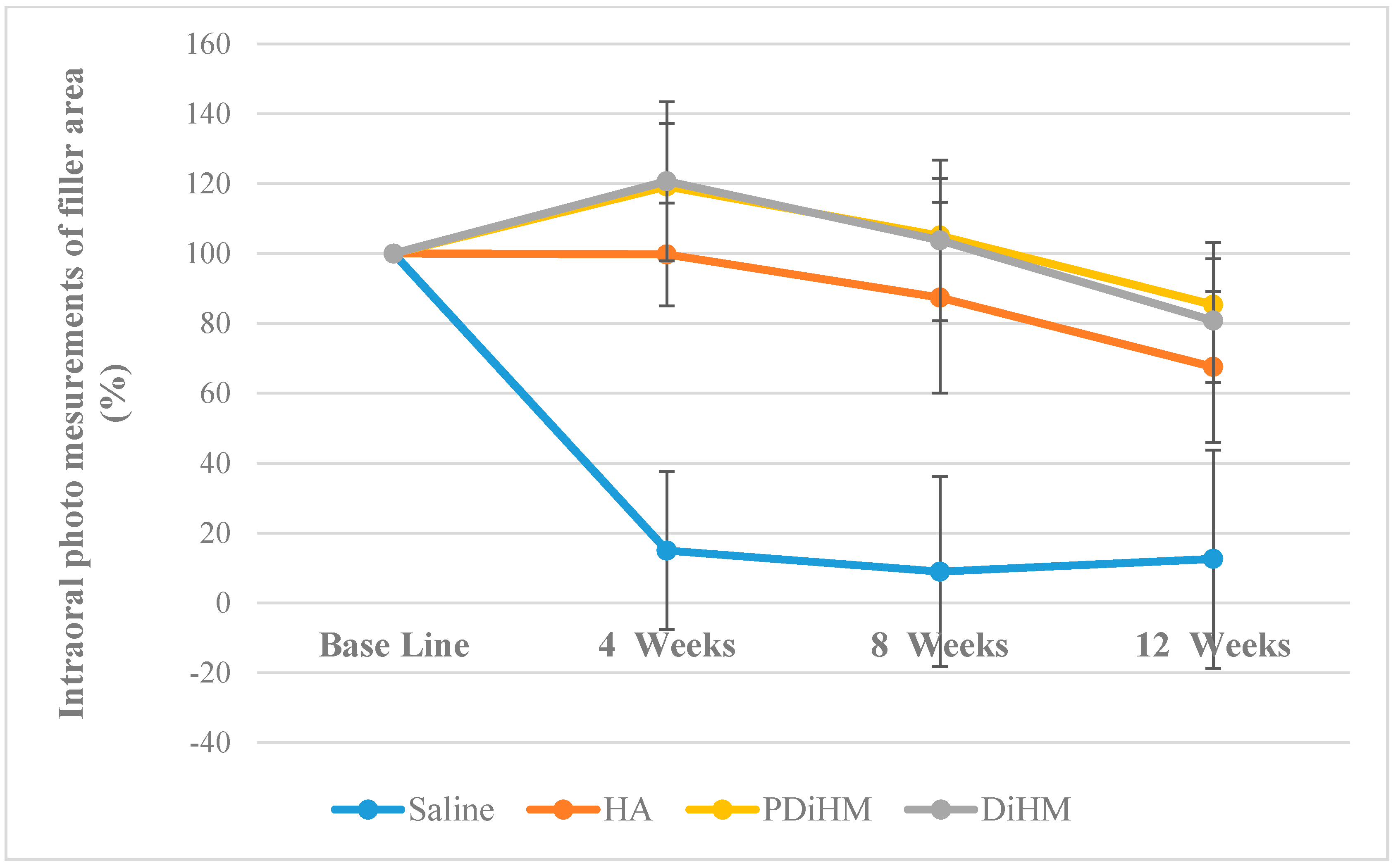
2.1. Photographic Measurements of the Filler Injection Area in Oral Submucosa
| Area (%) | Group | n | Mean | SD | F (p) | Duncan | Time F (p) | Interaction Effect F (p) | Group Duncan |
|---|---|---|---|---|---|---|---|---|---|
| Base line | Saline | 9 | 100.00 | - | - | - | 39.636 *** (0.000) | 17.968 *** (0.000) | Saline < HA < DiHM, PDiHM |
| HA | 10 | 100.00 | - | ||||||
| DiHM | 10 | 100.00 | - | ||||||
| PDiHM | 9 | 100.00 | - | ||||||
| 4-week | Saline | 9 | 14.96 | 22.60 | 58.577 *** (0.000) | Saline < HA < DiHM, PDiHM | |||
| HA | 10 | 99.75 | 14.70 | ||||||
| DiHM | 10 | 120.70 | 22.74 | ||||||
| PDiHM | 9 | 119.22 | 18.08 | ||||||
| 8-week | Saline | 9 | 8.93 | 27.24 | 33.025 *** (0.000) | Saline < HA DiHM, PDiHM | |||
| HA | 10 | 87.39 | 27.32 | ||||||
| DiHM | 10 | 103.78 | 23.02 | ||||||
| PDiHM | 9 | 105.12 | 16.42 | ||||||
| 12-week | Saline | 9 | 12.55 | 31.22 | 20.025 *** (0.000) | Saline < HA DiHM, PDiHM | |||
| HA | 10 | 67.53 | 21.62 | ||||||
| DiHM | 10 | 80.79 | 17.70 | ||||||
| PDiHM | 9 | 85.36 | 17.88 |
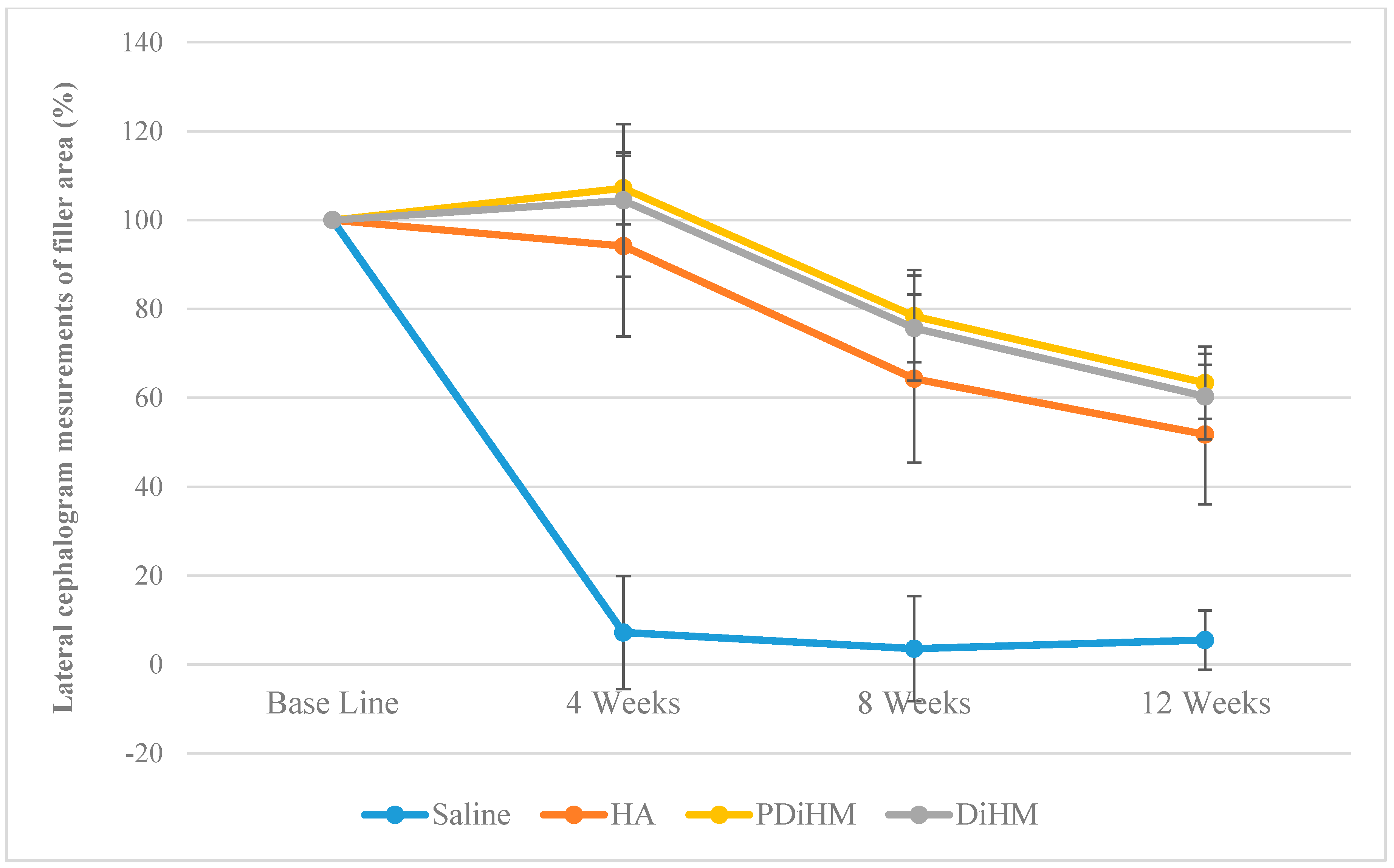
2.2. Radiographic Measurements of the Filler Injection Area in Cranial Subcutaneous Tissue
| Area (%) | Group | n | Mean | SD | F (p) | Duncan | Time F (p) | Interaction Effect F (p) | Group Duncan |
|---|---|---|---|---|---|---|---|---|---|
| Base line | Saline | 9 | 100.00 | - | - | - | 207.702 *** (0.000) | 34.243 *** (0.000) | Saline < HA < DiHM, PDiHM |
| HA | 10 | 100.00 | - | ||||||
| DiHM | 10 | 100.00 | - | ||||||
| PDiHM | 9 | 100.00 | - | ||||||
| 4-week | Saline | 9 | 7.19 | 12.72 | 86.048 *** (0.000) | Saline < HA, DiHM, PDiHM | |||
| HA | 10 | 94.16 | 20.33 | ||||||
| DiHM | 10 | 104.43 | 17.21 | ||||||
| PDiHM | 9 | 107.17 | 8.08 | ||||||
| 8-week | Saline | 9 | 3.53 | 11.83 | 59.429 *** (0.000) | Saline < HA, DiHM < PDiHM | |||
| HA | 10 | 64.31 | 18.93 | ||||||
| DiHM | 10 | 75.69 | 11.80 | ||||||
| PDiHM | 9 | 78.40 | 10.35 | ||||||
| 12-week | Saline | 9 | 5.49 | 6.69 | 57.097 *** (0.000) | Saline < HA, DiHM < PDiHM | |||
| HA | 10 | 51.75 | 15.69 | ||||||
| DiHM | 10 | 60.30 | 9.63 | ||||||
| PDiHM | 9 | 63.37 | 8.13 |
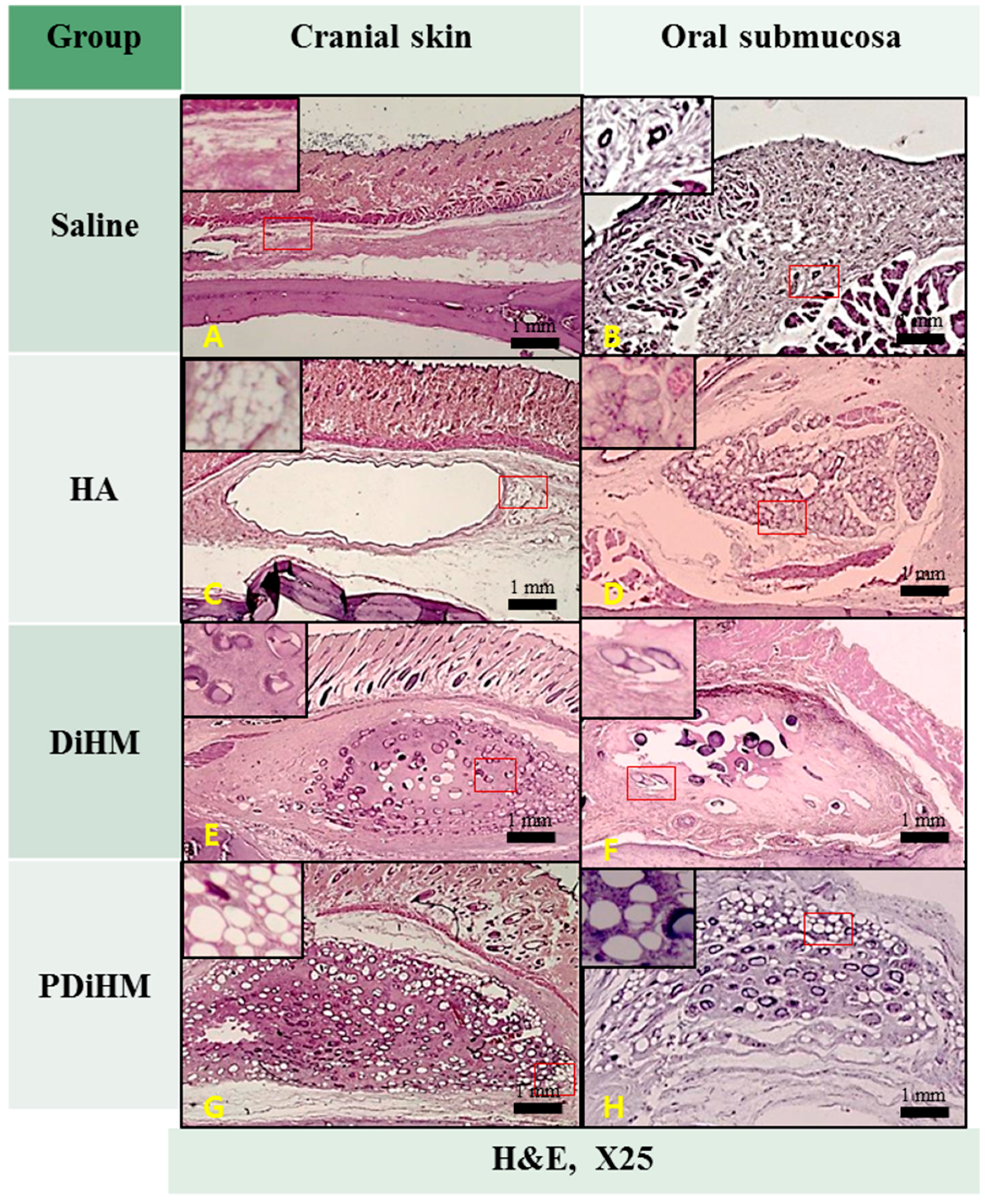
2.3. Histomorphologic Examination
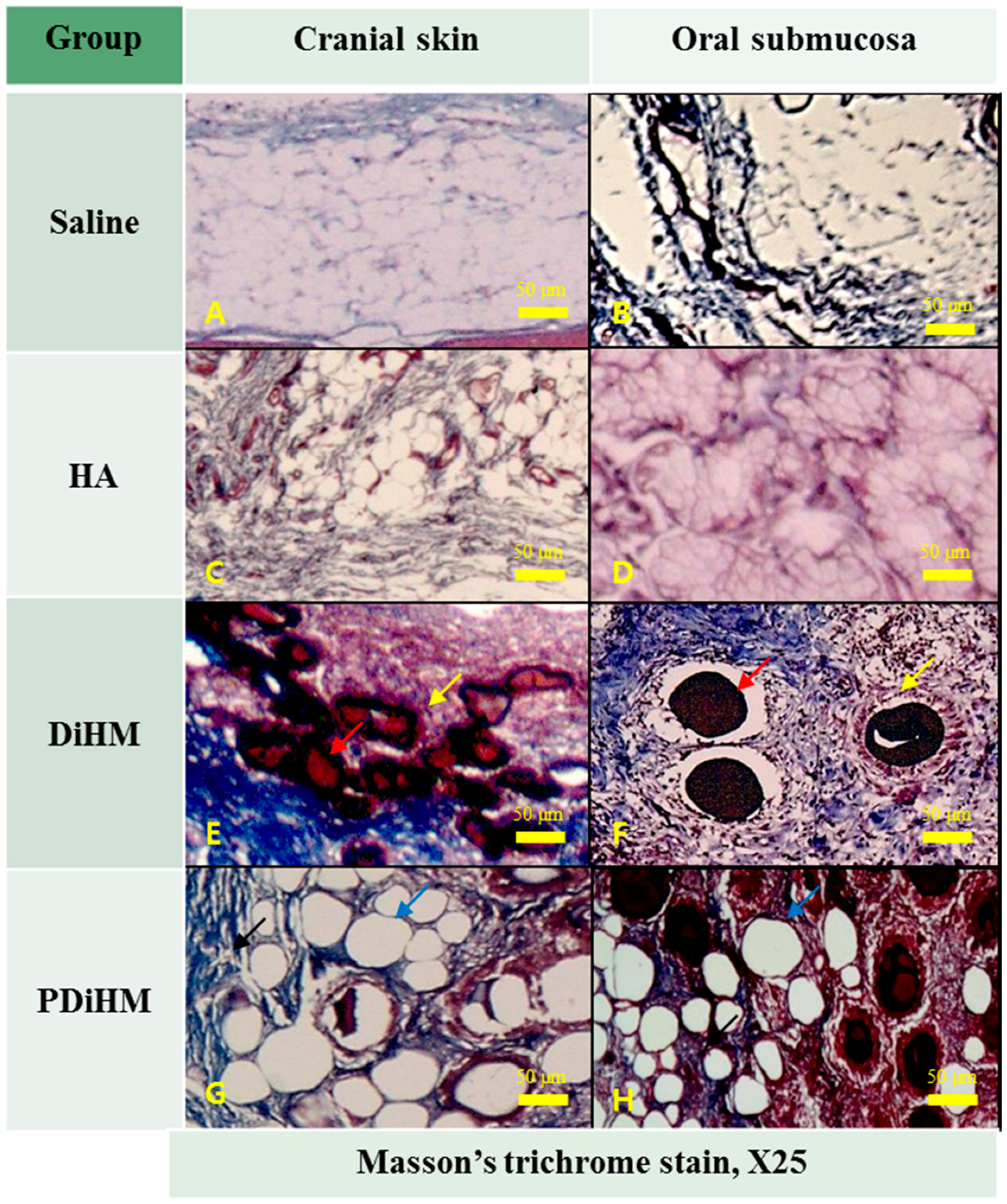
3. Discussion
4. Materials and Method
4.1. Test Animals and Groups
4.2. Injection of Filler Materials
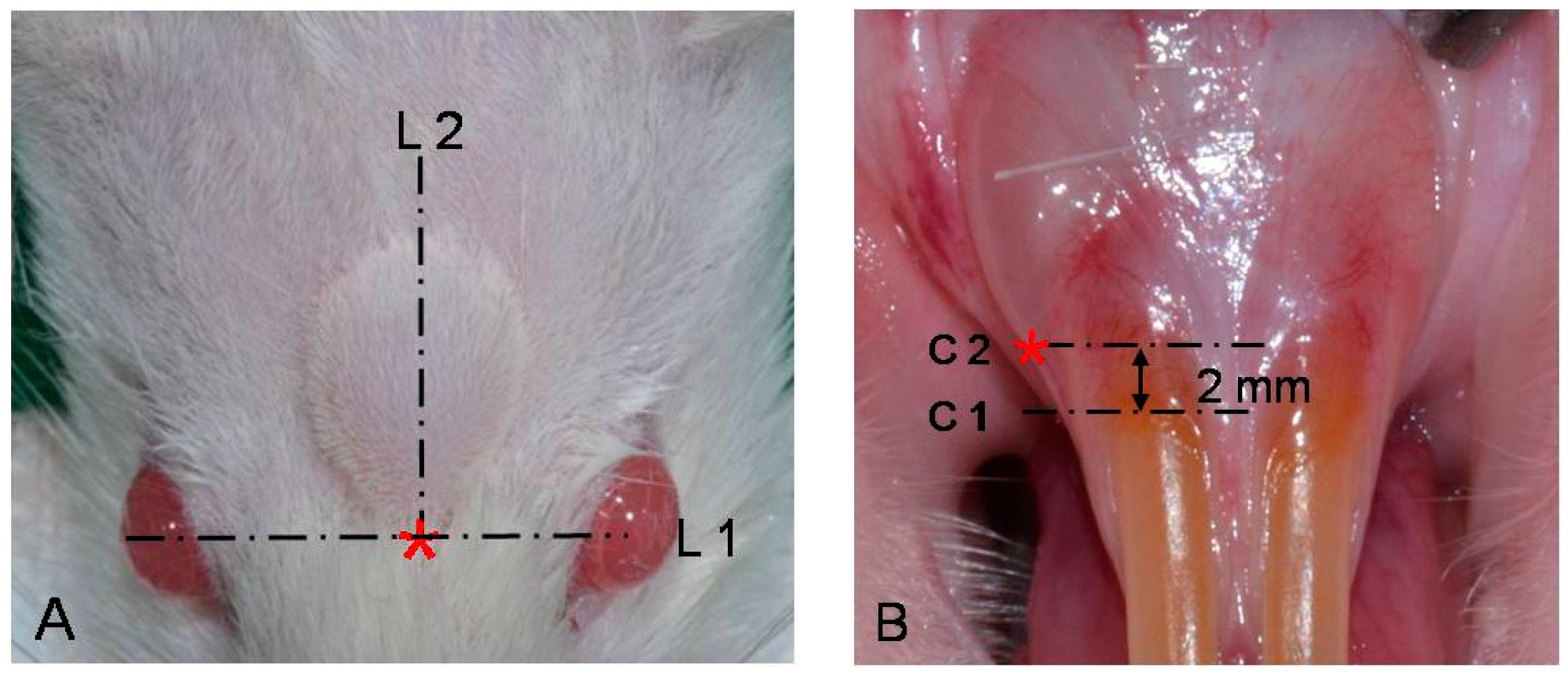
4.3. Observation of Systemic Signs
4.4. Measurements of Augmentation Area
4.4.1. Photographic Examination for Filler Effect in Oral Submucosa
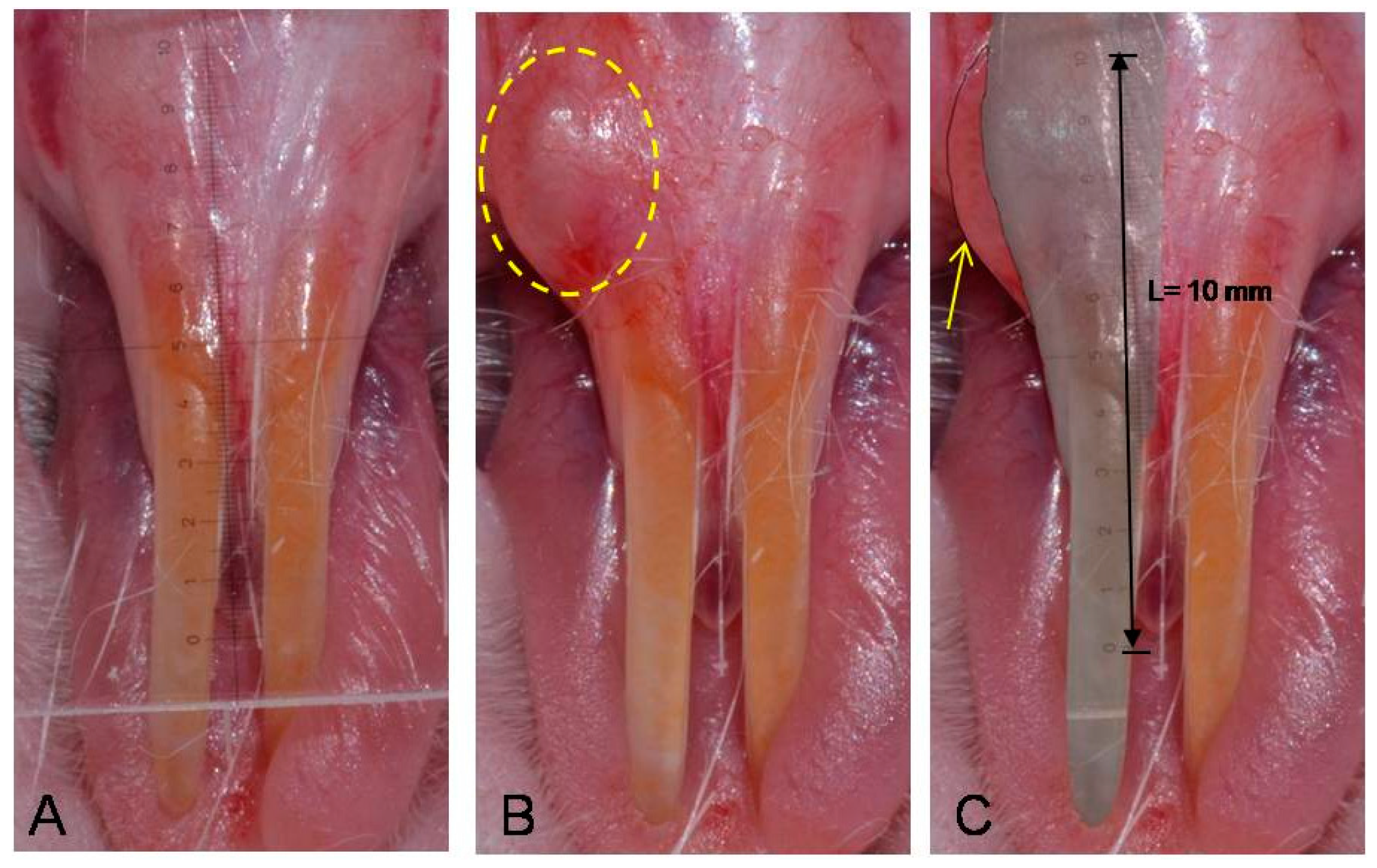
4.4.2. Radiographic Examination for Filler Effect in Cranial Subcutaneous Tissue
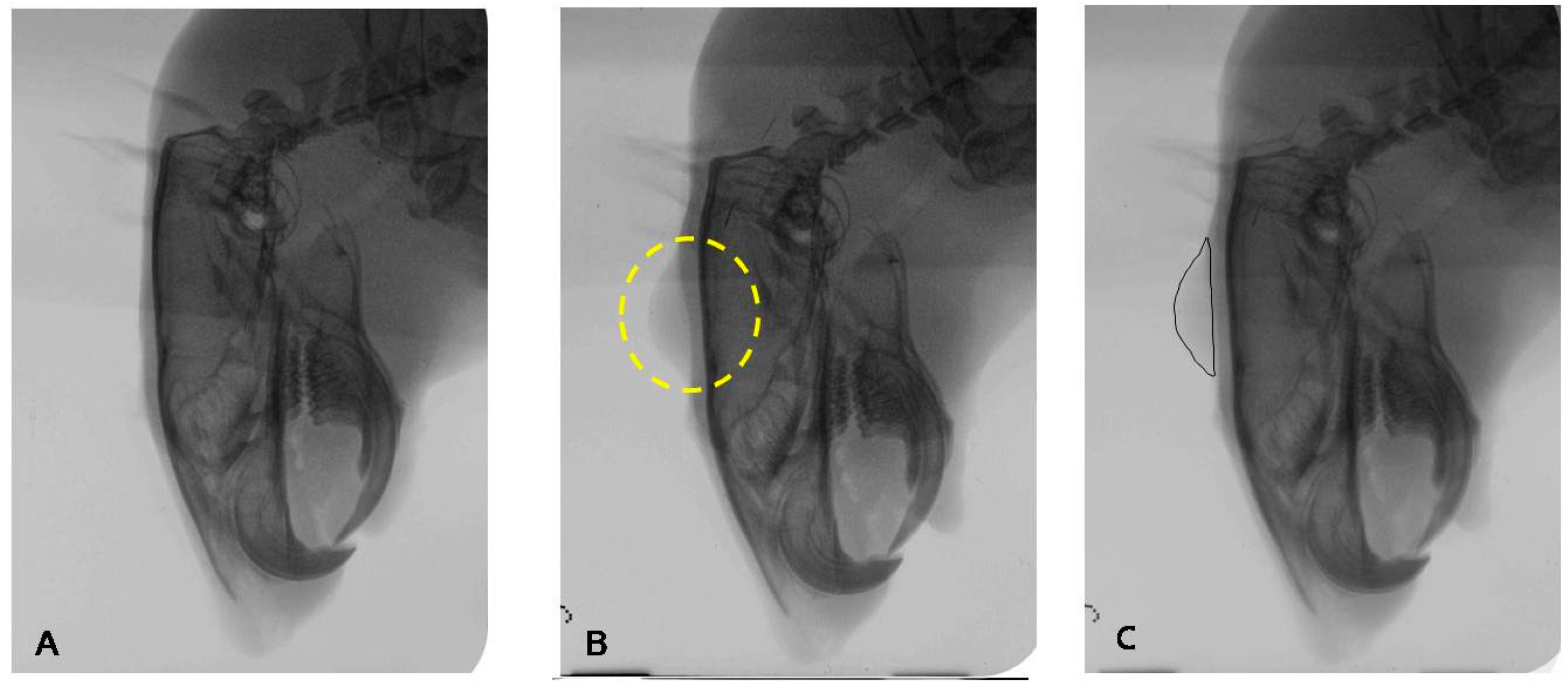
4.4.3. Histomorphologic Examination
4.4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thoma, D.S.; Benić, G.I.; Zwahlen, M.; Hämmerle, C.H.F.; Jung, R.E. A systematic review assessing soft tissue augmentation techniques. Clin. Oral Implant. Res. 2009, 20, 146–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seibert, J.S.; Salama, H. Alveolar ridge preservation and reconstruction. Periodontology 1996, 11, 69–84. [Google Scholar] [CrossRef]
- Seibert, J.S. Treatment of moderate localized alveolar ridge defects. Preventive and reconstructive concepts in therapy. Dent. Clin. N. Am. 1993, 37, 265–280. [Google Scholar] [PubMed]
- Harris, R.J. Soft tissue ridge augmentation with an acellular dermal matrix. Int. J. Periodontics Restor. Dent. 2003, 23, 87–92. [Google Scholar]
- Mahn, D.H. Esthetic soft tissue ridge augmentation using an acellular dermal connective tissue allograft. J. Esthet. Restor. Dent. 2003, 15, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Abrams, L. Augmentation of the deformed residual edentulous ridge for fixed prosthesis. Compend. Contin. Educ. Gen. Dent. 1980, 1, 205–213. [Google Scholar] [PubMed]
- Aichelmann-Reidy, M.E.; Yukna, R.A.; Evans, G.H.; Nasr, H.F.; Mayer, E.T. Clinical evaluation of acellular allograft dermis for the treatment of human gingival recession. J. Periodontol. 2001, 72, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.P.; Gainza, C.S.; Farthing, G.G.; Newbold, D.A. Improved technique for localized ridge augmentation: A report of 21 cases. J. Periodontol. 1985, 56, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Oates, T.W.; Robinson, M.; Gunsolley, J.C. Surgical therapies for the treatment of gingival recession. A systematic review. J. Periodontol. 2003, 8, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Griffin, T.J.; Cheung, W.S.; Zavras, A.I.; Damoulis, P.D. Postoperative complications following gingival augmentation procedures. J. Periodontol. 2006, 77, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Gabitov, I.; Stepanov, M.; Kois, J.; Smidt, A.; Becker, B.E. Minimally invasive treatment for papillae deficiencies in the esthetic zone: A pilot study. Clin. Implant Dent. Relat. Res. 2010, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.K.; Scheyer, E.T. A randomized, double-blind, placebo-controlled study to determine the safety and efficacy of cultured and expanded autologous fibroblast injections for the treatment of interdental papillary insufficiency associated with the papilla priming procedure. J. Periodontol. 2007, 78, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Kretlow, J.D.; Young, S.; Klouda, L.; Wong, M.; Mikos, A.G. Injectable biomaterials for regenerating complex craniofacial tissues. Adv. Mater. 2009, 21, 3368–3393. [Google Scholar] [CrossRef] [PubMed]
- Elson, M. Soft tissue augmentation: A review. Dermatol. Surg. 1995, 21, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Dadzie, O.E.; Mahalingam, M.; Parada, M.; El Helou, T.; Philips, T.; Bhawan, J. Adverse cutaneous reactions to soft tissue fillers—A review of the histological features. J. Cutan. Pathol. 2008, 35, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Carpintero, I.; Candelas, D.; Ruiz-Rodríguez, R. Dermal fillers: Types, indications, and complications. Actas Dermo Sifiliogr. (Engl. Ed.) 2010, 101, 381–393. [Google Scholar] [CrossRef]
- Lemperle, G.; Morhenn, V.; Charrier, U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthet. Plast. Surg. 2003, 27, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Johl, S.S.; Burgett, R.A. Dermal filler agents: A practical review. Curr. Opin. Ophthalmol. 2006, 17, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Monheit, G.D.; Coleman, K.M. Hyaluronic acid fillers. Dermatol. Ther. 2006, 19, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Lupo, M.P. Hyaluronic acid fillers in facial rejuvenation. Semin. Cutan. Med. Surg. 2006, 25, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.P.I. Animal-based hyaluronic acid fillers: Scientific and technical considerations. Plast. Reconstr. Surg. 2007, 120, 27S–32S. [Google Scholar] [CrossRef] [PubMed]
- Cena, R.B.; Park, J.G.; Kim, H.J.; Son, K.Y.; Kim, D.S.; Kang, M.I.; Park, S.I.; Moon, D.G.; Yang, D.Y.; Yu, D.S.; et al. Effects of crosslinked dextran in hydroxylpropyl methylcellulose on soft tissue augmentation in rats. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2014, 102, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.W.; Jin, B.K.; Lee, S.H.; Park, J.H.; Ryu, J.M.; Yun, S.P.; Park, S.W.; Kim, H.S.; Moon, C.J.; Suh, G.H.; et al. Effect of PMMA and cross-linked dextran mixture on bio-safety and volume in rat. Tissue Eng. Regen. Med. 2010, 7, 57–63. [Google Scholar]
- Alkan, M.; Ciftci, A.; Talim, B.; Senocak, M.; Caglar, M.; Buyukpamukcu, N. Histological response to injected dextranomer-based implant in a rat model. Pediatr. Surg. Int. 2007, 23, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Wischke, C.; Borchert, H.-H.; Zimmermann, J.; Siebenbrodt, I.; Lorenzen, D.R. Stable cationic microparticles for enhanced model antigen delivery to dendritic cells. J. Control. Release 2006, 114, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhang, X.; Shen, Y.I.; Sebastian, R.; Dickinson, L.E.; Fox-Talbot, K.; Reinblatt, M.; Steenbergen, C.; Harmon, J.W.; Gerecht, S. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc. Natl. Acad. Sci. USA 2011, 108, 20976–20981. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Dormans, J.A.M.A.; van Steenbergen, M.J.; Verharen, H.W.; Hennink, W.E. Tissue response in the rat and the mouse to degradable dextran hydrogels. J. Biomed. Mater. Res. Part A 2007, 83A, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Frazer, R.Q.; Byron, R.T.; Osborne, P.B.; West, K.P. PMMA: An essential material in medicine and dentistry. J. Long Term Effect Med. Implants 2005, 15, 629–639. [Google Scholar] [CrossRef]
- Cohen, S.R.; Berner, C.F.; Busso, M.; Clopton, P.; Hamilton, D.; Romano, J.J.; Rullan, P.P.; Thaler, M.P.; Ubogy, Z.; Vecchione, T.R. Five-year safety and efficacy of a novel polymethylmethacrylate aesthetic soft tissue filler for the correction of nasolabial folds. Dermatol. Surg. 2007, 33, S222–S230. [Google Scholar] [PubMed]
- Lemperle, G.; Gauthier-Hazan, N.; Lemperle, M. PMMA-microspheres (Artecoll) for long-lasting correction of wrinkles: Refinements and statistical results. Aesthet. Plast. Surg. 1998, 22, 356–365. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- De Silva, D.J.; Olver, J.M. Hydroxypropyl methylcellulose (HPMC) lubricant facilitates insertion of porous spherical orbital implants. Ophthal. Plast. Reconstr. Surg. 2005, 21, 301–302. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huh, J.-B.; Kim, J.-H.; Kim, S.; Lee, S.-H.; Shim, K.M.; Kim, S.E.; Kang, S.S.; Jeong, C.-M. Effects of PMMA and Cross-Linked Dextran Filler for Soft Tissue Augmentation in Rats. Int. J. Mol. Sci. 2015, 16, 28523-28533. https://doi.org/10.3390/ijms161226112
Huh J-B, Kim J-H, Kim S, Lee S-H, Shim KM, Kim SE, Kang SS, Jeong C-M. Effects of PMMA and Cross-Linked Dextran Filler for Soft Tissue Augmentation in Rats. International Journal of Molecular Sciences. 2015; 16(12):28523-28533. https://doi.org/10.3390/ijms161226112
Chicago/Turabian StyleHuh, Jung-Bo, Joo-Hyun Kim, Soyun Kim, So-Hyoun Lee, Kyung Mi Shim, Se Eun Kim, Seong Soo Kang, and Chang-Mo Jeong. 2015. "Effects of PMMA and Cross-Linked Dextran Filler for Soft Tissue Augmentation in Rats" International Journal of Molecular Sciences 16, no. 12: 28523-28533. https://doi.org/10.3390/ijms161226112