Development and Characterization of Novel Films Based on Sulfonamide-Chitosan Derivatives for Potential Wound Dressing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chitosan Derivative Films
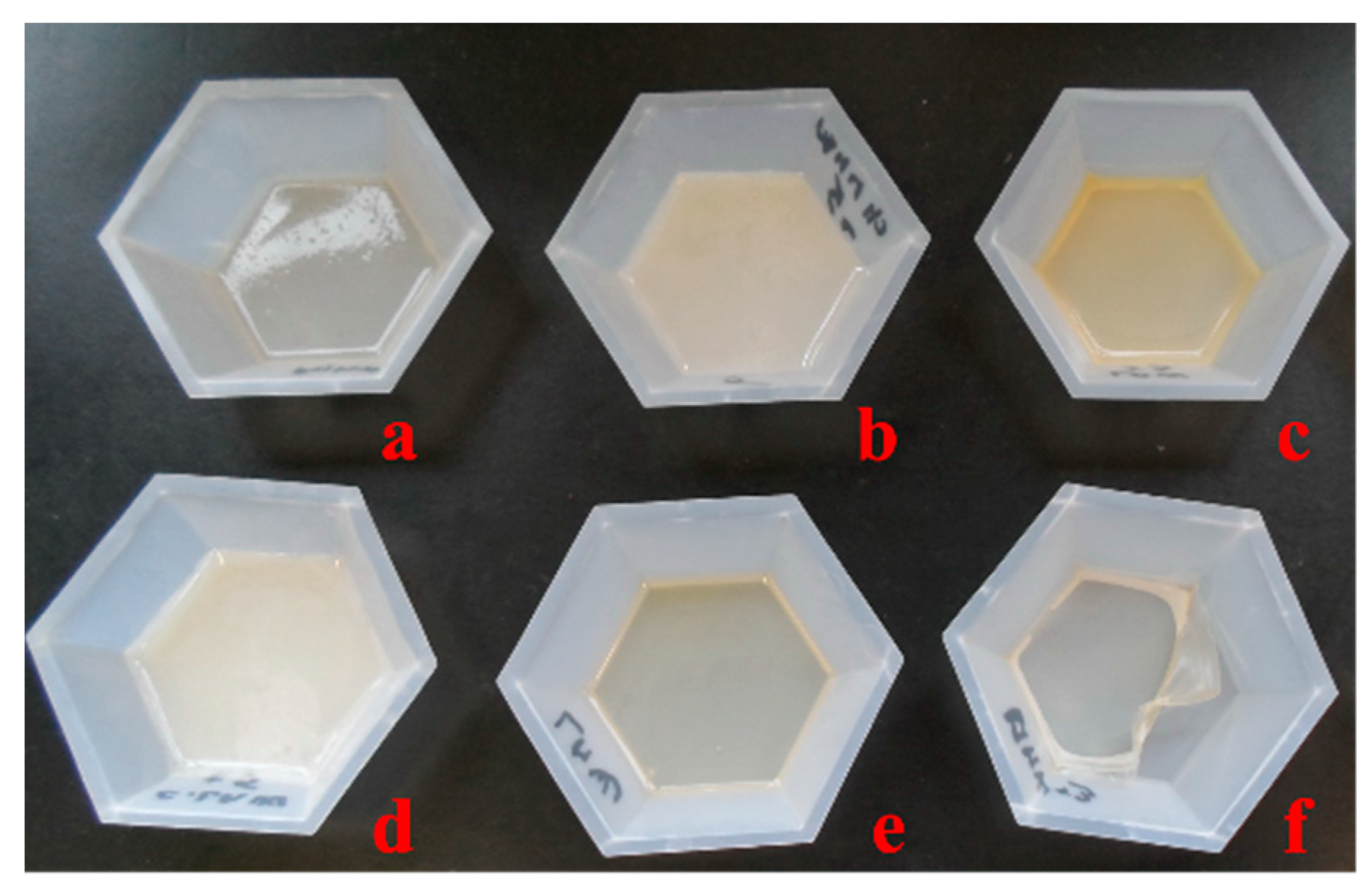
2.2. Surface Morphology
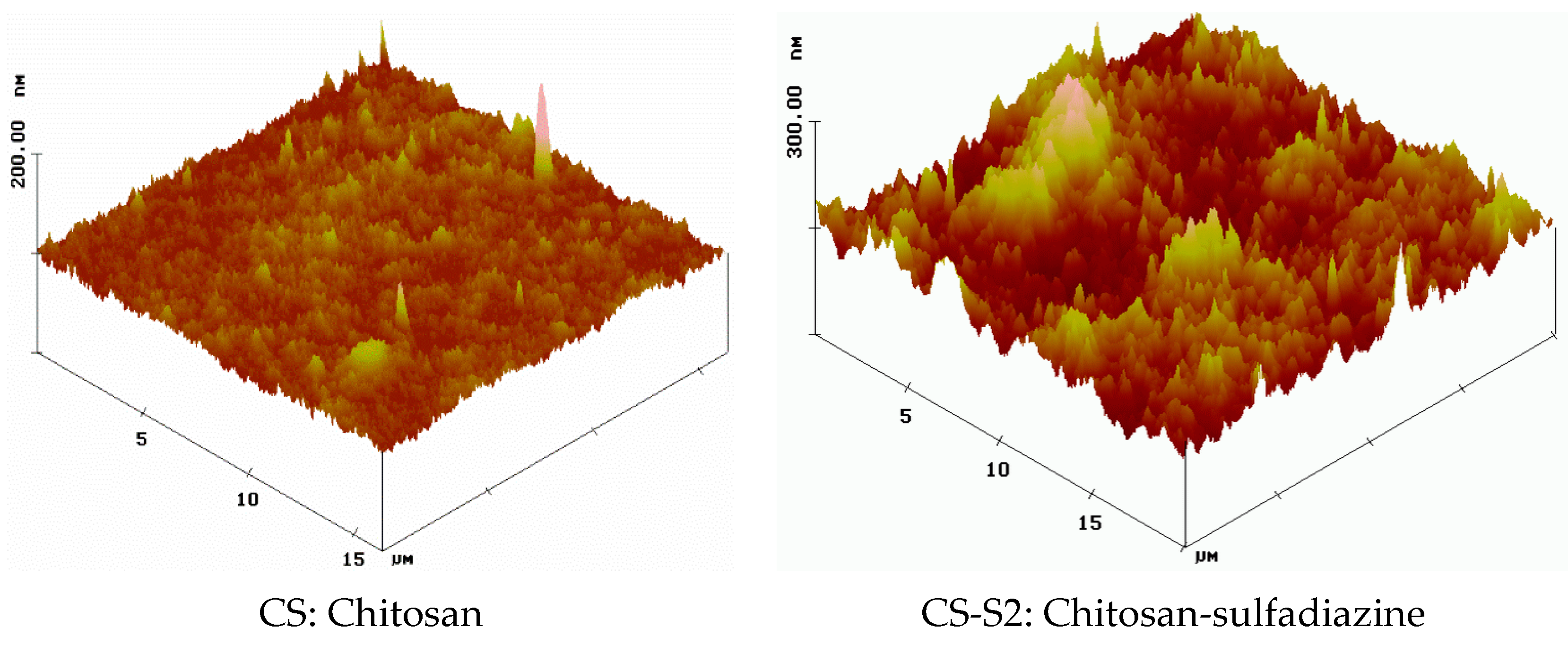
2.3. Contact Angle Measurement
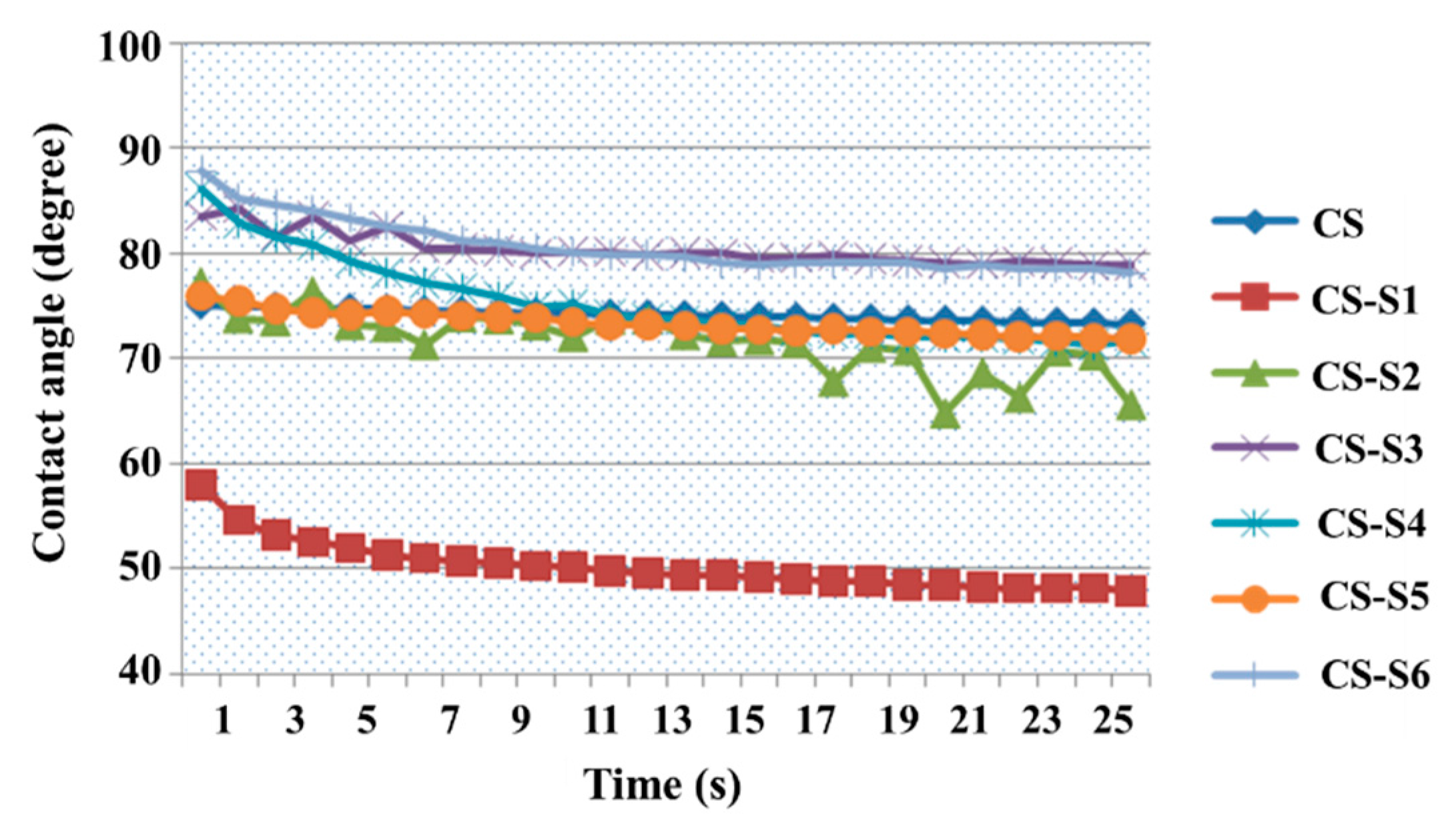

2.4. The Mechanical Properties of Films
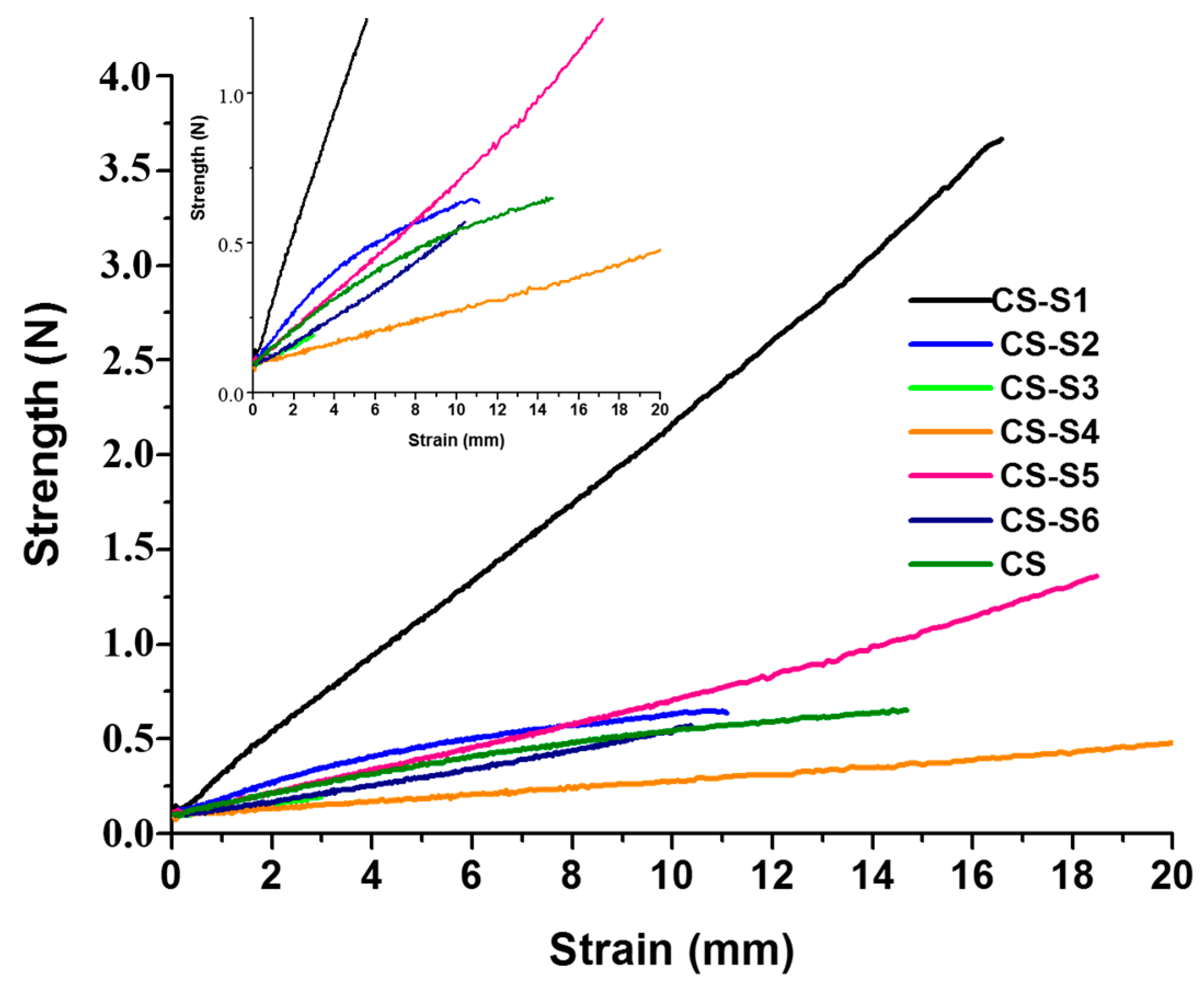
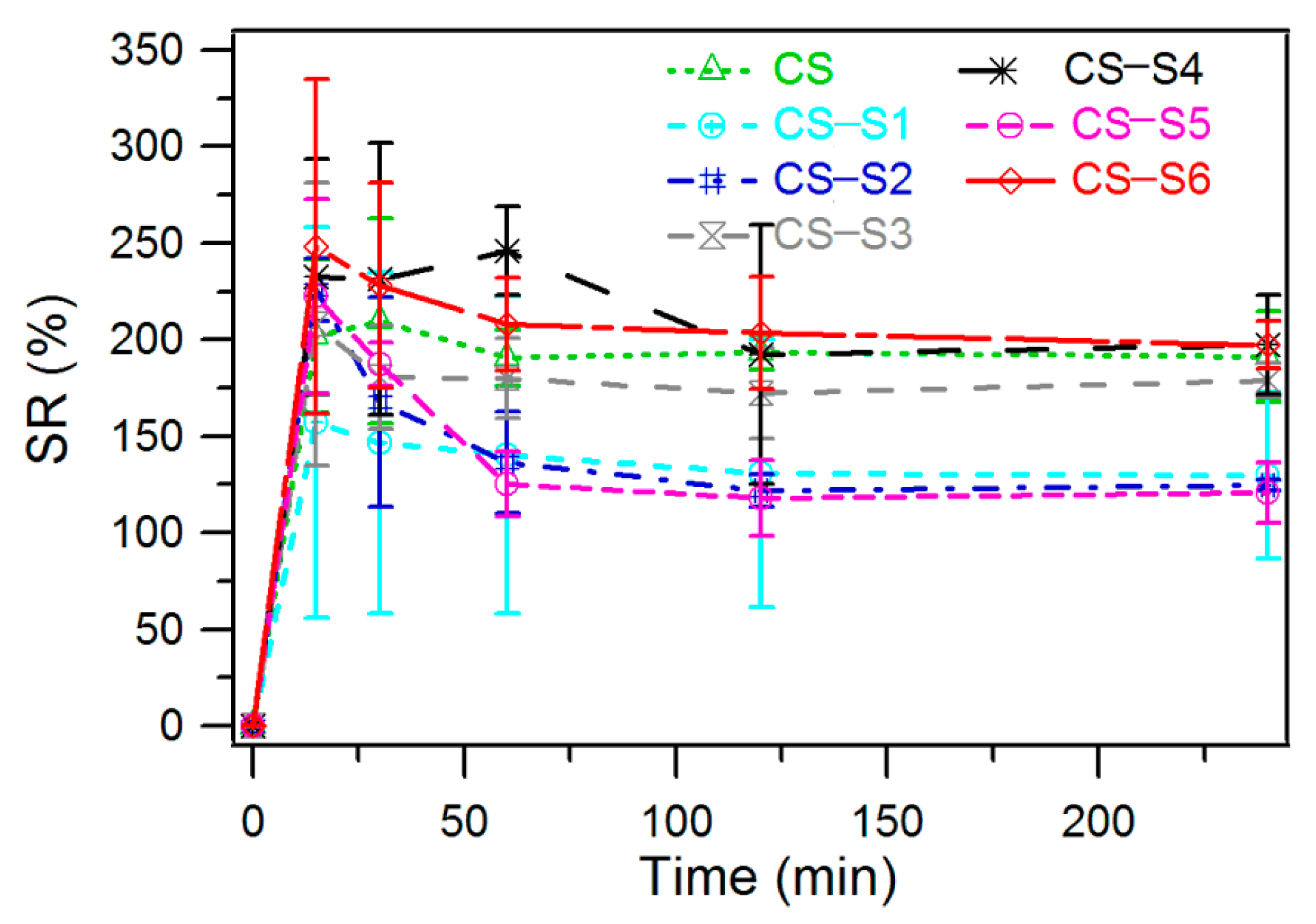
2.5. Swelling Ratio Measurement
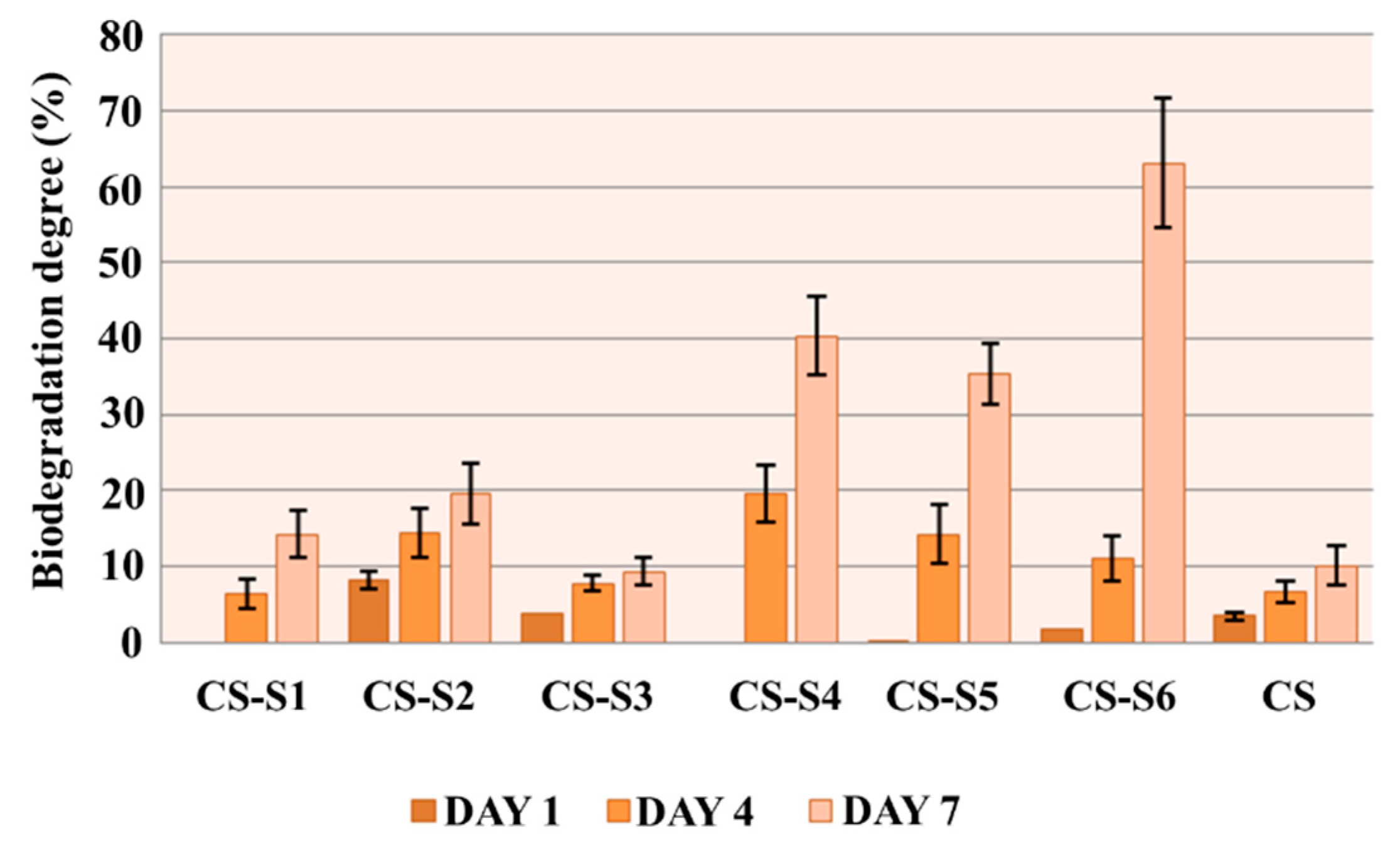
2.6. In Vitro Biodegradation
2.7. Antioxidant Assays
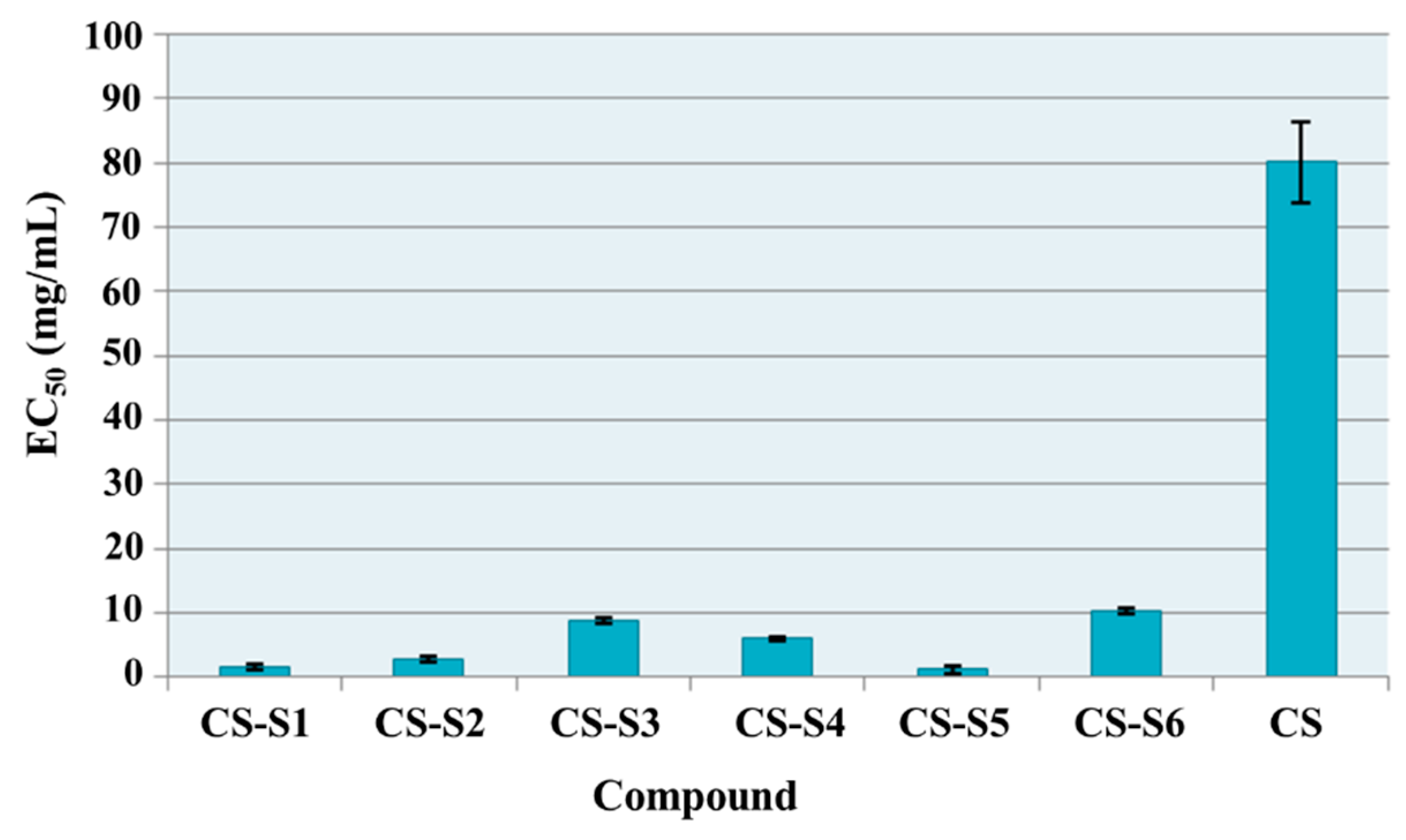
2.7.1. Total Antioxidant Capacity
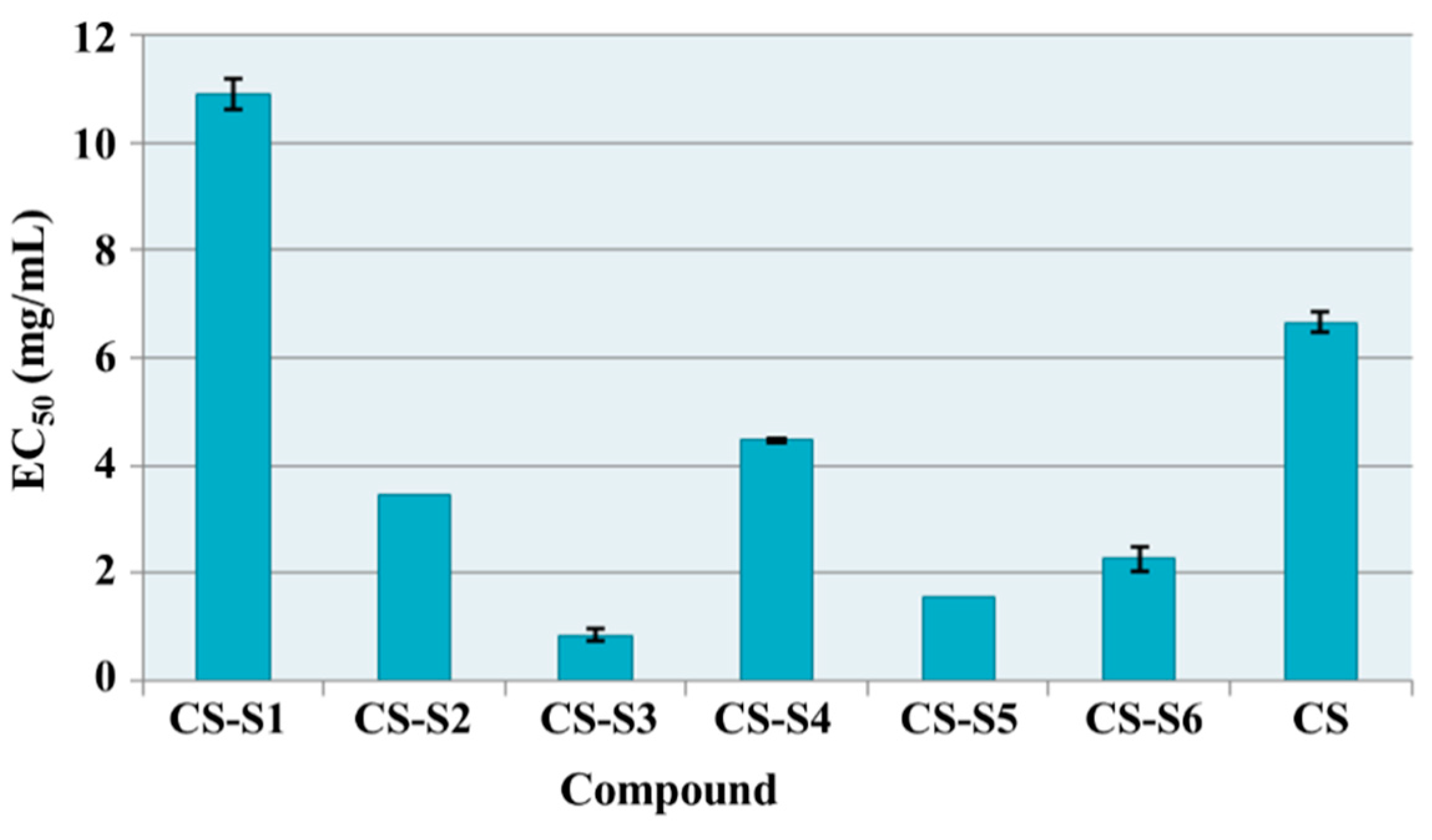
2.7.2. Reducing Power
2.7.3. DPPH (1,1-diphenyl-2-picrylhydrazyl) Radical Scavenging Ability
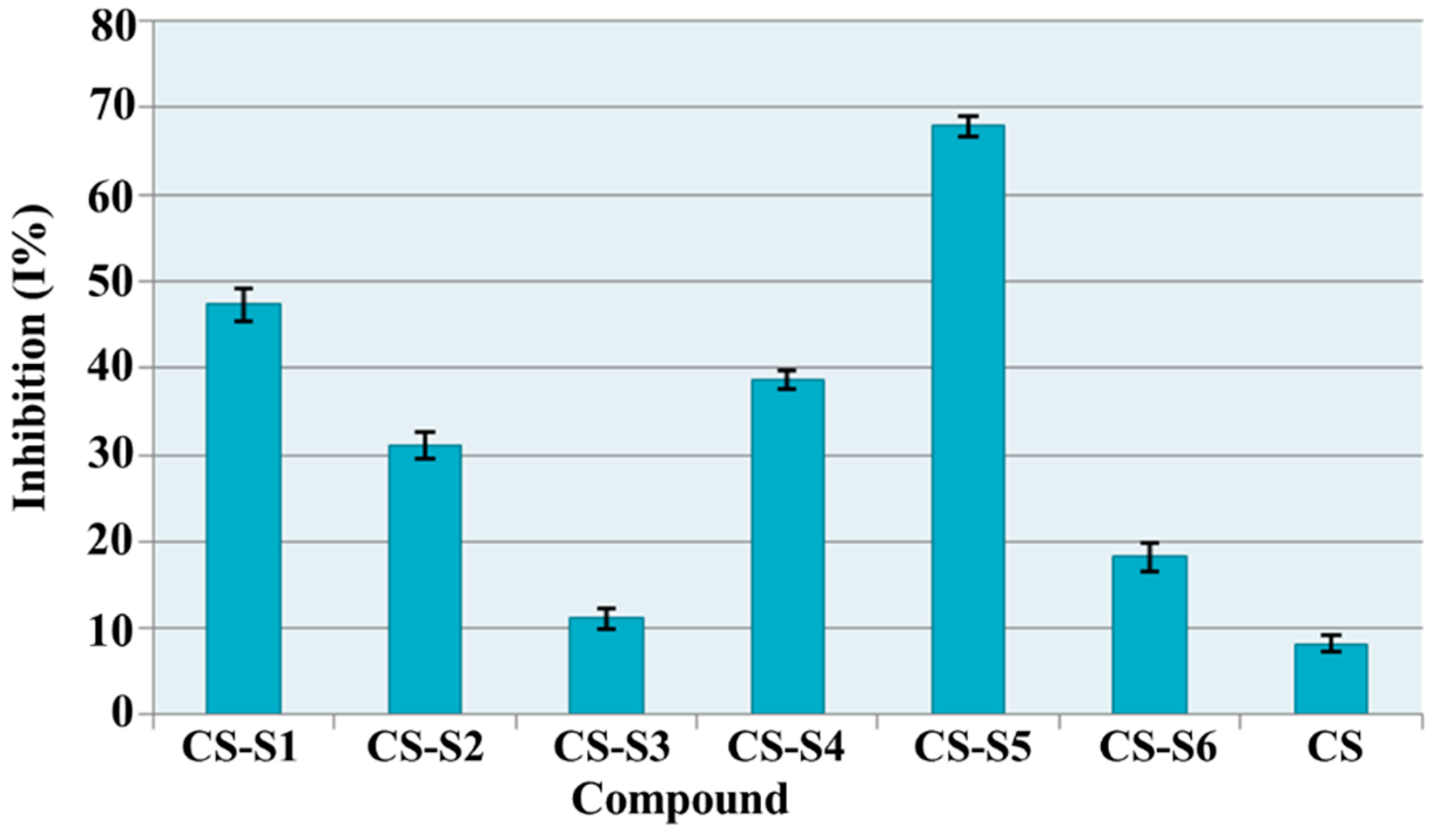
2.8. Antimicrobial Assays
Diameter of Inhibition Area
| Film | Diameter of Inhibition Area (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| S.a. | S.l. | B.c. | B.s. | E.c. | C.a. | C.g. | C.s. | |
| CS | 16 | 28 | 20 | 20 | 15 | 14 | 0 | 0 |
| CS-S1 | 15 | 30 | 22 | 18 | 19 | 10 | 12 | 17 |
| CS-S2 | 17 | 30 | 27 | 25 | 28 | 20 | 25 | 32 |
| CS-S3 | 18 | 30 | 15 | 20 | 19 | 11 | 0 | 0 |
| CS-S4 | 17 | 30 | 27 | 22 | 22 | 0 | 25 | 32 |
| CS-S5 | 15 | 30 | 20 | 18 | 17 | 0 | 0 | 0 |
| CS-S6 | 17 | 28 | 16 | 18 | 18 | 0 | 0 | 9 |
| Control | Diameter of Inhibition Area (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| S.a. | S.l. | B.c. | B.s. | E.c. | C.a. | C.g. | C.s. | |
| Nitrofurantoine 300 µg/disc | 19 | 8 | 12 | 20 | 20 | n.d. | n.d. | n.d. |
| Nystatine 100 µg/disc | n.d. | n.d. | n.d. | n.d. | n.d. | 29 | 26 | 31 |
3. Experimental Section
3.1. Materials
3.2. Preparation of Chitosan Derivatives Films
3.3. Surface Morphology
3.4. Contact Angle Measurement
3.5. The Mechanical Properties
3.6. Swelling Ratio
3.7. In Vitro Biodegradation
3.8. Antioxidant Assays
3.8.1. Total Antioxidant Capacity
3.8.2. Ferric Reducing Power
3.8.3. DPPH (1,1-diphenyl-2-picrylhydrazyl) Radical Scavenging Ability
3.9. Antimicrobial Assays
3.9.1. Diameter of Inhibition Area
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Balan, V.; Verestiuc, L. Strategies to improve chitosan hemocompatibility: A review. Eur. Polym. J. 2014, 53, 171–188. [Google Scholar] [CrossRef]
- Zhanga, D.; Zhoua, W.; Weib, Bing.; Wangb, X.; Tanga, R.; Nieb, J.; Wang, J. Carboxyl-modified poly(vinyl alcohol)-crosslinked chitosan hydrogelfilms for potential wound dressing. Carbohydr. Polym. 2015, 125, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.F.; Facchi, S.P.; Follmann, H.D.M.; Pereira, A.G.B.; Rubira, A.F.; Muniz, E.C. Antimicrobial activity of chitosan derivatives containing N-quaternized moieties in its backbone: A review. Int. J. Mol. Sci. 2014, 15, 20800–20832. [Google Scholar] [CrossRef] [PubMed]
- Noel, S.P.; Courtney, H.; Bumgardner, J.D.; Haggard, W.O. Chitosan films: A potential local drug delivery system for antibiotics. Clin. Orthop. Relat. Res. 2008, 466, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Kloster, G.A.; Marcovich, N.E.; Mosiewicki, M.A. Composite films based on chitosan and nanomagnetite. Eur. Polym. J. 2015, 66, 386–396. [Google Scholar] [CrossRef]
- He, Q.; Ao, Q.; Gong, Y.; Zhang, X. Preparation of chitosan films using different neutralizing solutions to improve endothelial cell compatibility. J. Mater. Sci. Mater. Med. 2011, 22, 2791–2802. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Marsh, K.S.; Rhim, J.W. Characteristics of different molecular weight chitosan films affected by the type of organic solvents. J. Food Sci. 2002, 67, 194–197. [Google Scholar] [CrossRef]
- Dragostin, O.; Lupaşcu, F.; Vasile, C.; Baican, M.; Tuchiluș, C.; Profire, L. Synthesis, characterisation and antimicrobial activity of new chitosan derivatives. In Proceedings of the BIOFUTURE 2011: Young European Biomaterial Scientists Designing: A View for the Future, Ghent, Belgium, 16–18 November 2011.
- Costa-Pinto, A.R.; Martins, A.M.; Castelhano-Carlos, M.J.; Correlo, V.M.; Sol, P.C.; Longatto-Filho, A.; Battacharya, M.; Reis, R.L.; Neves, N.M. In vitro degradation and in vivo biocompatibility of chitosan-poly(butylene succinate) fiber mesh scaffolds. J. Bioact. Compat. Polym. 2014, 29, 137–151. [Google Scholar] [CrossRef]
- Menda, J.P.; Reddy, T.; Deepika, R.; Pandima, D.M.; Sastry, T.P. Preparation and characterization of wound healing composites of chitosan, aloe vera and Calendula officinalis—A comparative study. Am. J. Phytomed. Clin. Ther. 2014, 2, 61–76. [Google Scholar]
- Causa, A.; Fillipone, G.; Acierno, D.; Domingo, C.; Salerno, A. Surface morphology, cristallinity and hidrophilicity of poly(Ɛ-caprolactone) films prepared via casting of ethyl lactate and ethyl acetate solutions. Macromol. Chem. Phys. 2015, 216, 49–58. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Vicente, A.A. Influence of α-tocopherol on physicochemical properties of chitosan based films. Food Hydrocoll. 2012, 27, 220–227. [Google Scholar] [CrossRef]
- Dragostin, O.M.; Samal, S.K.; Dash, M.; Lupașcu, F.; Dubruel, P.; Profire, L. Physico-chemical characterization of polymeric matrices based on functionalized chitosan. In Proceeding of the Advanced Materials for Biomedical Applications, Ghent, Belgium, 2014.
- Hasmann, A.; Wehrschuetz-Sigl, E.; Kanzler, G.; Gewessler, U.; Hulla, E.; Schneider, K.P.; Binder, B.; Schintler, M.; Guebitz, M. Novel peptidoglycan-based diagnostic for detection of wound infection. Diagn. Microbiol. Infect. Dis. 2011, 71, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hu, P.; Ren, X.; Worley, S.D.; Huang, T.S. Antimicrobial N-halamine modified chitosan films. Carbohydr. Polym. 2013, 92, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Rubilar, J.F.; Cruz, R.M.S.; Silva, H.D.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Physico-mechanical properties of chitosan films with carvacrol and grape seed extract. J. Food Eng. 2013, 115, 466–474. [Google Scholar] [CrossRef]
- Samal, S.K.; Kaplan, D.L.; Chiellini, E. Ultrasound sonication effects on silk fibroin protein. Macromol. Mat. Eng. 2013, 298, 1201–1208. [Google Scholar] [CrossRef]
- Rotta, J.; Ozório, R.A.; Kehrwald, A.M.; Mariz de Oliveira Barra, G.; Dias de Melo Castanho Amboni, R.; Barreto, P.L.M. Parameters of color, transparency, water solubility, wettability and surface free energy of chitosan/hydroxypropyl-methylcellulose (HPMC) films plasticized with sorbitol. Mater. Sci. Eng. C 2009, 29, 619–623. [Google Scholar] [CrossRef]
- ASTM D1708–13. In Standard Test Method for Tensile Properties of Plastics by Use of Microtensile Specimens; ASTM International: West Conshohocken, PA, USA, 2013.
- Baran, E.T.; Tuzlakoğlu, K.; Mano, J.F.; Reis, R.L. Enzymatic degradation behavior and cytocompatibility of silk fibroin-starch-chitosan conjugate membranes. Mater. Sci. Eng. 2012, 32, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Melo-Silveira, R.F.; Fidelis, G.P.; Costa, M.S.S.P.; Telles, C.B.S.; Dantas-Santos, N.; Elias, S.; Ribeiro, V.B.; Barth, A.L.; Macedo, A.J.; Leite, E.L.; et al. In vitro antioxidant, anticoagulant and antimicrobial activity and inhibition of cancer cell proliferation by xylan extracted from corn cobs. Int. J. Mol. Sci. 2012, 13, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Lupascu, F.G.; Dragostin, O.M.; Foia, L.; Lupascu, D.; Profire, L. The synthesis and the biological evaluation of new thiazolidin-4-one derivatives containing a xanthine moiety. Molecules 2013, 18, 9684–9703. [Google Scholar] [CrossRef] [PubMed]
- Dragostin, O.M.; Lupascu, F.; Vasile, C.; Mares, M.; Nastasa, V.; Moraru, R.F.; Pieptu, D.; Profire, L. Synthesis and biological evaluation of new 2-azetidinones with sulfonamide structures. Molecules 2013, 18, 4140–4157. [Google Scholar] [CrossRef] [PubMed]
- Osorio, M.; Aravena, J.; Vergara, A.; Taborga, L.; Baeza, E.; Catalán, K.; González, C.; Carvajal, M.; Carrasco, H.; Espinoza, L. Synthesis and DPPH radical scavenging activity of prenylated phenol derivatives. Molecules 2012, 17, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Nyanhongo, G.S.; Sygmund, G.; Ludwig, R.; Prasetyo, E.N.; Guebitz, G.M. An antioxidant regenerating system for continuous quenching of free radicals in chronic wounds. Eur. J. Pharm. Biopharm. 2013, 83, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute Antimicrobial Susceptibility Testing Standards. In Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragostin, O.M.; Samal, S.K.; Lupascu, F.; Pânzariu, A.; Dubruel, P.; Lupascu, D.; Tuchilus, C.; Vasile, C.; Profire, L. Development and Characterization of Novel Films Based on Sulfonamide-Chitosan Derivatives for Potential Wound Dressing. Int. J. Mol. Sci. 2015, 16, 29843-29855. https://doi.org/10.3390/ijms161226204
Dragostin OM, Samal SK, Lupascu F, Pânzariu A, Dubruel P, Lupascu D, Tuchilus C, Vasile C, Profire L. Development and Characterization of Novel Films Based on Sulfonamide-Chitosan Derivatives for Potential Wound Dressing. International Journal of Molecular Sciences. 2015; 16(12):29843-29855. https://doi.org/10.3390/ijms161226204
Chicago/Turabian StyleDragostin, Oana Maria, Sangram Keshari Samal, Florentina Lupascu, Andreea Pânzariu, Peter Dubruel, Dan Lupascu, Cristina Tuchilus, Cornelia Vasile, and Lenuta Profire. 2015. "Development and Characterization of Novel Films Based on Sulfonamide-Chitosan Derivatives for Potential Wound Dressing" International Journal of Molecular Sciences 16, no. 12: 29843-29855. https://doi.org/10.3390/ijms161226204
APA StyleDragostin, O. M., Samal, S. K., Lupascu, F., Pânzariu, A., Dubruel, P., Lupascu, D., Tuchilus, C., Vasile, C., & Profire, L. (2015). Development and Characterization of Novel Films Based on Sulfonamide-Chitosan Derivatives for Potential Wound Dressing. International Journal of Molecular Sciences, 16(12), 29843-29855. https://doi.org/10.3390/ijms161226204









