ITSN2L Interacts with and Negatively Regulates RABEP1
Abstract
:1. Introduction
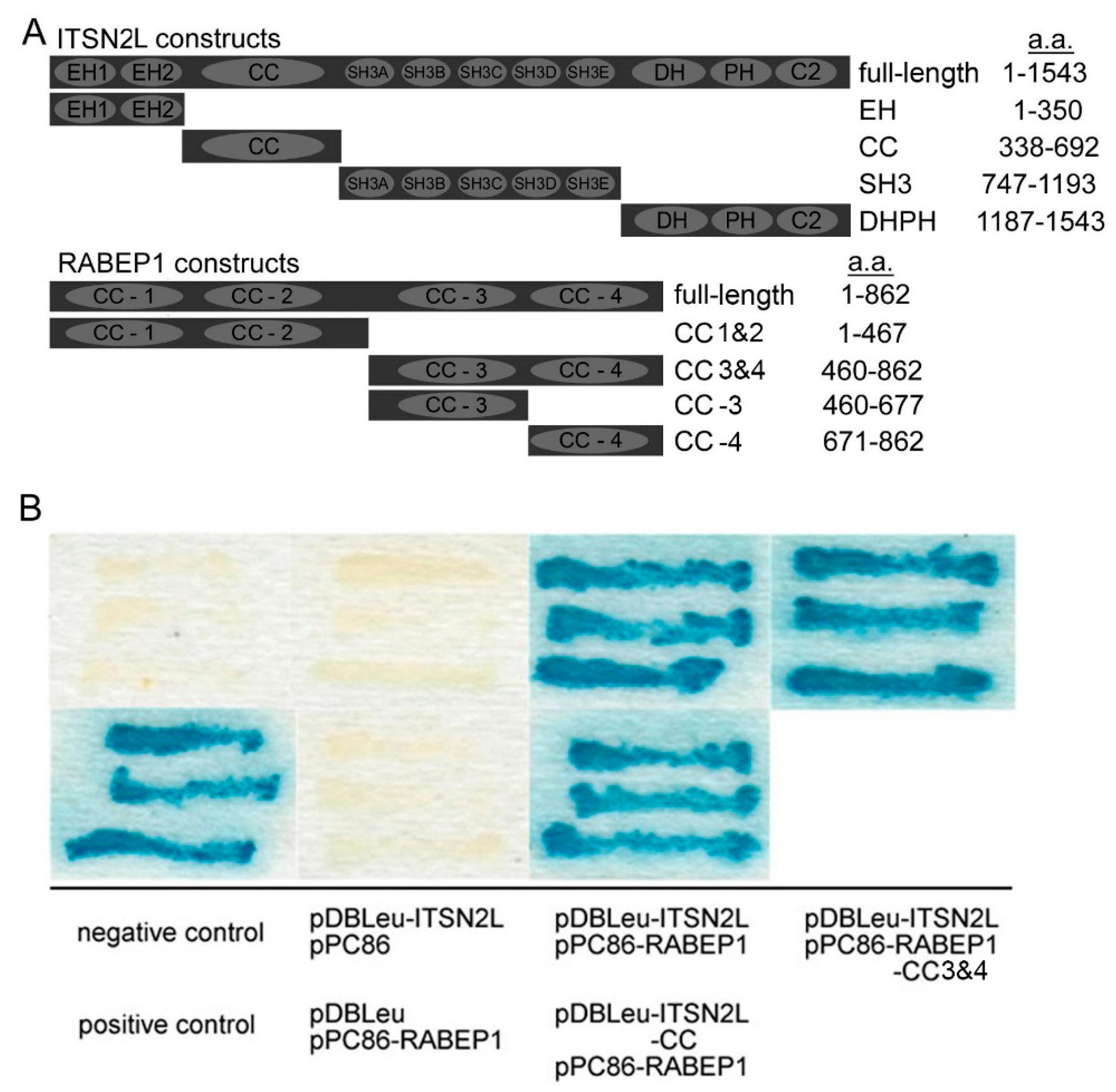

2. Results
2.1. ITSN2L Interacts with RABEP1 Both in Vitro and Vivo
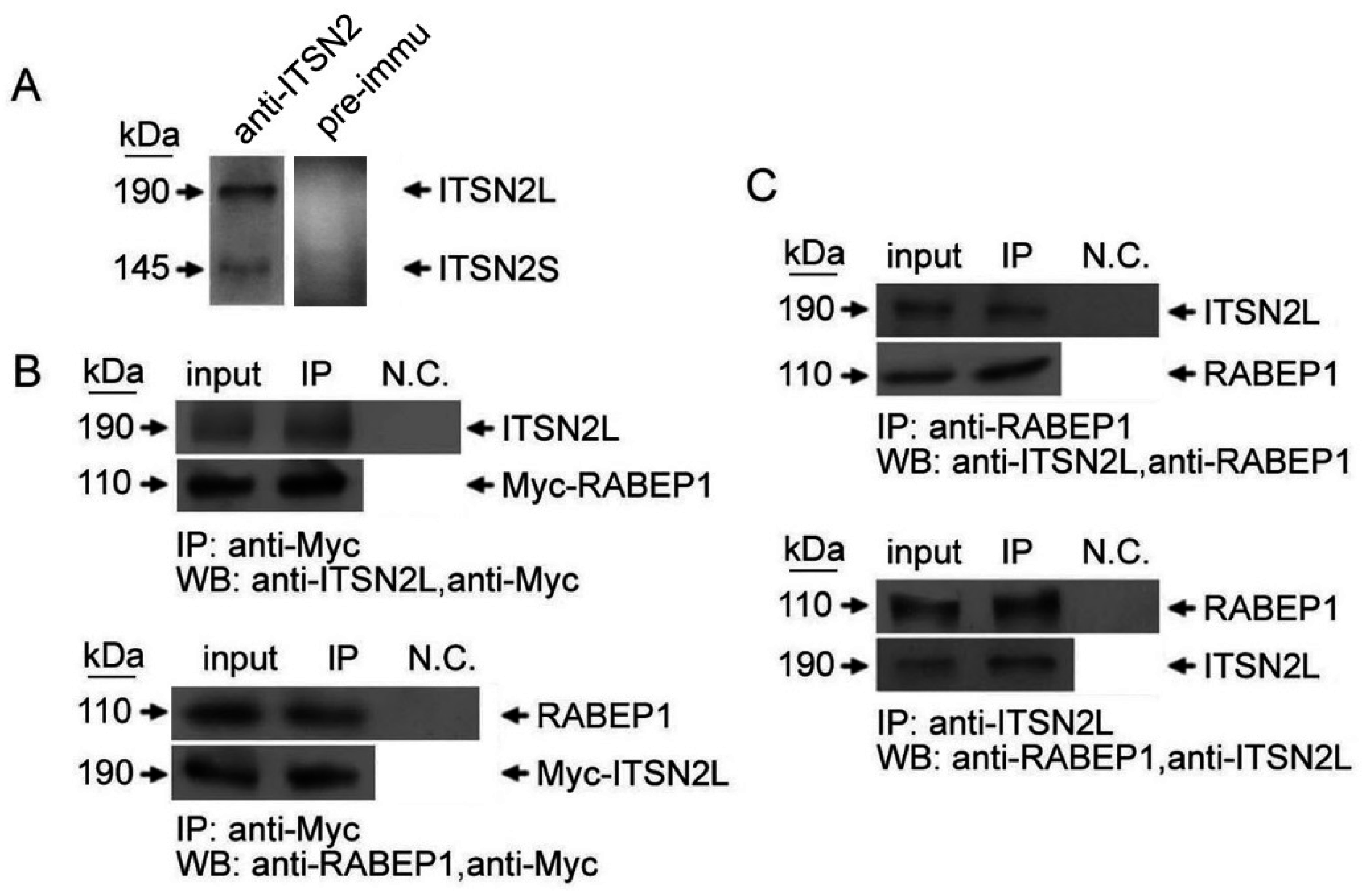
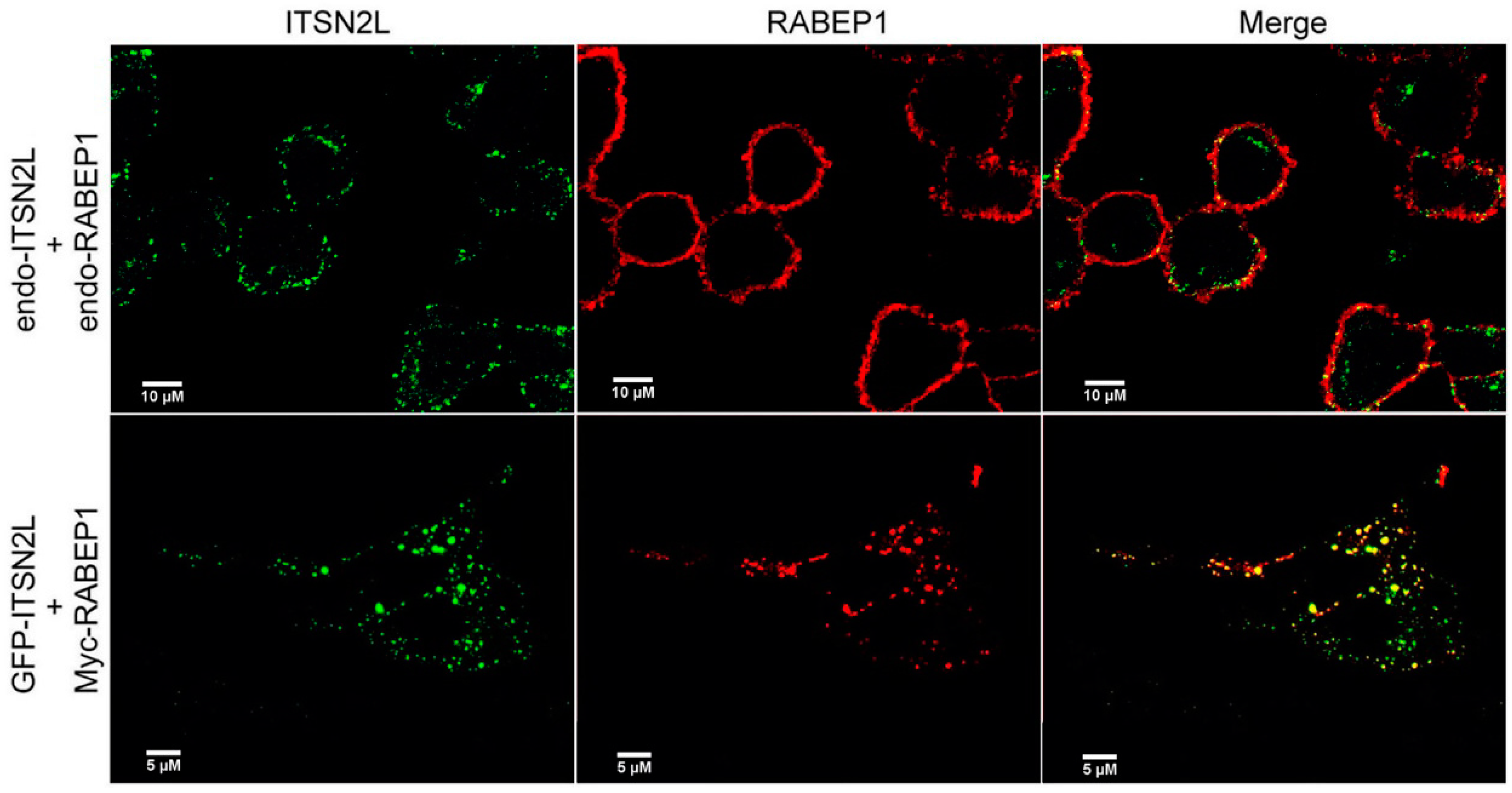
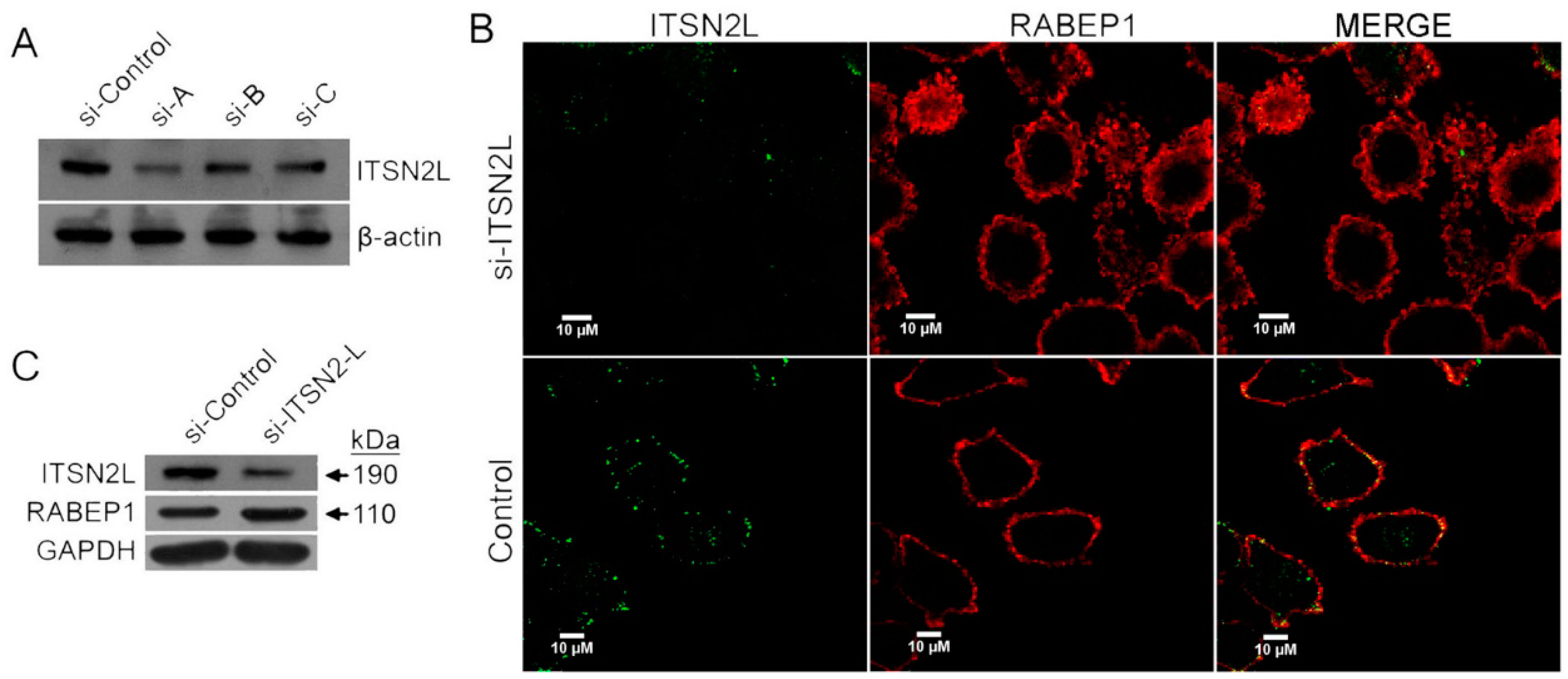
2.2. ITSN2L Co-Localizes with RABEP1
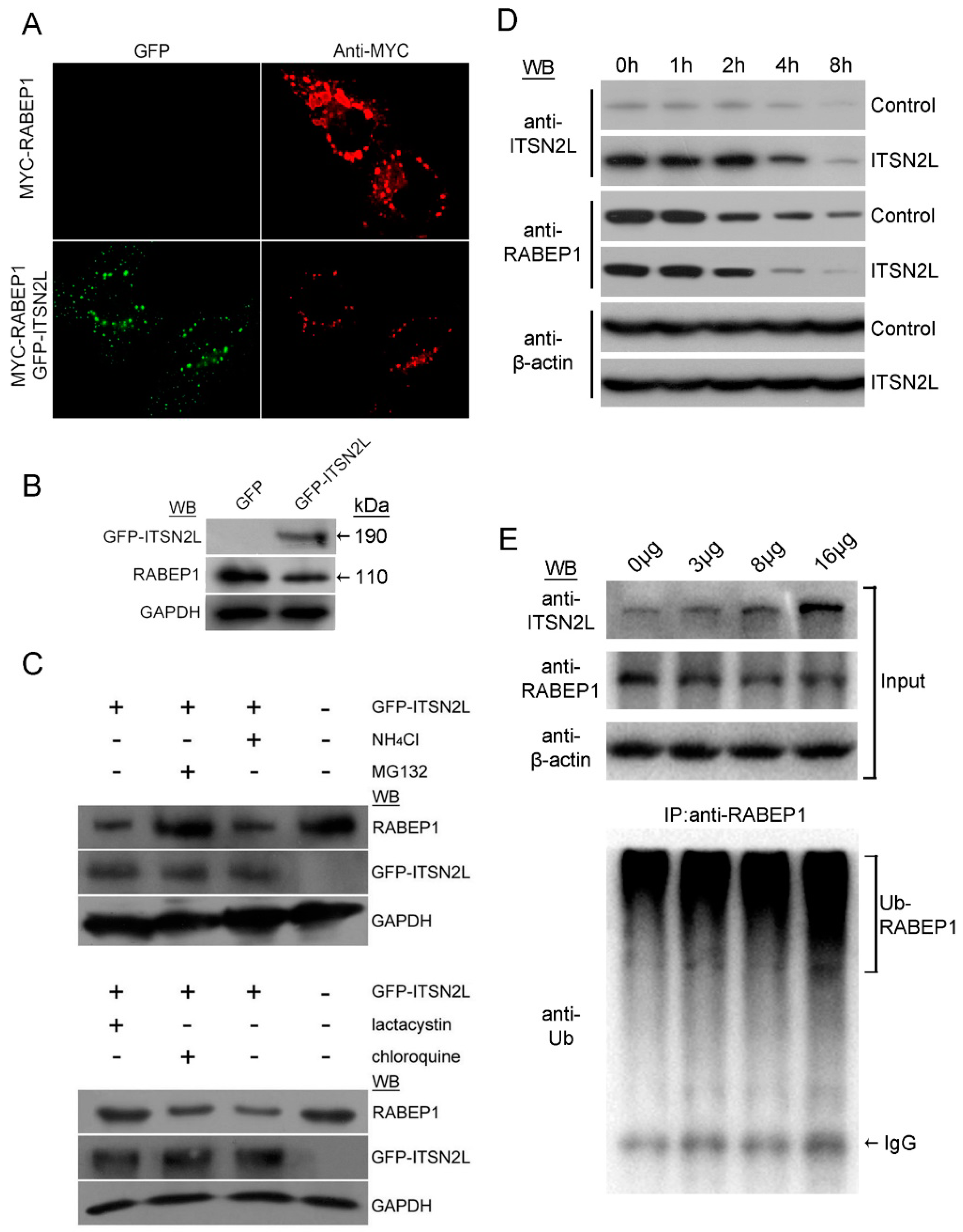
2.3. ITSN2L Acts as a Negative Regulator of RABEP1
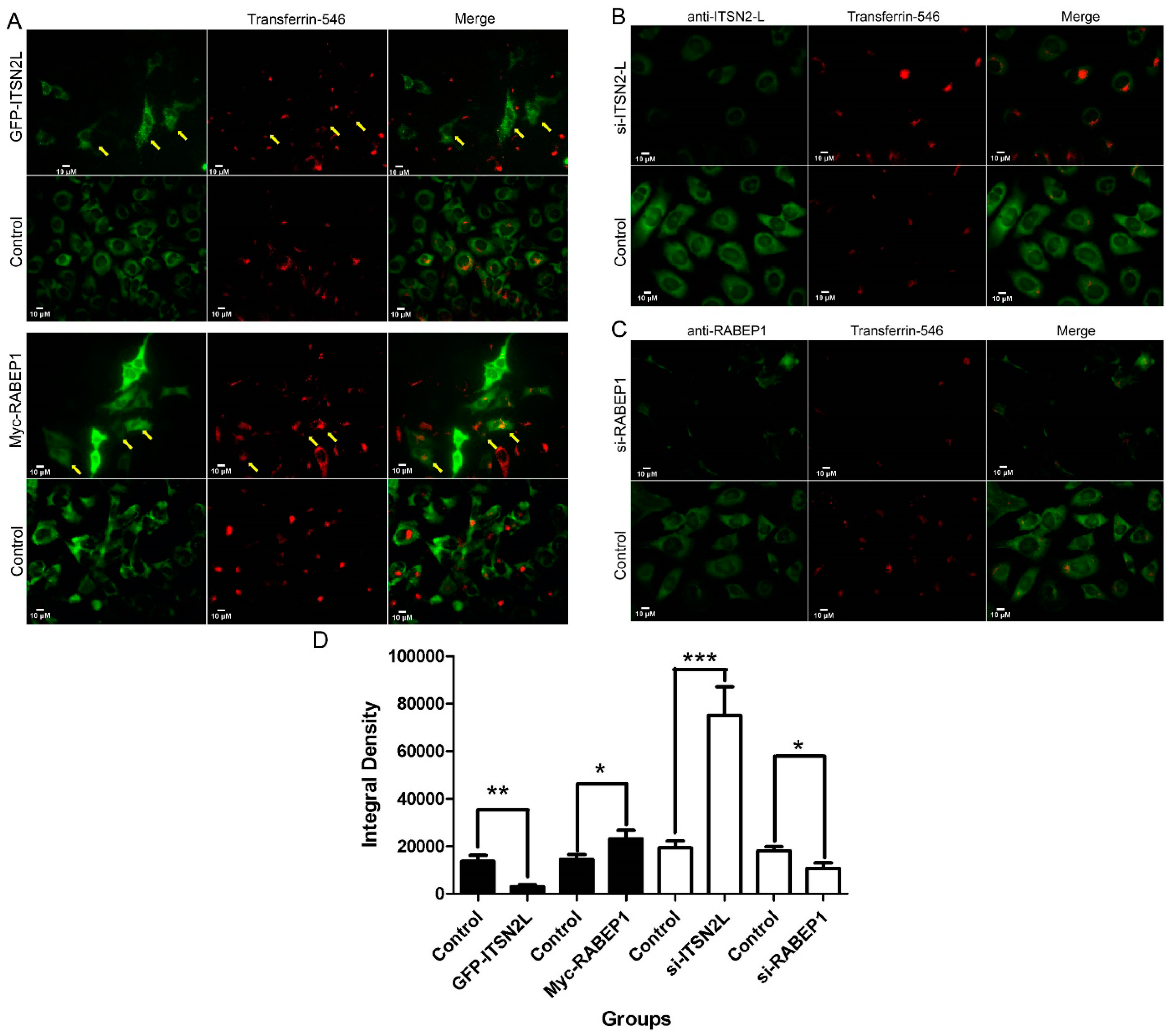
2.4. ITSN2L and RABEP1 Play Opposite Roles in Regulating Endocytosis
3. Discussion
4. Experimental Section
4.1. Yeast Two-Hybrid Screens
4.2. GST Pull-down Assay
4.3. Immunoprecipitation and Western Blot Analysis
4.4. Immunofluorescent Analysis
4.5. Transferrin (Tf) Internalization Assay
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yamabhai, M.; Hoffman, N.G.; Hardison, N.L.; McPherson, P.S.; Castagnoli, L.; Cesareni, G.; Kay, B.K. Intersectin, a novel adaptor protein with two Eps15 homology and five Src homology 3 domains. J. Biol. Chem. 1998, 273, 31401–31407. [Google Scholar] [CrossRef] [PubMed]
- Sengar, A.S.; Wang, W.; Bishay, J.; Cohen, S.; Egan, S.E. The EH and SH3 domain Ese proteins regulate endocytosis by linking to dynamin and Eps15. EMBO J. 1999, 18, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Schoch, S.; Sudhof, T.C. EHSH1/intersectin, a protein that contains EH and SH3 domains and binds to dynamin and SNAP-25. A protein connection between exocytosis and endocytosis? J. Biol. Chem. 1999, 274, 18446–18454. [Google Scholar] [CrossRef] [PubMed]
- Roos, J.; Kelly, R.B. Dap160, a neural-specific Eps15 homology and multiple SH3 domain-containing protein that interacts with Drosophila dynamin. J. Biol. Chem. 1998, 273, 19108–19119. [Google Scholar] [CrossRef] [PubMed]
- Mohney, R.P. Intersectin Activates Ras but Stimulates Transcription through an Independent Pathway Involving JNK. J. Biol. Chem. 2003, 278, 47038–47045. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.P.; Mohney, R.P.; Dunn, S.; Das, M.; Scappini, E.; O’Bryan, J.P. Intersectin regulates epidermal growth factor receptor endocytosis, ubiquitylation, and signaling. Mol. Pharmacol. 2006, 70, 1643–1653. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H.; Misra, S. Signaling and subcellular targeting by membrane-binding domains. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 49–79. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.W.; Verstreken, P.; Bellen, H.J. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron 2004, 43, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.K.; Hussain, N.K.; de Heuvel, E.; Kurakin, A.; Abi-Jaoude, E.; Quinn, C.C.; Olson, M.F.; Marais, R.; Baranes, D.; Kay, B.K.; et al. The endocytic protein intersectin is a major binding partner for the Ras exchange factor mSos1 in rat brain. EMBO J. 2000, 19, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Tsyba, L.; Nikolaienko, O.; Dergai, O.; Dergai, M.; Novokhatska, O.; Skrypkina, I.; Rynditch, A. Intersectin multidomain adaptor proteins: Regulation of functional diversity. Gene 2011, 473, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.K.; Yamabhai, M.; Ramjaun, A.R.; Guy, A.M.; Baranes, D.; O’Bryan, J.P.; Der, C.J.; Kay, B.K.; McPherson, P.S. Splice variants of intersectin are components of the endocytic machinery in neurons and nonneuronal cells. J. Biol. Chem. 1999, 274, 15671–15677. [Google Scholar] [CrossRef] [PubMed]
- Pucharcos, C.; Estivill, X.; de la Luna, S. Intersectin 2, a new multimodular protein involved in clathrin-mediated endocytosis. FEBS Lett. 2000, 478, 43–51. [Google Scholar] [CrossRef]
- Klein, I.K.; Predescu, D.N.; Sharma, T.; Knezevic, I.; Malik, A.B.; Predescu, S. Intersectin-2L regulates caveola endocytosis secondary toCdc42-mediated actin polymerization. J. Biol. Chem. 2009, 284, 25953–25961. [Google Scholar] [CrossRef] [PubMed]
- Tebar, F.; Sorkina, T.; Sorkin, A.; Ericsson, M.; Kirchhausen, T. Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J. Biol. Chem. 1996, 271, 28727–28730. [Google Scholar] [PubMed]
- Hussain, N.K.; Jenna, S.; Glogauer, M.; Quinn, C.C.; Wasiak, S.; Guipponi, M.; Antonarakis, S.E.; Kay, B.K.; Stossel, T.P.; Lamarche-Vane, N.; et al. Endocytic protein intersectin-l regulates actin assembly via Cdc42 and N-WASP. Nat. Cell Biol. 2001, 3, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Pucharcos, C.; Casas, C.; Nadal, M.; Estivill, X.; de la Luna, S. The human intersectin genes and their spliced variants are differentially expressed. Biochim. Biophys. Acta 2001, 1521, 1–11. [Google Scholar] [CrossRef]
- McGavin, M.K.; Badour, K.; Hardy, L.A.; Kubiseski, T.J.; Zhang, J.; Siminovitch, K.A. The intersectin 2 adaptor links Wiskott Aldrich Syndrome protein (WASp)-mediated actin polymerization to T cell antigen receptor endocytosis. J. Exp. Med. 2001, 194, 1777–1787. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fraticelli, A.E.; Vergarajauregui, S.; Eastburn, D.J.; Datta, A.; Alonso, M.A.; Mostov, K.; Martín-Belmonte, F.; et al. The Cdc42 GEF Intersectin 2 controls mitotic spindle orientation to form the lumen during epithelial morphogenesis. J. Cell Biol. 2010, 189, 725–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenmark, H.; Vitale, G.; Ullrich, O.; Zerial, M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell 1995, 83, 423–432. [Google Scholar] [CrossRef]
- Vitale, G.; Rybin, V.; Christoforidis, S.; Thornqvist, P.; McCaffrey, M.; Stenmark, H.; Zerial, M. Distinct Rab-binding domains mediate the interaction of Rabaptin-5 with GTP-bound Rab4 and Rab5. EMBO J. 1998, 17, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Zerial, M.; Stenmark, H. Rab GTPases in vesicular transport. Curr. Opin. Cell Biol. 1993, 5, 613–620. [Google Scholar] [CrossRef]
- Pfeffer, S.R.; Dirac-Svejstrup, A.B.; Soldati, T. Rab GDP dissociation inhibitor: Putting rab GTPases in the right place. J. Biol. Chem. 1995, 270, 17057–17059. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, H.; Lippé, R.; McBride, H.M.; Rubino, M.; Woodman, P.; Stenmark, H.; Rybin, V.; Wilm, M.; Ashman, K.; Mann, M.; et al. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 1997, 90, 1149–1159. [Google Scholar] [CrossRef]
- Haas, A.K.; Fuchs, E.; Kopajtich, R.; Barr, F.A. A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nat. Cell Biol. 2005, 7, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.H.; Shoarinejad, F.; Jin, F.; Golemis, E.A.; Yeung, R.S. The tuberous sclerosis 2 gene product, tuberin, functions as a Rab5 GTPase activating protein (GAP) in modulating endocytosis. J. Biol. Chem. 1997, 272, 6097–6100. [Google Scholar] [CrossRef] [PubMed]
- Luan, P.; Balch, W.E.; Emr, S.D.; Burd, C.G. Molecular dissection of guanine nucleotide dissociation inhibitor function in vivo. J. Biol. Chem. 1999, 274, 14806–14817. [Google Scholar] [CrossRef] [PubMed]
- Gorvel, J.P.; Chavrier, P.; Zerial, M.; Gruenberg, J. rab5 controls early endosome fusion in vitro. Cell 1991, 64, 915–925. [Google Scholar] [CrossRef]
- Ding, X.F.; Zhou, F.L.; Luo, Z.C.; Liu, Q.; Yan, F.; Xiang, S.L. Human Intersectin 2 (ITSN2) binds to Eps8 protein and enhances its degradation. Biochem. Mol. Biol. Rep. 2012, 45, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.; Vigneron, S.; Brioudes, E.; Labbe, J.-C.; Lorca, T.; Castro, A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc. Natl. Acad. Sci. USA 2010, 107, 12564–12569. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.K. Intersectin Can Regulate the Ras/MAP Kinase Pathway Independent of Its Role in Endocytosis. J. Biol. Chem. 2000, 275, 29894–29899. [Google Scholar] [CrossRef] [PubMed]
- Evergren, E.; Gad, H.; Walther, K.; Sundborger, A.; Tomilin, N.; Shupliakov, O. Intersectin is a negative regulator of dynamin recruitment to the synaptic endocytic zone in the central synapse. J. Neurosci. 2007, 27, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Predescu, S.A.; Predescu, D.N.; Knezevic, I.; Klein, I.K.; Malik, A.B. Intersectin-1s regulates the mitochondrial apoptotic pathway in endothelial cells. J. Biol. Chem. 2007, 282, 17166–17178. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, H.; Aasland, R.; Toh, B.H.; D’Arrigo, A. Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger. J. Biol. Chem. 1996, 271, 24048–24054. [Google Scholar] [PubMed]
- Lim, C.S.; Seet, B.T.; Ingham, R.J.; Gish, G.; Matskova, L.; Winberg, G.; Ernberg, I.; Pawson, T. The K15 protein of Kaposi’s sarcoma-associated herpesvirus recruits the endocytic regulator intersectin 2 through a selective SH3 domain interaction. Biochemistry 2007, 46, 9874–9885. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bouhours, M.; Gracheva, E.O.; Liao, E.H.; Xu, K.; Sengar, A.S.; Xin, X.; Roder, J.; Boone, C.; Richmond, J.E.; et al. ITSN-1 controls vesicle recycling at the neuromuscular junction and functions in parallel with DAB-1. Traffic 2008, 9, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Swanton, E.; Bishop, N.; Woodman, P. Human rabaptin-5 is selectively cleaved by caspase-3 during apoptosis. J. Biol. Chem. 1999, 274, 37583–37590. [Google Scholar] [CrossRef] [PubMed]
- Cosulich, S.C.; Horiuchi, H.; Zerial, M.; Clarke, P.R.; Woodman, P.G. Cleavage of rabaptin-5 blocks endosome fusion during apoptosis. EMBO J. 1997, 16, 6182–6191. [Google Scholar] [CrossRef] [PubMed]
- Rios, E.J.; Piliponsky, A.M.; Ra, C.; Kalesnikoff, J.; Galli, S.J. Rabaptin-5 regulates receptor expression and functional activation in mast cells. Blood 2008, 112, 4148–4157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Roche, O.; Yan, M.S.; Finak, G.; Evans, A.J.; Metcalf, J.L.; Hast, B.E.; Hanna, S.C.; Wondergem, B.; Furge, K.A.; et al. Regulation of endocytosis via the oxygen-sensing pathway. Nat. Med. 2009, 15, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Deneka, M.; Neeft, M.; Popa, I.; van Oort, M.; Sprong, H.; Oorschot, V.; Klumperman, J.; Schu, P.; van der Sluijs, P. Rabaptin-5alpha/rabaptin-4 serves as a linker between rab4 and gamma(1)-adaptin in membrane recycling from endosomes. EMBO J. 2003, 22, 2645–2657. [Google Scholar] [CrossRef] [PubMed]
- Puri, C. Loss of myosin VI no insert isoform (NoI) induces a defect in clathrin-mediated endocytosis and leads to caveolar endocytosis of transferrin receptor. J. Biol. Chem. 2009, 284, 34998–35014. [Google Scholar] [CrossRef] [PubMed]
- Levchenko, A.; Bruck, J.; Sternberg, P.W. Scaffold proteins may biphasically affect the levels of mitogen-activated protein kinase signaling and reduce its threshold properties. Proc. Natl. Acad. Sci. USA 2000, 97, 5818–5823. [Google Scholar] [CrossRef] [PubMed]
- Offenhäuser, N.; Borgonovo, A.; Disanza, A.; Romano, P.; Ponzanelli, I.; Iannolo, G.; di Fiore, P.P.; Scita, G. The eps8 family of proteins links growth factor stimulation to actin reorganization generating functional redundancy in the Ras/Rac pathway. Mol. Biol. Cell 2004, 15, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Olchowik, M.; Miaczyńska, M. Effectors of GTPase Rab5 in endocytosis and signal transduction. Postepybiochemisty 2009, 55, 171–180. [Google Scholar]
- Lippe, R.; Miaczynska, M.; Rybin, V.; Runge, A.; Zerial, M. Functional synergy between Rab5 effector Rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex. Mol. Biol. Cell 2001, 12, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Yan, F.; He, Z.; Liu, S.; Cheng, Y.; Wei, K.; Gan, S.; Yuan, J.; Wang, S.; Xiao, Y.; et al. ITSN2L Interacts with and Negatively Regulates RABEP1. Int. J. Mol. Sci. 2015, 16, 28242-28254. https://doi.org/10.3390/ijms161226091
Yang X, Yan F, He Z, Liu S, Cheng Y, Wei K, Gan S, Yuan J, Wang S, Xiao Y, et al. ITSN2L Interacts with and Negatively Regulates RABEP1. International Journal of Molecular Sciences. 2015; 16(12):28242-28254. https://doi.org/10.3390/ijms161226091
Chicago/Turabian StyleYang, Xiaoxu, Feng Yan, Zhicheng He, Shan Liu, Yeqing Cheng, Ke Wei, Shiquan Gan, Jing Yuan, Shang Wang, Ye Xiao, and et al. 2015. "ITSN2L Interacts with and Negatively Regulates RABEP1" International Journal of Molecular Sciences 16, no. 12: 28242-28254. https://doi.org/10.3390/ijms161226091




