Discovery and Current Status of Evaluation System of Bioavailability and Related Pharmaceutical Technologies for Traditional Chinese Medicines—Flos Lonicerae Japonicae—Fructus Forsythiae Herb Couples as an Example
Abstract
:1. Introduction
2. Identification of Active Compounds in Traditional Chinese Medicines (TCMs)
2.1. Classic Separation and Analysis
2.2. Spectrum-Effect Relationships
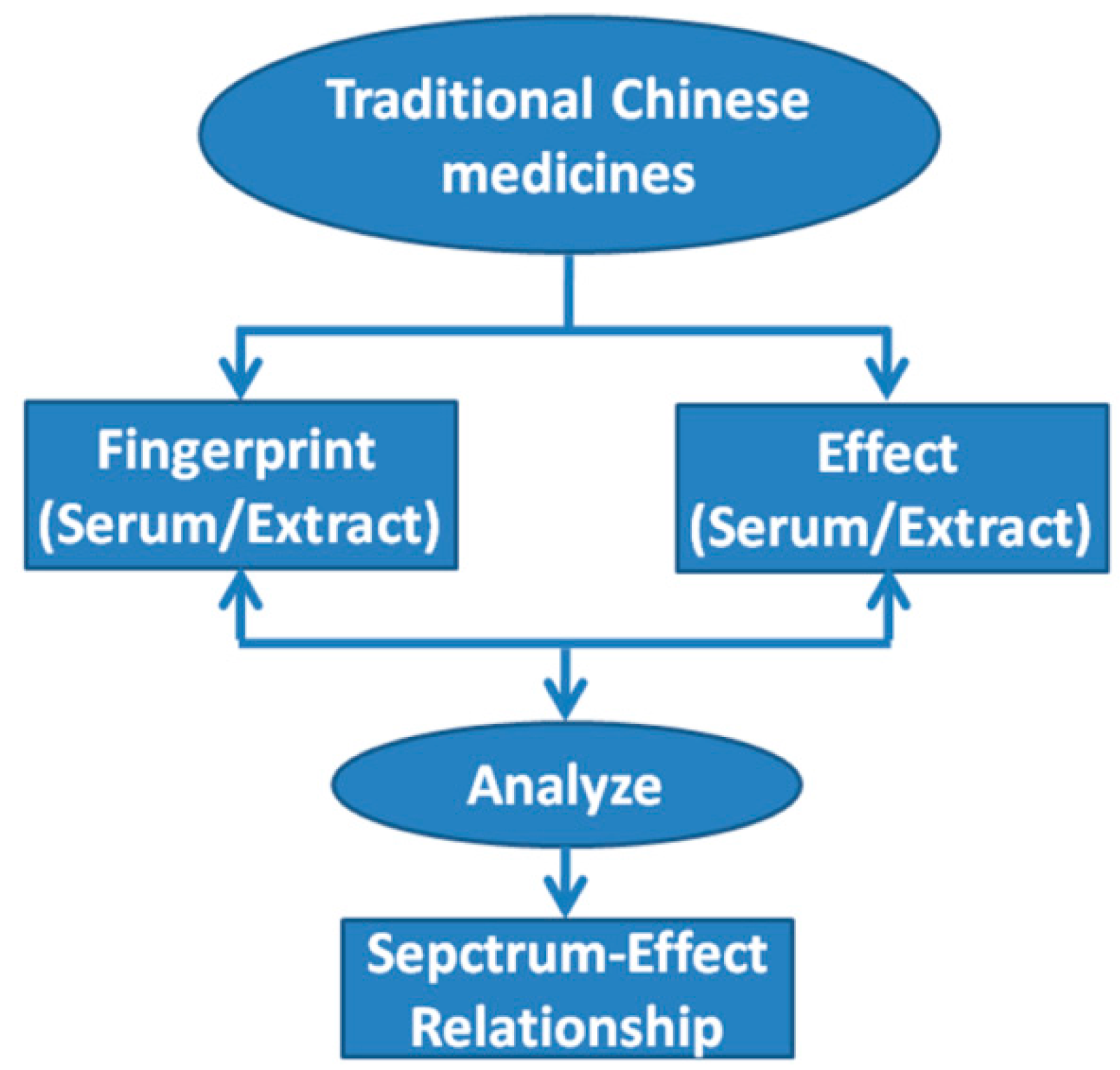
| Names | TCMs Composition | Fingerprint | Pharmacology | Experimentalmodel | Analytical Method | Active Components | Reference |
|---|---|---|---|---|---|---|---|
| Cichorium intybus L. | - | HPLC-DAD-MS | Anti uric acid | Quails | CCA | Aesculin, chlorogenic acid, chicoric acid, isochlorogenic acid A/B/C and 13,14-seco-stigma5(6), 14(15)-diene-3α-ol | [16] |
| Tripterygium glycosides | Tripterygium wilfordii | HPLC | Anti-inflammatory, immunosuppressive activities | mice spleen cells | GRA | Peak 5, peak 10 | [17] |
| Radix Astragali | - | HPLC-PDA-ELSD | Anti-gastric effect | Mice, GES-1 cell | GRA | Ononin, astragaloside III, astragaloside IV | [19] |
| Rhizoma Coptidis | - | UPLC-PDA/HPLC-DAD | Antibacterial effect/Anti-MRSA activity/Anti-inflammatory | Escherichia coli/Broth microdilution/RAW264.7 mouse macrophage cells | HCA, CCA, PCA, PLS | Berberine, jateorrhizine, palmatine, coptisine, epiberine | [18,20,28] |
| Da Cheng Qi Tang | Rhizoma Rhei, Cortex Magnoliae officinalis, Fructus Aurantii Immaturus | HPLC-DAD | Purgative effect | Mice | HCA | Hesperidin, aloe-emodin, honokiol, rhein, magnolol, emodin, sennoside A | [21] |
| Polygonum cuspidatum | - | HPLC-DAD-FICL | Anti-oxidant effect | H2O2 scavenging activities | CA | Piceid, resveratrol, torachrysone-8-O-glucoside, questin/physcion, peak1, peak 10 | [22] |
| Acalypha australis Linn. | - | UPLC/MS, semi-preparative HPLC | Antibacterial effect | Agar-diffusion method; Broth microdilution method | - | Gallic acid, peak 6, peak 9–11 | [23] |
| Zathoxylum nitidum | - | IR | Antitumor effect | 7901, Hela cells | MLR | Nitidine chloride | [24] |
| Morinanepalensis | - | HPLC-ELSD | NO inhibition | RAW264.7 cell | PLS | Peak 2, peak 4–6, peak 10, peak 12, peak 13 | [25] |
| Rheum species | - | UPLC-PDA | Anti-HIV activity(Ribonuclease H) | enzyme activity | BA | Catechin, epicatechin, aloe-emodinmonoglucoside, Peak (tR = 21.28 min) | [26] |
| Rabbiteye blueberry | - | HPLC-DAD | Antioxidant effect | DPPH radical scavenging | HCA | Delphinins, anthocyanidin-3-glucosides | [27] |
| EtOAC extracts of Radix Isatidis | - | HPLC-DAD | Antibacterial effect | Escherichia coli | HCA, MLR, PCA | Salicylic acid | [29] |
| Radix Aconiti, Radix Aconiti Singularis, Radix Aconiti Kusnezoffii, Radix Aconiti Lateralis Preparata, Radix Aconiti Brachypodi | - | UPLC-PDA | Antibacterial effect | Escherichia coli | CCA | Hypaconitine, peak 1, peak 3 | [30] |
| Polygonum orientale | - | UPLC-PDA | Anti-oxidative injury | H9c2 myocardial cell | BA | Peak 3–5, peak 11–14, peak 18, peak 19, peak 21–25 | [31] |
| Qizhiweitong Granules | Radix bupleuri, Rhizoma Corydalis, Fructus Aurantii, Rhizoma Cyperi, Radix Paeoniae Alba, Radix glycyrrhizae Preparata | HPLC-DAD | Promoting gastrointestinal motility | Small intestine smooth muscle cells | GRA, BP neural network | Naringin, neohesperidin, hesperidin, neoponcirin, narirutin, liquiritinapioside, albiflorin analogues, neoeriocitrin, glycyrrhizin | [32] |
| ZuoJin Wan | Coptis chinensis Franch.Evodia rutaecarpa (Juss.) Benth. | HPLC-DAD | Biothermo-logical effect | Escherichia coli | CCA | Evodiamine, palmatine hydrochloride, berberine hydrochloride | [33] |
| Suanzaoren decoction | Semen Ziziphi Spinosae, poria, rhizoma Chuanxiong, rhizome Anemarrhenae, radix glycyrrhizae | HPLC-PDA | Sedative effect | Mice | Correlation and regressive analysis | Spinosin, ferulic acid, mangiferin, glycyrrhizic acid, peak 3, peak 8, peak 9, peak 16, peak 21, peak 34, peak 42, peak 46, peak 47 | [34] |
| Platycladi cacumen | - | HPLC-MS/MS | Hemostatic activities | New Zealand rabbit | CCA | Cecarbon | [35] |
| Radix Hedysari | - | HPLC | Anti-hepatic fibrosis | Mice | GRA, PLS, | Adenosine, calycosin | [36] |
| Saffron | - | HPLC-DAD | Antioxidants | DPPH | MCA | Crocins-1, crocins-2, crocins-3 | [37] |
| Flos Sophorae | - | HPLC-MS/MS | Hemostatic activities | New Zealand rabbit | CCA | Huaicarbon A, huaicarbon B | [38] |
| Aconitum carmichaelii Debeaus | - | UPLC-ELSD | Mitochondria growth promoting effect | Rat | CCA | Mesaconitine, benzoylaconitine, benzoylhypacotine | [39] |
| Artificial Calculus bovis | - | UPLC-ELSD | Antibacterial effect | Escherichia coli | HCA, MLR, PCA | Cholic acid, taurocholate sodium, chenodeoxycholic acid | [40] |
| Belamcanda chinensis leaf | - | HPLC-DAD | Hypoglycemic effect | Rat | - | Flavonoids (tectoridin, swertisin) | [41] |
| Da-Huang-Fu-Zi-Tang | Rheum officinale Baill., Aconitum carmichaelii Debx., Asarum sieboldii Miq. | UHPLC-ESI-Q-TOF-MS | Anti-acute pancreatitis effect | AR42J cell | CCA | Talatisamine, rhein glucoside, rhein isomer methylation, hypaconine, hydroxyl-chrysophanol, emodin glucuronide conjugation, chysophanol glucuronide conjugation | [43] |
2.3. Knock-in and Knock-out
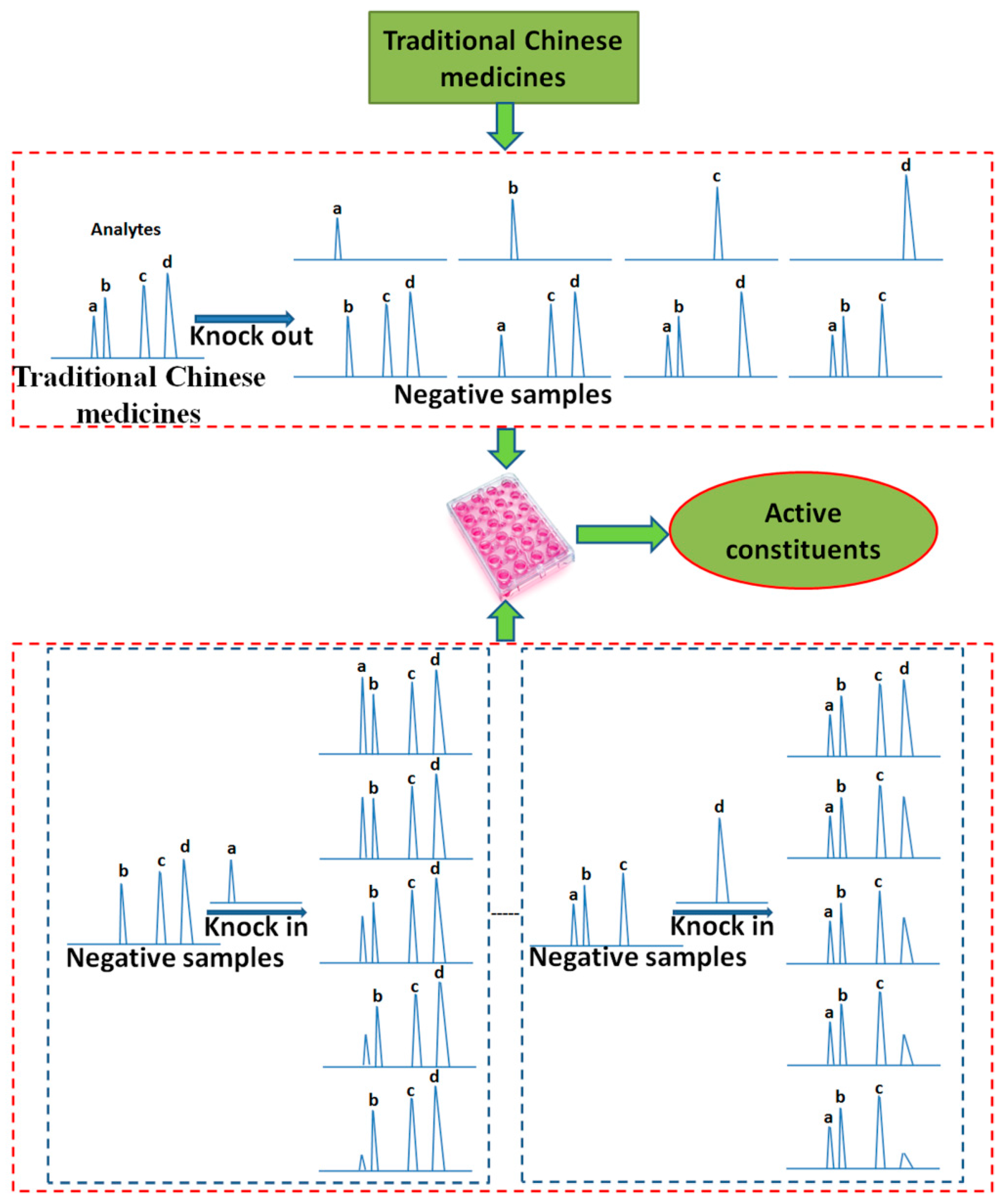
| Names | Knock-in or Knock-out Components | Pharmacology | Experimental Model | Active Components | Reference |
|---|---|---|---|---|---|
| Rhizoma Coptidis | Berberine, palmatine, coptisine, epiberberine, jateorrhizine, columbamine | Growth inhibition of shigelladysenteriae | Microcalorimetry | Berberine, coptisine | [46,47] |
| Herba Epimedii | Epimedin A, epimedin B, epimedin C, icariin | Cell proliferation, differentiation | Third generation rat osteoblasts | Epimedin A, epimedin B, epimedin C, icariin | [48] |
| Calculus bovis | Bilirubin, bilirubin conjugate, glycocholic acid, cholic acid, chenodeoxycholic acid, hyodeoxycholic acid, sodium taurocholic acid, deoxycholic acid | Inhibition of hydrogen peroxide-induced damage | SH-SY5Y | Bilirubin, bilirubin conjugate, glycocholic acid, cholic acid | [50,51] |
| Flos Lonicerae Japonicae | Isochlorogenic acids, chlorogenic acid, flavones, iridoid glycosides | Anti-virus, anti-bacteria | Vero cell, Escherichia coli | Isochlorogenic acids | [52] |
| Rhizoma Curcumae Longae | Curcumin, demethoxycurcumin, bisdemethoxycurcumin | Anti-oxidant activity, anti-coagulant effect, anti-oxidant stress damage | DPPH, rabbit, PC12 | Curcumin > demethoxycurcumin > bisdemethoxycurcumin | [53,54] |
| Radix puerariae | Puerarin, daidzin, daidzein, compound X | Anti-oxidant damage | HUVEC | Puerarin, compound X | [55] |
| Shenmai formulae | Panoxadiol, panaxotriol, ophiopogonpolysaccharide, ophiopogonin | Antitumor effect | S180 bearing mice | Panoxadiol, panaxotriol, ophiopogonpolysaccharide | [49] |
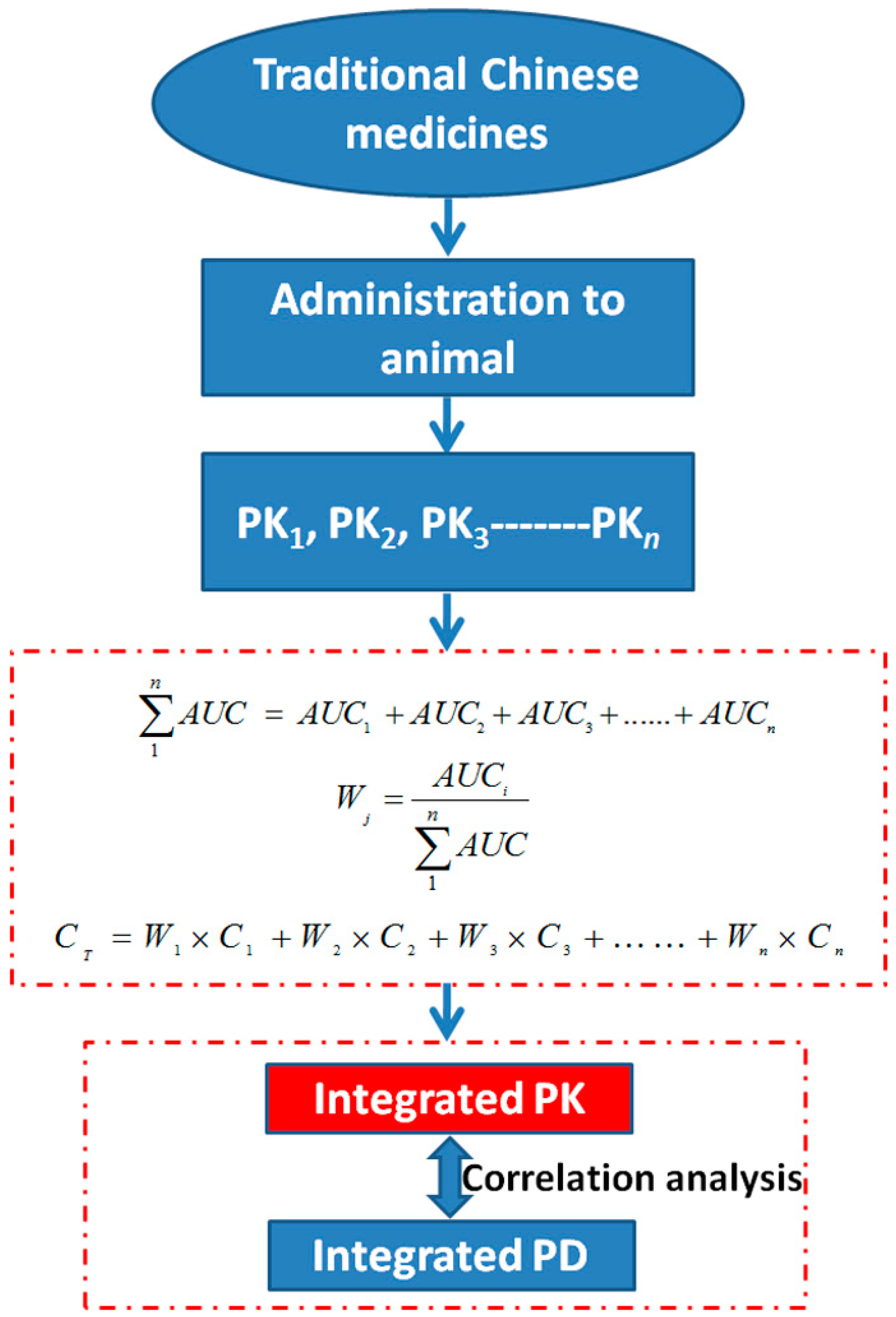
2.4. Pharmacokinetics (PK)-Pharmacodynamics (PD)

| Names | TCMs Composition | PK Ingredients | PD | Analytical Method | Active Components | Reference |
|---|---|---|---|---|---|---|
| Da-Huang-Fu-Zi-Tang | Rheum officinale Baill., Aconitum carmichaelii Debx., Asarum sieboldii Miq. | Talatisamine emodin isomer | Anti-acute pancreatitis effect in AR42J cell | CCA AUE-lgAUC E-logC Winnonlin | Talatisamin chysophanol glucuronide conjugation | [43] |
| Qingkailing injection | Cholalic acid, Concha margaritifera, Hyodeoxycholic acid, Gardeniae Fructus, Cornu bubali, Radix isatidis, Baicalin, Flos Lonicerae Japonicae | Baicalin, geniposide, cholalic acid, hyodeoxycholic acid, chlorogenic acid, neochlorogenic acid | Temperature changes in rat | Baicalin, geniposide | [57] | |
| Yin-Teng-Gu-Bi-Kang Precription | Radix Salviae Miltiorrhiae, Angelicae Sinensis Radix, Paeoniae Radix Alb, Celastrus orbiculatus Thunb. | Tanshinone IIA | MDA in rat’s serum | Tanshinone IIA | [58] | |
| Shengmai injection | Red ginseng, ophiopogon japonicas (Thunb.) Ker-Gawl, schisandra chinensis | Ginsenoside (Rg1, Rb1) | NO in rat’s serum | Ginsenoside (Rg1, Rb1) | [59] | |
| Rhizoma Curculiginis | — | Orcinol glucoside | SOD, GSH, GSH-PX in plasma | Orcinol glucoside | [60] | |
| Schisandra chinensis alcoholic extract | — | Schisandrin, gomisin D, gomisin O, tigloylgomisin H, angeloylgomisin Q, gomisin G, gomisin B, angeloylgomisin P, schisantherin A, gomisin E, schisantherin D, deoxyschizandrin, gomisin R, γ-schisandrin, angeloylisogomisin O, angeloylgomisin O, 6-O-benzoyl gomisin O, 7-8-dihydroxy-schizandrin, PeaktR (42.0 min) | ALT in rat’s serum | Schisandrin, schisantherin A, deoxyschizandrin, γ-schisandrin, 7-8-dihydroxy-schizandrin, PeaktR (42.0 min) | [61] | |
| Radix et Rhizoma Rhei | — | Aloe Emodin, rhein, emodin, chrysophanol | Amylase, endotoxin, TNF-α, diamineoxidase in beagle dog’s serum; Temperature changes and NO in rat in vivo | Rhein | [62] | |
| Tea polyphenols | — | Epigallocatechingallate, epicatechingallate, epigallocatechin, epicatechin | MDA in rat’s liver | Epigallocatechi-n gallate, epicatechingallate, epigallocatechi-n, epicatechin | [63] |
3. Evaluation System of Bioavailability Establishment for TCMs
| Names | TCMs Composition | Integrated Ingredients | Integrated Method | Pharmacology | Correlation Analysis | Reference |
|---|---|---|---|---|---|---|
| Rhododendri Mollis Flos | - | Rhodojaponin (I, II, III) | Weighting factor based on AUC | Myocardial damage (LDH, CK-MB) | - | [65] |
| Panax Notoginseng Saponins1 | - | Panax Notoginsenoside R1, Ginsenosides Rg1, Rb1, Re, Rd | - | - | [66,67] | |
| Huanglian-Zhizi couplet medicine | Rhizoma Coptidis, Fructus Gardeniae | Gardenia acid, geniposide | Antioxidant efficacy (SOD) | E-C | [68] | |
| Huang-Lian-Jie-Du-Tang | Rhizomacoptidis, Radix scutellariae, Cortex phellodendri, Fructusgardeniae | Berberine, palmatine, baicalin, baicalein, geniposide | Anti-ischemia | - | [69] | |
| Schisandra lignans | - | Schisandrin, schisantherin A, deoxyschisandrin, γ-schisandrin | Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) | E-C | [70] | |
| Jiao-Tai-Wan | Rhizomacoptidis powder, Cortex cinnamomi powder | Berberine, palmatine, coptisine, epiberberine, jatrorrhizine | - | - | [71] | |
| Huang-Lian-Jie-Du-Tang | Rhizomacoptidis, Radix scutellariae, Cortex phellodendri, Fructusgardeniae | Groenlandicine, berberine, palmatine, epiberberine, jatrorrhizine, columbamine | - | - | [72] | |
| Total coumarins in Radix Angelicae dahuricae | - | Bergapten, imperatorin…isoimperatorin | - | - | [73] | |
| Tea polyphenols | - | Epigallcocatechingallate, Epicatechingallate, Epigallocatechin, Epicatechin | Anti-lipid peroxidation in vitro of mouse liver homogenate | E-logC | [74] | |
| Gegen-Qinlian Decoction | Radix Puerariae, Radixscutellariae, Coptidisrhizome, Radixglycyrrhizae | Puerarin, Daidzein, Baicalin, Baicalein, Wogonoside, Wogonin, Glycyrrhizin, Liquiritin, Berberine, Jateorhizine, Palmatine | - | - | [75] |
4. Pharmaceutical Technology Applications to Improve the Bioavailability of Active Components in TCMs
5. Study on the Evaluation System of Bioavailability Establishment and Related Pharmaceutical Technologies—Flos Lonicerae Japonicae—Fructus Forsythiae Herb Couples as an Example
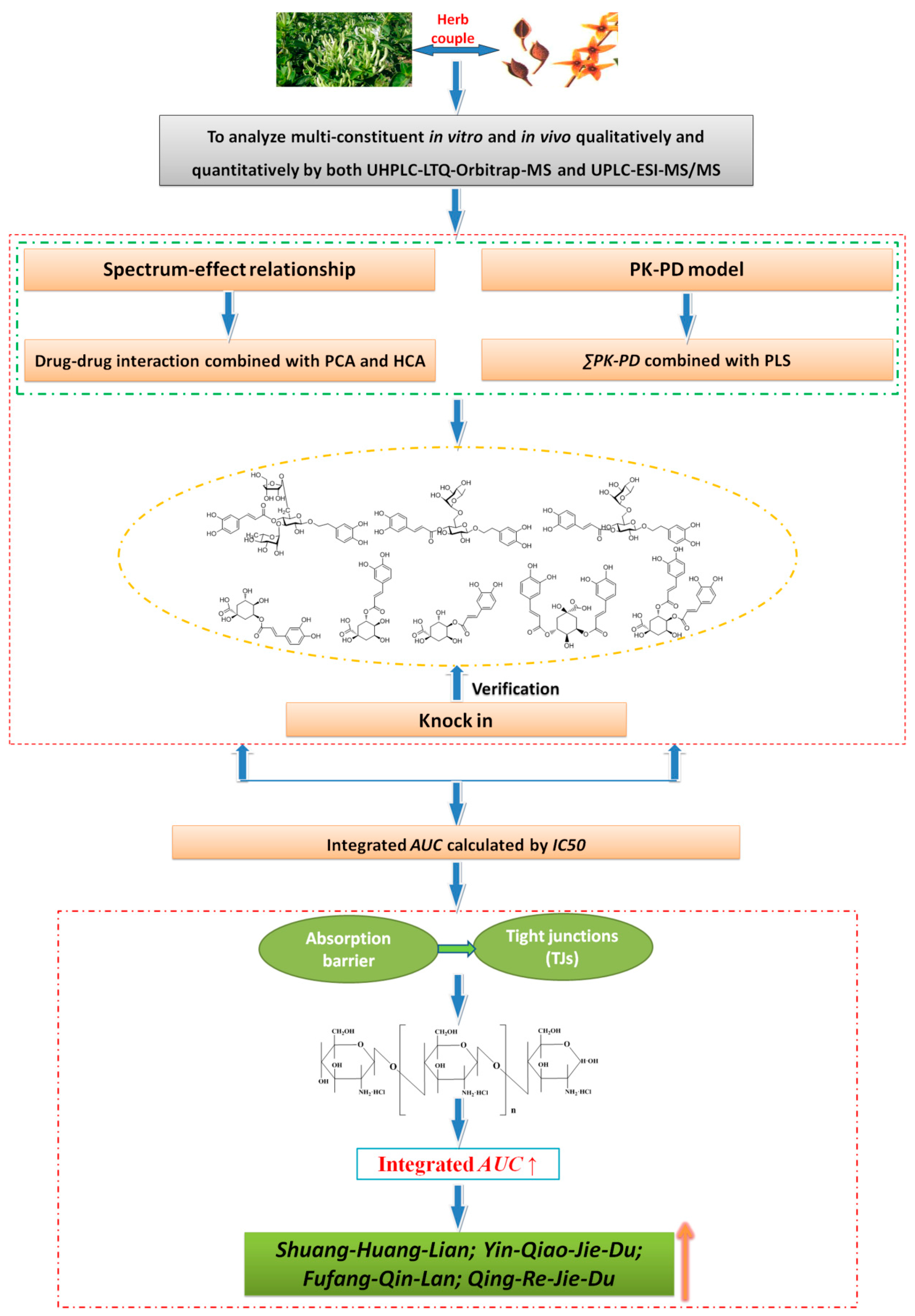
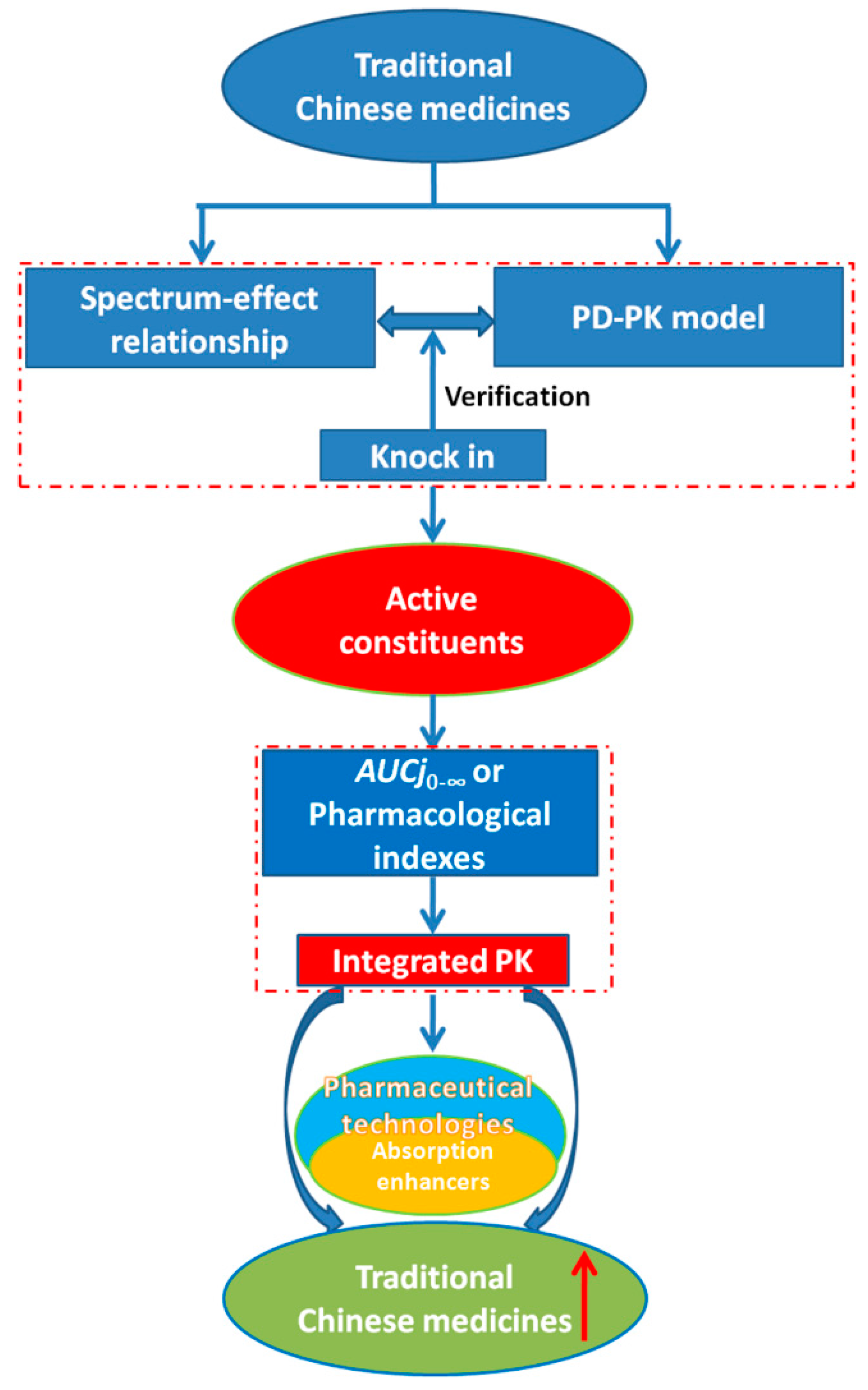
6. Conclusions and Future Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yi, Y.D.; Chang, I.M. An overview of traditional Chinese medicine herbal formulae and a proposal of a new code system for expressing the formula titles. Evid. Based Complement. Alternat. Med. 2004, 1, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.F. 3.2 billon dollars exports for traditional Chinese medicine in China. J. Trad. Chin. Med. Manag. 2015, 23, 125. [Google Scholar]
- Zhou, Y.L. The clinical application and adverse reaction of Shuang-Huang-Lian preparations, Nei Mongol Journal of Traditional Chinese Medicine. Nei Mongol J. Trad. Chin. Med. 2010, 13, 101–102. [Google Scholar]
- White, N.J. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob. Agents Chemother. 1997, 41, 1413–1422. [Google Scholar] [PubMed]
- Hollman, A. Drugs for atrial fibrillation. Digoxin comes from Digitalis lanata. BMJ 1996, 312, 912. [Google Scholar] [CrossRef] [PubMed]
- Matyášová, E.; Novák, J.; Stránská, I.; Hejtmánková, A.; Skalický, M.; Hejtmánková, K.; Hejnák, V. Production of morphine and variability of significant characters of Papaver somniferum L. Plant Soil Environ. 2011, 57, 423–428. [Google Scholar]
- Trung Bui-Khac, T.; Dupuis, N. Process for Extraction and Purification of Paclitaxel from Natural Sources. U.S. Patent 6452024 B1, 26 May 2000. [Google Scholar]
- Johnson, I.S.; Armstrong, J.G.; Gorman, M.; Burnett, J.P., Jr. The vinca alkaloids: A new class of oncolytic agents. Cancer Res. 1963, 23, 1390–1427. [Google Scholar] [PubMed]
- Hu, L.S. Application of ginkgo leaf total lactones in preparation of medicament for preventing or treating deafness and tinnitus. C.N. Patent 102078343 B, 3 October 2012. [Google Scholar]
- Mukhtar, H.; Ahmad, N. Tea polyphenols: Prevention of cancer and optimizing health. Am. J. Clin. Nutr. 2000, 71, 1698S–1702S. [Google Scholar] [PubMed]
- Li, R.; Yan, Z.Y.; Li, W.J.; Xu, T.; Tan, R.A.; Pan, L.; Li, Y.M.; Ma, Y.L. The establishment of chromatographic pharmacodynamics. Educ. Chin. Med. 2002, 21, 62. [Google Scholar]
- Liang, Y.; Xie, P.; Chau, F. Chromatographic fingerprinting and related chemometric techniques for quality control of traditional Chinese medicines. J. Sep. Sci. 2010, 33, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B. Efficacy, safety, quality control, marking and regulatory guidelines for herbal medicine (phytotherapeutic agents). Braz. J. Med. Biol. Res. 2000, 33, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Beccaro, G.L.; Cerutti, A.K.; Mellano, M.G.; Bounous, G. Bud extracts as new phytochemical source for herbal preparations-quality control and standardization by analytical fingerprint. In Phytochemicals—Isolation, Characterisation and Role in Human Health; Rao, V., Rao, L.G., Eds.; InTech: Rijeka, Croazia, 2015; pp. 187–218. [Google Scholar]
- Gad, H.A.; El-Ahmady, S.H.; Abou-Shoer, M.I.; Al-Azizi, M.M. Application of chemometrics in authentication of herbal medicines: A review. Phytochem. Anal. 2013, 24, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.S.; Zhang, B.; Lin, Z.J.; Wang, X.J.; Zhou, Y.; Sun, X.X.; Xiao, M.L. Relationship between high-performance liquid chromatography fingerprints and uric acid-lowering activities of Cichorium intybus L. Molecules 2015, 20, 9455–9467. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Lin, B.; Liu, Z.H.; Yang, L.N.; Liu, X.M.; Song, H.T. Fingerprint and spectrum-effect relationships on Tripterygium glycosides preparation. Zhongguo Zhong Yao Za Zhi 2015, 40, 1479–1483. [Google Scholar] [PubMed]
- Li, J.Y.; Wang, X.B.; Luo, J.G.; Kong, L.Y. Seasonal variation of alkaloid contents and anti-inflammatory activity of Rhizoma coptidis based on fingerprints combined with chemometrics methods. J. Chromatogr. Sci. 2015, 53, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Zhao, L.G.; Liang, J.; Guo, L.; Yang, Y.L.; Hu, F.; Zhu, R.J.; Feng, S.L. Component analysis and structure identification of active substances for anti-gastric ulcer effects in Radix Astragali by liquid chromatography and tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 960, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.Y.; Yan, D.; Yang, M.H. Study of the anti-MRSA activity of Rhizoma coptidis by chemical fingerprinting and broth microdilution methods. Chin. J. Nat. Med. 2014, 12, 393–400. [Google Scholar] [CrossRef]
- Xie, R.F.; Zhou, X.; Shi, Z.N.; Li, Y.M.; Li, Z.C. Study on spectrum-effect relationship of rhizoma Rhei, cortex Magnoliae Officinalis, fructus Aurantii Immaturus and their formula. J. Chromatogr. Sci. 2013, 51, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.P.; Zhang, C.L.; Qi, J.; Sun, L.Q.; Qin, M.J.; Yu, B.Y. The Spectrum-Effect integrated fingerprint of Polygonum cuspidatum based on HPLC-diode array detection-flow injection-chemiluminescence. Chin. J. Nat. Med. 2013, 11, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhang, L.F.; Zhang, X.; Li, S.M.; Xue, F.Q. Tracing antibacterial compounds from Acalypha australis Linn. by spectrum-effect relationships and semi-preparative HPLC. J. Sep. Sci. 2013, 36, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.L.; Qin, Y.; Cai, J.; Zheng, J.M.; Ye, Y.H.; Liu, H.G.; Huang, Z.S. Infrared fingerprint of Zathoxylum nitidum and its effect on inhibition of tumor cell. J. Infrared Millim. 2013, 32, 91–96. [Google Scholar] [CrossRef]
- Luo, P.; Liu, Y.; Lv, L.Y.; Zhang, Z.F. Spectrum-effect correlation analysis of traditional Tibetan medicine “Morina nepalensis” on nitric oxide production inhibition. Zhongguo Zhong Yao Za Zhi 2013, 38, 2882–2885. [Google Scholar] [PubMed]
- Ma, P.; Zhang, X.Y.; Xu, L.J.; Wang, Z.; Xiao, P.G. Spectrum-effect relation between anti-HIV 1 activities and ultra-performance liquid chromatography fingerprints of Rheum species. Zhongguo Zhong Yao Za Zhi 2013, 38, 2434–2437. [Google Scholar] [PubMed]
- Sun, L.Q.; Ding, X.P.; Qi, J.; Yu, H.; He, S.A.; Zhang, J.; Ge, H.X.; Yu, B.Y. Antioxidant anthocyanins screening through spectrum–effect relationships and DPPH-HPLC-DAD analysis on nine cultivars of introduced rabbiteye blueberry in China. Food Chem. 2012, 132, 759–765. [Google Scholar] [CrossRef]
- Kong, W.J.; Zhao, Y.L.; Xiao, X.H.; Wang, J.B.; Li, H.B.; Li, Z.L.; Jin, C.; Liu, Y. Spectrum-effect relationships between ultra performance liquid chromatography fingerprints and anti-bacterial activities of Rhizoma coptidis. Anal. Chim. Acta 2009, 634, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.J.; Zhao, Y.L.; Shan, L.M.; Xiao, X.H.; Guo, W.Y. Investigation on the spectrum-effect relationships of EtOAC extract from Radix Isatidis based on HPLC fingerprints and microcalorimetry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 871, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Y.; Zhao, Y.L.; Liu, T.T.; Sun, X.J.; Li, R.S.; Zhang, P.; Xiao, X.H. Spectrum-effect relationships between UPLC fingerprints and bioactivities of five Aconitum L. plants. Thermochim. Acta 2013, 558, 61–66. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Chen, H.; Wang, Y.L.; Wang, A.M.; Huang, Y. Study on fingerprint-pharmacology correlation of protective effect of Polygonum orientale on myocardial cell oxidative injury induced by H2O2. Zhongguo Zhong Yao Za Zhi 2012, 37, 2585–2588. [Google Scholar]
- Bao, Y.R.; Wang, S.; Meng, X.S.; Yang, X.X.; Cui, Y.L. Establishment of spectrum-effect relationship network model of Qizhiweitong granules promoting gastrointestinal motility. Zhong Yao Cai 2014, 37, 828–832. [Google Scholar] [PubMed]
- Kong, W.J.; Zhao, Y.L.; Shan, L.M.; Xiao, X.H.; Guo, W.Y. Spectrum-effect relationships between HPLC fingerprints and biothermo-logical activity of Zuojinwan and its similar formulas. Acta Chem. Sin. 2008, 66, 2533–2538. [Google Scholar]
- Li, Y.J.; Bi, K.S. Study on the therapeutic material basis of traditional Chinese medicinal preparation suanzaoren decoction. Chem. Pharm. Bull. (Tokyo) 2006, 54, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, H.; Wu, H.; Pan, Y.; Wang, K.; Liu, L.; Jin, Y.; Zhang, C. A novel reduplicate strategy for tracing hemostatic compounds from heating products of the flavonoid extract in platycladi cacumen by spectrum-effect relationships and column chromatography. Molecules 2015, 20, 16970–16986. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, X.; Chen, Y.; Hong, Y.; Feng, S. Spectrum-effect relationship on anti-hepatic fibrosis effect of Radix Hedysari. Se Pu 2015, 33, 413–418. [Google Scholar] [PubMed][Green Version]
- Tong, Y.; Zhu, X.; Yan, Y.; Liu, R.; Gong, F.; Zhang, L.; Hu, J.; Fang, L.; Wang, R.; Wang, P. The influence of different drying methods on constituents and antioxidant activity of saffron from China. Int. J. Anal. Chem. 2015, 2015, 953164. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, H.; Wu, H.; Pan, Y.; Wang, K.; Liu, L.; Jin, Y.; Zhang, C. Tracing novel hemostatic compounds from heating products of total flavonoids in Flos Sophorae by spectrum-effect relationships and column chromatography. J. Sep. Sci. 2015, 38, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhao, Y.; Wang, J.; Liu, T.; Zhang, B.; Gong, M.; Li, J.; Liu, H.; Han, B.; Zhang, Y.; et al. Spectrum-effect relationships between UPLC fingerprints and bioactivities of crude secondary roots of Aconitum carmichaelii Debeaux (Fuzi) and its three processed products on mitochondrial growth coupled with canonical correlation analysis. J. Ethnopharmacol. 2014, 153, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Zang, Q.C.; Wang, J.B.; Kong, W.J.; Jin, C.; Ma, Z.J.; Chen, J.; Gong, Q.F.; Xiao, X.H. Searching for the main anti-bacterial components in artificial Calculus bovis using UPLC and microcalorimetry coupled with multi-linear regression analysis. J. Sep. Sci. 2011, 34, 3330–3338. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, C.M.; Dai, R.J.; Li, L.; Yu, Y.H.; Li, Y.; Meng, W.W.; Zhang, L.; Zhang, Y.; Deng, Y.L. Combination of HPLC chromatogram and hypoglycemic effect identifies isoflavones as the principal active fraction of Belamcanda chinensis leaf extract in diabetes treatment. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Canterino, S.; Bounous, G. Effect of agronomic and environmental conditions on chemical composition of tree-species buds used for herbal preparations. Vegenos 2012, 25, 21–29. [Google Scholar]
- Liu, X.; Wang, X.L.; Wu, L.; Li, H.; Qin, K.M.; Cai, H.; Pei, K.; Liu, T.; Cai, B.C. Investigation on the spectrum-effect relationships of Da-Huang-Fu-Zi- Tang in rats by UHPLC-ESI-Q-TOF-MS method. J. Ethnopharmacol. 2014, 154, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Ganzera, M. Quality control of herbal medicines by capillary electrophoresis: Potential, requirements and applications. Electrophoresis 2008, 29, 3489–3503. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.H.; Yan, D.; Yuan, H.L.; Wang, J.B.; Cheng, J. Novel patterns of efficient components recognition and quality control for Chinese material medica based on constituent Knock-out/Knock-in. Zhong Cao Yao 2009, 40, 1345–1348. [Google Scholar]
- Yan, D.; Li, J.; Xiong, Y.; Zhang, C.; Luo, J.; Han, Y.; Wang, R.; Jin, C.; Qian, H.; Li, J. Promotion of quality standard of herbal medicine by constituent removing and adding. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X. A Quality Evaluation Strategy for Active Constituents Recognition and Quality Control of Traditional Chinese Medicine (Rhizoma coptidis) by Constituent Knock-out/Knock-in Strategy. Master’s Thesis, University of Science and Technology Kunming, Kunming, China, 2013. [Google Scholar]
- Jin, J.; Li, Y.; Kipletting Tanui, E.; Han, L.; Jia, Y.; Zhang, L.; Wang, Y.; Zhang, X.; Zhang, Y. Fishing and knockout of bioactive compounds using a combination of high-speed counter-current chromatography (HSCCC) and preparative HPLC for evaluating the holistic efficacy and interaction of the components of Herba Epimedii. J. Ethnopharmacol. 2013, 147, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.Y.; Wang, Y.; Fan, X.H.; Qu, H.B.; Cheng, J.Y. Discovering active components from traditional Chinese medicine by component-Knock out approach. Zhongguo Zhong Yao Za Zhi 2009, 34, 336–339. [Google Scholar] [PubMed]
- Yan, C.X. Study on the Model for Efficient Component Recognition of Calculus Bovis Based on Target Constituents “Knock-out & Knock-in”. Master’s Thesis, University of Science and Technology Kunming, Kunming, China, 2013. [Google Scholar]
- Kong, W.J. A New Pattern for Efficient Constituents Recognition and Quality Controlof Traditional Chinese Drug (Calculus bovis) by Component “Knock-out/Knock-in” strategy. Ph.D. Thesis, Chengdu University of Traditional Chinese Medicine, Chengdu, China, 2011. [Google Scholar]
- Zhang, T.T. Novel Pattern of Efficient Components Recognition and Quality Control for Flos Lonicerae Japonicae Based on Constituent Knock-out/Knock-in. Master’s Thesis, Chengdu University of Traditional Chinese Medicine, Chengdu, China, 2011. [Google Scholar]
- Li, X.F. Novel Pattern of Efficient Components Recognition and Quality Control for Curcuma longa L. Based on Constituent Knock-out/Knock-in. Master’s Thesis, Hunan University of Traditional Chinese Medicine, Hunan, China, 2011. [Google Scholar]
- He, J. The Anti-Oxidative Efficient Component Recognition for Curcuma longa L. Based on the Quality Control Pattern of Constituent Knock-out/Knock-in. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2011. [Google Scholar]
- Li, S.M.; Tan, R.; Gu, J.; Zeng, H.S.; Xiao, X.H. The anti-atherosclerosis efficient component recognition for radix puerariae based on the quality control pattern of constituent Knock out. Ning Xia Yi Ke Da Xue Xue Bao 2011, 33, 104. [Google Scholar]
- Sheiner, L.B.; Stanski, D.R.; Vozeh, S.; Miller, R.D.; Ham, J. Simultaneous modeling of pharmacokinetics and pharmacodynamics: Application to d-tubocurarine. Clin. Pharmacol. Ther. 1979, 25, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Peng, L. Study on the PK-PD Characteristics with Antipyretic Effect Following Intravenous Administration of Qingkailing Injection. Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, 2014. [Google Scholar]
- Wang, Y.Q.; Yan, J.Y.; Li, S.X.; Luo, K.; Peng, M.J.; Xie, Y.; Xu, F. Study on pharmacokinetics-pharmacodynamics correlation of Yin Teng Gu Bi Kang prescription. Zhong Yao Cai 2014, 37, 473–477. [Google Scholar] [PubMed]
- Zhan, S.Y.; Shao, Q.; Li, Z.; Wang, Y.; Fan, X.H. Study on PK-PD characteristics of ginsenoside Rg1 and Rb1 in rats with myocardial ischemia following intravenous administration of Shengmai injection. Zhongguo Zhong Yao Za Zhi 2014, 39, 1300–1305. [Google Scholar] [PubMed]
- Yan, L. Study on the PK-PD Characteristics of Curculigo in Rats. Master’s Thesis, Qufu Normal University, Qufu, China, 2014. [Google Scholar]
- Wang, B.L.; Hu, J.P.; Sheng, L.; Chen, H.; Li, Y. Chemical-pharmacokinetic (PK)-pharmacodynamic (PD) fingerprints study of schisandra chinensis alcoholic extraction. Yao Xue Xue Bao 2013, 48, 734–740. [Google Scholar] [PubMed]
- Yang, Y.M. Pharmacokinetic-Pharmacodynamic Model of the Rhubarb Anthraquinone Treat on Intestinal Barrier Injury. Master’s Thesis, Chengdu University of Traditional Chinese Medicine, Chengdu, China, 2011. [Google Scholar]
- Li, Q.S. Multicomponent Pharmacokinetics and Antioxidation Pharmacodynamics of Tea Polyphenols in Rats as well as Their Correlations. Master’s Thesis, Dalian Medical University, Dalian, China, 2010. [Google Scholar]
- Hao, H.P.; Zhang, C.N.; Wang, G.J. Thoughts and experimental exploration on pharmacokinetic study of herbal medicines with multiple-components and targets. Yao Xue Xue Bao 2009, 44, 270–275. [Google Scholar] [PubMed]
- Dong, L.C.; Zhang, X.H.; Ma, J.; Luo, N.; Song, W.; Li, P.; Li, H.J. The integrated pharmacokinetics of major rhodojaponins correlates with the cardiotoxicity after oral administration of Rhododendri Mollis Flos extract in rats. J. Ethnopharmacol. 2014, 157, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, P.; Wang, Z.; Cheng, Y.; Wu, H.; Yang, B.; Du, S.; Lu, Y. Brain distribution pharmacokinetics and integrated pharmacokinetics of Panax Notoginsenoside R1, Ginsenosides Rg1, Rb1, Re and Rd in rats after intranasal administration of Panax Notoginseng Saponins assessed by UPLC/MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 969, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Hao, H.P.; Wang, G.J.; Guo, S.J.; Yan, L.; Lin, X. Integrated pharmacokinetic study of multiple effective components contained in total Panax Notoginsenosides. Chin. J. Nat. Med. 2008, 6, 377–381. [Google Scholar] [CrossRef]
- Pan, L.; Zhou, J.; Zhu, H.; Wang, W.; Zhang, M.; Tian, X.; Lu, J.; Zeng, M. Study on integrated pharmacokinetics of gardenia acid and geniposide: Time-antioxidant efficacy after oral administration of Huanglian-Zhizi couplet medicine from Huang-Lian-Jie-Du-Tang in MCAO rats. Am. J. Chin. Med. 2014, 42, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Qian, Z.; Li, H.; Guo, L.; Pan, L.; Zhang, Q.; Tang, Y. Integrated pharmacokinetics of major bioactive components in MCAO rats after oral administration of Huang-Lian-Jie-Du-Tang. J. Ethnopharmacol. 2012, 141, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hao, H.; Kang, A.; Liang, Y.; Xie, T.; Sun, S.; Dai, C.; Zheng, X.; Xie, L.; Li, J.; et al. Integral pharmacokinetics of multiple lignan components in normal, CCl4-induced hepatic injury and hepatoprotective agents pretreated rats and correlations with hepatic injury biomarkers. J. Ethnopharmacol. 2010, 131, 290–299. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, G.; Cai, H.; Sun, X.; Hou, W.; Zhang, P.; Xie, Z.; Liao, Q. Integrated pharmacokinetics of five protoberberine-typealkaloidsin normal and insomnic rats after single and multiple oral administration of Jiao-Tai-Wan. J. Ethnopharmacol. 2014, 154, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.T.; Yang, X.W.; Zhang, Y.; Liu, J.X. Pharmacochemistry and integrated pharmacokinetics of sixalkaloidsafter oral administration of huang-lian-jie-du-tang decoction. J. Asian Nat. Prod. Res. 2014, 16, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Y.; Zhang, F.L.; Liang, S.; Koomson, E.; Edmond, S.; He, X. Integral pharmacokinetic study of multiple components in total coumarins in Radix angelicae dahuricae. In Proceedings of the 10th Conference on Drugs and Xenobiotics Metabolism of the Chinese Pharmacological Society, Nanjing, China, 21 September 2012.
- Li, Q.S.; Xi, H.; Han, G.Z.; Wang, C.Y.; Lü, L.; Zou, L.L.; Li, N. Integrated pharmacokinetic study of multiple effective components of tea polyphenols and its correlation with anti-free radical pharmacodynamics in rats. Yao Xue Xue Bao 2012, 47, 863–869. [Google Scholar] [PubMed]
- Zhang, Q.Y.; Xu, L.H.; Li, B.T.; Luo, H.; Tang, X.L.; Xu, G.L. Classified and integrated pharmacokinetic study of multiple effective components contained in Gegen-Qinlian decoction. Zhong Guo Lin Chuang Yao Li Xue Yu Zhi Liao Xue 2011, 16, 51–56. [Google Scholar]
- Löbenberg, R.; Amidon, G.L. Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur. J. Pharm. Biopharm. 2000, 50, 3–12. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, X.; Zu, Y.; Zhang, X.; Zu, B.; Zhang, X. Preparation and Characterization of Micronized Artemisinin via a Rapid Expansion of Supercritical Solutions (RESS) Method. Int. J. Mol. Sci. 2012, 13, 5060–5073. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Lei, Z.J.; Zhang, S.Y.; Zhang, Z.Y. Micronization of magnolia bark extract by RESS as well as dissolution and pharmacokinetics evaluation. Yao Xue Xue Bao 2009, 44, 532–539. [Google Scholar] [PubMed]
- Yen, F.L.; Wu, T.H.; Lin, L.T.; Cham, T.M.; Lin, C.C. Nanoparticles formulation of Cuscuta chinensis prevents acetaminophen-induced hepatotoxicity in rats. Food Chem. Toxicol. 2008, 46, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.F.; Wan, J.; Wang, Y.; Li, Y.; Ma, Y.Q.; Yang, M.; Hu, P.Y.; Yuan, H.L.; Wang, C.H. d-α-tocopherol acid polyethylene glycol 1000 succinate, an effective stabilizer during solidification transformation of baicalin nanosuspensions. Int. J. Pharm. 2013, 443, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.F.; Li, Y.; Wan, J. Study on formability of solid nanosuspensions during nanodispersion and solidification: I. Novel role of stabilizer/drug property. Int. J. Pharm. 2013, 454, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Dang, Y.; Lin, G.; Yao, Y.; Li, G.; Ji, G.; Shen, H.; Xie, Y. Effects of stabilizing agents on the development of myricetin nanosuspensiion and its characterization: An in vitro and in vivo evaluation. Int. J. Pharm. 2014, 477, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Yu, X.; Guo, Y.; Wang, Y.; Kuang, H.; Wang, X. Honokiolnanosuspensions: Preparation, increased oral bioavailability and dramatically enhanced biodistribution in thecardio-cerebro-vascularsystem. Colloids Surf. B Biointerfaces 2014, 116, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.L.; Kok, S.H.; Bian, Z.X.; Lam, K.H.; Tang, J.C.; Lee, K.K.; Gambari, R.; Chui, C.H. d-Glucose as a modifying agent in gelatin/collagen matrix and reservoir nanoparticles for Calendula officinalis delivery. Colloids Surf. B Biointerfaces 2014, 117, 227–283. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-Y.; Han, J.; Jin, S.-X.; Lv, Q.-Y.; Bai, J.-X.; Chen, H.G.; Li, R.-S.; Wu, W.; Yuan, H.-L. Characterization and evaluation in vivo of baicalin-nanocrystals prepared by an ultrasonic-homogenization-fluridbed drying method. Chin. J. Nat. Med. 2014, 12, 71–80. [Google Scholar] [PubMed]
- Jin, S.Y.; Yuan, H.L.; Jin, S.X.; Lv, Q.Y.; Bai, J.X.; Han, J. Preparation of baicalin nanocrystal pellets and preliminary study on its pharmacokinetics. Zhongguo Zhong Yao Za Zhi 2013, 38, 1156–1159. [Google Scholar] [PubMed]
- Li, Y.; Wang, Y.; Yue, P.F.; Hu, P.Y.; Wu, Z.F.; Yang, M.; Yuan, H.L. A novel high-pressure precipitation tandem homogenization technology for drug nanocrystals production-acase study with ursodeoxycholic acid. Pharm. Dev. Technol. 2014, 19, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Miao, X.; Cao, F.; Li, S.; Ai, N.; Chang, Q.; Lee, S.M.; Zheng, Y. Nanosuspension development of scutellarein as an active and rapid orally absorbed precursor of its BCS class IV glycoside scutellarin. J. Pharm. Sci. 2014, 103, 3576–3584. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, S.; Chang, Q.; Zhang, L.; Wang, G.; Chen, W.; Miao, X.; Zheng, Y. A strategy for the improvement of the bioavailability and antiosteoporosis activity of BCS IV flavonoid glycosides through the formulation of their lipophilic aglycone into nanocrystals. Mol. Pharm. 2013, 10, 2534–2542. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, G.; Zhang, B.; Li, H.; Nie, Q.; Zang, C.; Zhao, X. Development of silymarin nanocrystals lyophilized power applying nanosuspension technology. Zhongguo Zhong Yao Za Zhi 2009, 34, 1503–1508. [Google Scholar] [PubMed]
- Wang, W.; Kang, Q.; Liu, N.; Zhang, Q.; Zhang, Y.; Li, H.; Zhao, B.; Chen, Y.; Lan, Y.; Ma, Q.; et al. Enhanced dissolution rate and oral bioavailability of Ginkgo biloba extract by preparing solid dispersion via hot-melt extrusion. Fitoterapia 2015, 102, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.; Deng, J.; Jia, X.; Zhou, J.; Lv, H. Solid dispersion of berberine-phospholipid complex/TPGS 1000/SiO2: Preparation, characterization and in vivo studies. Int. J. Pharm. 2014, 465, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Yun, F.; Kang, A.; Shan, J.; Zhao, X.; Bi, X.; Li, J.; Di, L. Preparation of osthole-polymersoliddispersions by hot-melt extrusion for dissolution and bioavailability enhancement. Int. J. Pharm. 2014, 465, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Duan, J.; Xie, Y.; Lin, G.; Luo, H.; Li, G.; Yuan, X. Effects of solid dispersion and self-emulsifying formulations on the solubility, dissolution, permeability and pharmacokinetics of isorhamnetin, quercetin and kaempferol in total flavones of Hippophae rhamnoides L. Drug Dev. Ind. Pharm. 2013, 39, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, G.; Yuan, X.; Cai, Z.; Rong, R. Preparation and in vitro evaluation of solid dispersions of total flavones of Hippophae rhamnoides L. AAPS Pharm. Sci. Tech. 2009, 10, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Liao, J.B.; Liang, Y.Z.; Xie, J.H.; Wu, Q.; Lai, X.P.; Chen, J.N.; Su, Z.R.; Lin, Z.X. Characterization of solid dispersions of Patchouli alcohol with different polymers: Effects on the inhibition of reprecipitation and the improvement of dissolution rate. Drug Dev. Ind. Pharm. 2015, 41, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Deng, K.Y.; Luo, J.B. Preparation and in vitro dissolution of the solid dispersions of cinnamon oil. Nan Fang Yi Ke Da Xue Xue Bao 2008, 28, 52–56. [Google Scholar] [PubMed]
- Ding, S.M.; Zhang, Z.H.; Song, J.; Cheng, X.D.; Jiang, J.; Jia, X.B. Enhanced bioavailability of apigenin viapreparation of a carbon nanopowder solid dispersion. Int. J. Nanomed. 2014, 9, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Zhang, Z.H.; Jin, X.; Jiang, Y.R.; Jia, X.B. Enhanced dissolution and oral bioavailability of tanshinone IIA base by solid dispersion system with low-molecular-weight Chitosan. J. Pharm. Pharmacol. 2013, 65, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.R.; Zhang, Z.H.; Huang, S.Y.; Lu, Y.; Ma, T.T.; Jia, X.B. Enhanced dissolution and stability of Tanshinone IIA base bysoliddispersion system with nano-hydroxyapatite. Pharmacogn. Mag. 2014, 10, 332–337. [Google Scholar] [PubMed]
- Jiang, Y.R.; Zhang, Z.H.; Huang, S.Y.; Lu, Y.; Ma, T.T.; Jia, X.B. An attempt to stabilize tanshinone IIA solid dispersion by the use of ternary systems with nano-CaCO3 and poloxamer 188. Pharmacogn. Mag. 2014, 10 (Suppl. 2), S311–S317. [Google Scholar]
- Jiang, Y.R.; Zhang, Z.H.; Ding, D.M.; Chen, X.Y.; Su, E.; Jia, X.B. Comparison of different preparation methods of tanshinone-porous silica solid dispersion. Zhongguo Zhong Yao Za Zhi 2013, 38, 3271–3276. [Google Scholar] [PubMed]
- Jiang, Y.R.; Zhang, Z.H.; Xia, H.J.; Jia, X.B. Study on solid dispersion of copovidone-based tanshinone IIA. Zhongguo Zhong Yao Za Zhi 2013, 38, 174–178. [Google Scholar] [PubMed]
- Jiang, Y.; Zhang, Z.; Lu, Y.; Tang, J.; Ma, T.; Jia, X. Study on solid dispersion of binary vector of tanshinone IIA. Zhongguo Zhong Yao Za Zhi 2012, 37, 1383–1387. [Google Scholar] [PubMed]
- Wang, C.; Nie, H.; Li, K.; Zhang, Y.X.; Shu, K.G.; Chen, X.J. Protective effect of baicalin solid dispersion on D-galactosamine induced acute hepatic injury in mice. Zhongguo Zhong Xi Yi Jie He Za Zhi 2014, 34, 71–74. [Google Scholar] [PubMed]
- Cong, W.; Shen, L.; Xu, D.; Zhao, L.; Ruan, K.; Feng, Y. Soliddispersion tablets of breviscapine with polyvinylpyrrolidone K30 for improved dissolution and bioavailability to commercial breviscapine tablets in beagle dogs. Eur. J. Drug Metab. Pharmacokinet. 2013, 39, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.Y.; Zhang, Z.H.; Jia, X.B. Study on andrographolide solid dispersion vectored by hydroxyapatite. Zhongguo Zhong Yao Za Zhi 2013, 38, 341–345. [Google Scholar] [PubMed]
- Hu, S.Y.; Zhang, Z.H.; Jiang, Y.R.; Ning, Q.; Liu, Q.Y.; Jia, X.B. Studies on sustained release solid dispersion of tripterine carried by HPMC-stearic acid. Zhongguo Zhong Yao Za Zhi 2012, 37, 3052–3055. [Google Scholar] [PubMed]
- Yue, P.F.; Yuan, H.L.; Li, X.Y.; Yang, M.; Zhu, W.F. Process optimization, characterization and evaluation in vivo of oxymatrine–phospholipid complex. Int. J. Pharm. 2010, 387, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, Z.H.; Sun, E.; Tan, X.B.; Zhu, F.X.; Jia, X.B. A novel drug-phospholipid complex loaded micelle for baohuoside I enhanced oral absorption: In vivo and in vivo evaluations. Drug Dev. Ind. Pharm. 2013, 39, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, Z.H.; Sun, E.; Qian, Q.; Tan, X.B.; Jia, X.B. Preparation of a nanoscale baohuoside I-phospholipid complex and determination of its absorption: In vivo and in vitro evaluations. Int. J. Nanomed. 2012, 7, 4907–4916. [Google Scholar]
- Wang, H.; Cui, Y.; Fu, Q.; Deng, B.; Li, G.; Yang, J.; Wu, T.; Xie, Y. A phospholipid complex to improve the oral bioavailability of flavonoids. Drug Dev. Ind. Pharm. 2014, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.J.; Zhang, Z.H.; Jin, X.; Hu, Q.; Chen, X.Y.; Jia, X.B. A novel drug-phospholipid complex enriched with micelles: Preparation and evaluation in vitro and in vivo. Int. J. Nanomed. 2013, 8, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Q.; Wang, L.P.; Ma, C.; Zhao, K.; Liu, Y.; Feng, N.P. Preparation and characterization of tetrandrine-phospholipid complex loaded lipid nanocapsules as potential oral carriers. Int. J. Nanomed. 2013, 8, 4169–4181. [Google Scholar]
- Zhou, H.; Wan, J.; Wu, L.; Yi, T.; Liu, W.; Xu, H.; Yang, X. A New strategy for enhancing the oral bioavailability of drugs with poor water-solubility and low liposolubility based on phospholipid complex and supersaturated SEDDS. PLoS ONE 2013, 8, e84530. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.J.; Wu, P.J.; Wei, P. Optimization on preparation of hawthorn fruit total flavonoids-phospholipid complex using Plackett-Burman design, central composite design and response surface methodology. Zhong Yao Cai 2010, 33, 437–441. [Google Scholar] [PubMed]
- Chen, Z.; Sun, J.; Liu, D.; Xiao, Y.; Cai, B. Preparation of multivariant-phospholipid complex of Ginkgo biloba extract. Zhongguo Zhong Yao Za Zhi 2010, 35, 2146–2150. [Google Scholar] [PubMed]
- Jia, D.S.; Zhao, J.L.; Shi, F.; Jia, X.B. Preparation of icaritin phytosomes and their solid dispersions. Zhong Cao Yao 2010, 41, 1449–1453. [Google Scholar]
- Jin, X.; Zhang, Z.H.; Sun, E.; Tan, X.B.; Zhu, F.X.; Li, S.L.; Jia, X.B. Preparation of icariside II-phospholipid complexand its absorption across Caco-2 cell monolayers. Pharmazie 2012, 67, 293–298. [Google Scholar] [PubMed]
- Wu, P.J.; Xu, R.C.; Su, Z.T.; Wei, P.; Lin, Y.J.; Yang, M.; Zheng, Q. The nasal mucosa permeability and toxicity of baicalin carrier systems liposomes, β-cyclodextrin inclusion compound, and phospholipid complex. Yao Xue Xue Bao 2009, 44, 417–424. [Google Scholar] [PubMed]
- Zhou, Q.; Wei, X.; Dou, W.; Chou, G.; Wang, Z. Preparation and characterization of inclusion complexes formed between baicalein and cyclodextrins. Carbohydr. Polym. 2013, 95, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhong, L.; Wei, X.; Dou, W.; Chou, G.; Wang, Z. Baicaleinand hydroxypropyl-γ-cyclodextrin complex in poloxamer thermal sensitive hydrogel for vaginal administration. Int. J. Pharm. 2013, 454, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.M.; Yu, S.C.; Tsai, F.J.; Tsai, Y. Enhancement of rhubarb extract solubility and bioactivity by 2-hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2013, 98, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xie, Y.; Hong, C.; Li, G.; Shen, H.; Ji, G. Dvelopment myricetin/hydroxypropyl-β-cyclodextrin inclusion complex: Preparation, characterization, and evaluation. Carbohydr. Polym. 2014, 110, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Tsao, J.Y.; Tsai, H.H.; Wu, C.P.; Lin, P.Y.; Su, S.Y.; Chen, L.D.; Tsai, F.J.; Tsai, Y. Release of paeonol-β-CD complex from thermo-sensitivepoly(N-isopropylacrylamide) hydrogels. Int. J. Pharm. 2010, 402, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, T.; Tao, J.; Ji, G.; Wang, S. Preparation, characterization, and pharmacokinetics of the inclusion complex of genipin-β-cyclodextrin. Drug Dev. Ind. Pharm. 2009, 35, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhang, Z.H.; Sun, E.; Jia, X.B. Effect of β-cyclodextrin complexation on solubility and enzymatic conversion of naringin. Int. J. Mol. Sci. 2012, 13, 14251–14261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Q.S.; Cui, Y.L.; Meng, F.C.; Lin, K.M. Changes in the intestinal absorption mechanism of icariin in the nanocavities of cyclodextrins. Int. J. Nanomed. 2012, 7, 4239–4249. [Google Scholar]
- Cui, L.; Zhang, Z.; Sun, E.; Jia, X.; Qian, Q. Effect of β-cyclodextrin complexation on solubility and enzymatic hydrolysis rate of icariin. J. Nat. Sci. Biol. Med. 2013, 4, 201–206. [Google Scholar] [PubMed]
- Zhang, Y.; Meng, F.C.; Cui, Y.L.; Song, Y.F. Enhancing effect of hydroxypropyl-β-Cyclodextrin ontheintestinal absorption process of genipin. J. Agric. Food Chem. 2011, 59, 10919–10926. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, W.; Yang, H.; Sun, W.; Gong, X.; Zhao, J.; Sun, Y.; Diao, G. A water-soluble inclusion complex of pedunculoside with the polymer β-cyclodextrin: A novel anti-inflammation agent with low toxicity. PLoS ONE 2014, 9, e101761. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, W.; Yang, H.; Sun, W.; Gong, X.; Zhao, J.; Sun, Y.; Diao, G. Preparation and evaluation of andrographolide-loaded microemulsion. J. Microencapsul. 2012, 29, 657–665. [Google Scholar]
- Wu, H.; Lu, C.; Zhou, A.; Min, Z.; Zhang, Y. Enhanced oral bioavailability of puerarin using microemulsion vehicle. Drug Dev. Ind. Pharm. 2009, 35, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.T.; Hu, X.B.; Liao, D.H.; Liu, X.Y.; Xiang, D.X. Mechanisms of microemulsion enhancing the oral bioavailability of puerarin: Comparison between oil-in-water and water-in-oil microemulsions using the single-pass intestinal perfusion method and a chylomicron flow blocking approach. Int. J. Nanomed. 2013, 8, 4415–4426. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Ji, L.; Wang, H.; Chen, Z.Q.; Zhang, Y.T.; Liu, Y.; Feng, N.P. Microemulsion-based novel transdermal delivery of tetramethylpyrazine: Preparation and evaluation in vitro and in vivo. Int. J. Nanomed. 2011, 6, 1611–1619. [Google Scholar]
- Zhang, Y.T.; Zhao, J.H.; Zhang, S.J.; Zhong, Y.Z.; Wang, Z.; Liu, Y.; Shi, F.; Feng, N.P. Enhanced transdermal delivery of evodiamine and rutaecarpine using microemulsion. Int. J. Nanomed. 2011, 6, 2469–2482. [Google Scholar]
- Qu, D.; He, J.; Liu, C.; Zhou, J.; Chen, Y. Triterpene-loaded microemulsion using Coix lacyma-jobi seed extract as oil phase for enhanced antitumor efficacy: Preparation and in vivo evaluation. Int. J. Nanomed. 2014, 9, 109–119. [Google Scholar]
- Shen, L.N.; Zhang, Y.T.; Wang, Q.; Xu, L.; Feng, N.P. Preparation and evaluation of microemulsion-based transdermal delivery of total flavones of rhizome arisaematis. Int. J. Nanomed. 2014, 9, 3453–3464. [Google Scholar]
- Wen, R.; Li, H.; Du, S.; Zhao, X.; Zhao, Z.; Bai, J.; Lu, Y. Preparation of Mpeg2000-PLA-modified Xingnaojing microemulsion and evaluation in mucosal irritation. J. Biomater. Sci. Polym. Ed. 2014, 25, 923–942. [Google Scholar] [CrossRef] [PubMed]
- He, J.J.; Chen, Y.; Du, M.; Cao, W.; Yuan, L.; Zheng, L.Y. Exploration of one-step preparation of Ganoderma lucidum multicomponent microemulsion. Yao Xue Xue Bao 2013, 48, 441–446. [Google Scholar] [PubMed]
- Chen, Y.; Lu, H.; Song, S.; Jia, X. Preparation of Ganoderma lucidum polysaccharides and triterpenes microemulsion and its anticancer effect in mice with transplant Heps tumors. Zhongguo Zhong Yao Za Zhi 2010, 35, 2679–2683. [Google Scholar] [PubMed]
- Liu, J.Y.; Han, Y.; Hu, J.H.; Wang, Z.T.; Chen, K.X. The preparation of paeonol transdermal delivery systems based on the microemulsion-based gels and its pharmacokinetics characters. Yao Xue Xue Bao 2012, 47, 244–249. [Google Scholar] [PubMed]
- Wang, L.; Guo, Q.; Zhang, Y.; Shi, Z. Preparation of Xiongbing microemulsion and its quality evaluation. Zhongguo Zhong Yao Za Zhi 2011, 36, 142–146. [Google Scholar] [PubMed]
- Gui, S.; Wu, L.; Pan, J.; Wen, Z.; Kai, W.; Wang, J. Study on preparation of berberine microemulsion and its absorption in intestine. Zhongguo Zhong Yao Za Zhi 2009, 34, 398–401. [Google Scholar] [PubMed]
- Gui, S.Y.; Wu, L.; Peng, D.Y.; Liu, Q.Y.; Yin, B.P.; Shen, J.Z. Preparation and evaluation of a microemulsion for oral delivery of berberine. Pharmazie 2008, 63, 516–519. [Google Scholar] [PubMed]
- Lü, F.Q.; Li, H.; Xu, W.; Zhang, X.; Huang, M.Q.; Zheng, J.; Chu, K.D. Preparation of self-microemulsion drug delivery system of the mixture of paeonol and borneol based on Xingbi Fang. Yao Xue Xue Bao 2013, 48, 1602–1610. [Google Scholar] [PubMed]
- Xuan, X.Y.; Wang, Y.J.; Tian, H.; Pi, J.X.; Sun, S.Z.; Zhang, W.L. Study on prescription of self-microemulsifying drug delivery system of Mangiferin phospholipid complex. Zhong Yao Cai 2012, 35, 1508–1511. [Google Scholar] [PubMed]
- Xie, Y.; Rong, R.; Li, G.; Yuan, X.; Wang, J. Studies on self-microemulsifying drug preparations of total flavones of Hippophae rhamnoides. Zhongguo Zhong Yao Za Zhi 2009, 34, 43–46. [Google Scholar] [PubMed]
- Zhang, J.; Li, Y.; Gao, W.; Repka, M.A.; Wang, Y.; Chen, M. Andrographolide-loaded PLGA-PEG-PLGA micelles to improve its bioavailability and anticancer efficacy. Expert Opin. Drug Deliv. 2014, 11, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wen, X.S.; Di, W. In vitro and in vivo evaluation of Triptolide-loaded pluronic P105 polymeric micelles. Arzneimittelforschung 2012, 62, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Fan, Y.; Wang, D.; Hu, Y.; Liu, J.; Zhao, X.; Guo, L.; Zhao, X.; Yuan, J.; Zhang, F. Optimization on preparation condition of epimedium polysaccharide lipsome and evaluation of its adjuvantactivity. Int. J. Biol. Macromol. 2012, 50, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Gao, Q.; Wang, D.Y.; Fan, Y.P.; Guo, L.W.; Zhao, X.N. Preparation conditions optimization of Epimedium polysaccharide liposome. Zhong Yao Cai 2011, 34, 1429–1433. [Google Scholar] [PubMed]
- Zhao, X.; Liu, J.; Hu, Y.; Fan, Y.; Wang, D.; Yuan, J.; Xu, L.; Cui, L.; Jing, Z. Optimization on condition of glycyrrhetinic acid liposome by RSM and the research of its immunological activity. Int. J. Biol. Macromol. 2012, 51, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, J.; Wang, D.; Song, X.; Hu, Y.; Zhang, C.; Zhao, X.; Nguyen, T.L. The preparation optimization and immune effect of epimedium polysaccharide-propolis flavone liposome. Carbohydr. Polym. 2013, 94, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, C.; Liu, Z.; Hu, Y.; Shi, C.; Yu, Y.; Zhao, X.; Liu, C.; Liu, J.; Wu, Y. Optimization on preparation conditions of Rehmannia glutinosa polysaccharide liposome and its immunological activity. Carbohydr. Polym. 2014, 104, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.G.; Dai, J.D.; Wu, F.G.; Zhang, X.H.; Li, W.H.; Wang, Y.R. Competitive molecular interaction among paeonol-loaded liposomes: Differential scanning calorimetry andsynchrotron X-ray diffraction studies. Int. J. Pharm. 2012, 438, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ma, F.; Wang, X.; Wang, F.; Liao, H. Formulation of liposomes gels of paeonol for transdermal drug delivery by Box-Behnken statistical design. J. Liposome Res. 2012, 22, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lu, Y.; Bo, R.; Huang, Y.; Hu, Y.; Liu, J.; Wu, Y.; Tao, Y.; Wang, D. The preparation of gypenosides liposomes and its effects on the peritoneal macrophages function in vitro. Int. J. Pharm. 2014, 460, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lu, Y.; Bo, R.; Huang, Y.; Hu, Y.; Liu, J.; Wu, Y.; Tao, Y.; Wang, D. Development of Salvianolic acid B-Tanshinone II A-Glycyrrhetinic acid compound liposomes: Formulation optimization and its effects on proliferation of hepatic stellate cells. Int. J. Pharm. 2014, 462, 11–18. [Google Scholar]
- Liu, D.; Hu, H.; Lin, Z.; Chen, D.; Zhu, Y.; Hou, S.; Shi, X. Quercetin deformable liposome: Preparation and efficacy against ultraviolet B induced skin damages in vitro and in vivo. J. Photochem. Photobiol. B 2013, 127, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, D.; Hu, Y.; Huang, Y.; Yu, Y.; Wang, D. The immunological enhancement activity of propolis flavonoids liposome in vitro and in vivo. Evid. Based Complement. Alternat. Med. 2014, 2014, 483513. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Lu, Y.; Abula, S.; Hu, Y.; Liu, J.; Fan, Y.; Zhao, X.; Wang, D.; Liu, X.; Liu, C. Optimization on preparation condition of propolis flavonoids liposome by response surface methodology and research of its immune enhancement activity. Evid. Based Complement. Alternat. Med. 2013, 2013, 505703. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, C.Q.; Lin, A.H.; Xu, F.; Wang, F.; Zhao, B.; Liu, X.; Chen, Z.P.; Cai, B.C. Brucine-loaded liposomes composed of HSPC and DPPC at different ratios: In vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2014, 40, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yan, G.J.; Hu, R.R.; Gu, Q.W.; Chen, M.L.; Gu, W.; Chen, Z.P.; Cai, B.C. Improved pharmacokinetics and reduced toxicity of brucine after encapsulation into stealth liposomes: Role of phosphatidylcholine. Int. J. Nanomed. 2012, 7, 3567–3577. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ning, Q.; Yu, D.N.; Li, W.G.; Deng, J. Improved oral bioavailability of breviscapine via a Pluronic P85-modified liposomal delivery system. J. Pharm. Pharmacol. 2014, 66, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Shen, L.N.; Wu, Z.H.; Zhao, J.H.; Feng, N.P. Evaluation of skin viability effect on ethosome and liposome-mediated psoralen delivery via cell uptake. J. Pharm. Sci. 2014, 103, 3120–3126. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Al-Suwayeh, S.A.; Hung, C.F.; Chen, C.C.; Fang, J.Y. Camptothecin-loaded liposomes with α-melanocyte-stimulating hormone enhnace cytotoxicity toward and cellular uptake by melanomas: An application of nanomedicine on natural product. J. Tradit. Complement. Med. 2013, 3, 102–109. [Google Scholar] [PubMed]
- Song, J.; Shi, F.; Zhang, Z.; Zhu, F.; Xue, J.; Tan, X.; Zhang, L.; Jia, X. Formulation and evaluation of celastrol-loaded liposomes. Molecules 2011, 16, 7880–7892. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Q.; Zhang, Z.; Yuan, L.; Liu, X.; Zhou, L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules 2012, 17, 5972–5987. [Google Scholar] [CrossRef] [PubMed]
- He, C.Q.; Hu, M.Y.; Zhang, H.; Chang, H.; Chen, J.; Cai, B.C. Study on preparation and thermosensitive release property of composite phospholipid liposomes containing total alkaloids from Strychnos nux-vomica. Zhongguo Zhong Yao Za Zhi 2013, 38, 1366–1370. [Google Scholar] [PubMed]
- He, C.Q.; Hu, M.Y.; Zhang, H.; Chang, H.; Chen, J.; Cai, B.C. Preparation of freeze-dried long-circulation oridonin liposomes and their pharmacokinetics in rats. Zhejiang Da Xue Xue Bao Yi Xue Ban 2013, 42, 638–643. [Google Scholar]
- Wu, M.; Liu, J.; Zhang, X. Preparation of nano-liposome enveloping Flos Magnoliae volatile oil. Zhong Xi Yi Jie He Xue Bao 2007, 5, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, P.; Sun, C.; Zhao, J.; Du, Y.; Shi, F.; Feng, N. Bioadhesion and enhanced bioavailability by wheat germ agglutinin-grafted lipid nanoparticles for oral delivery of poorly water-soluble drug bufalin. Int. J. Pharm. 2011, 419, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, T.; Chen, R.; Hu, Y.; Chen, M.; Wang, Y. Emod in loaded solid lipid nanoparticles: Preparation, characterization and antitumor activity studies. Int. J. Pharm. 2012, 430, 2012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, X.; Ye, T.; Chen, F.; Sun, X.; Kong, J.; Yang, X.; Pan, W.; Li, S. Design, characterization, and in vitro cellular inhibition and uptake of optimized genistein-loaded NLC for the prevention of posterior capsular opacification using response surface methodology. Int. J. Pharm. 2013, 454, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, Y.J.; Peng, D.Y.; Li, Q.L.; Wang, X.S.; Wang, D.L.; Chen, W.D. Solid lipid nanoparticles as delivery systems for Gambogenic acid. Colloids. Surf. B Biointerfaces 2013, 102, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bi, C.; Chan, H.M.; Sun, S.; Zhang, Q.; Zheng, Y. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloids. Surf. B Biointerfaces 2013, 111, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Zhao, Y.; Li, X.J.; Jiang, Z.Z.; Zhang, L.; Liu, S.H.; Li, X.M.; Zhang, L.Y.; Yang, S.Y. Comparison of toxicokinetic and tissue distribution of triptolide-loaded solid lipid nanoparticles vs. free triptolide in rats. Eur. J. Pharm. Sci. 2012, 47, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Peng, F.; Liu, W.; Wan, J.; Wan, C.; Xu, H.; Lam, C.W.; Yang, X. Nanostructured lipid carriers as a novel oral delivery system for triptolide: Induced changes in pharmacokinetics profile associated with reduced toxicity in male rats. Int. J. Nanomed. 2014, 9, 1049–1063. [Google Scholar]
- Liu, Z.; Zhang, X.; Wu, H.; Li, J.; Shu, L.; Liu, R.; Li, L.; Li, N. Preparation and evaluation of solid lipid nanoparticles of baicalin for ocular drug delivery system in vitro and in vivo. Drug Dev. Ind. Pharm. 2011, 37, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, Q.; Lin, T.; Li, R.; Zhu, T.; Zhou, K.; Ji, Z.; Song, J.; Jia, B.; Zhang, C.; et al. PEGylated nanostructured lipid carriers (PEG-NLC) as a novel drug delivery system for biochanin A. Drug Dev. Ind. Pharm. 2014, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Yang, C.R.; Yang, K.L.; Li, K.X.; Hu, H.Y.; Chen, D.W. Preparation and characterization of nanostructured lipid carriers loaded traditional Chinese medicine, zedoary turmeric oil. Drug Dev. Ind. Pharm. 2010, 36, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Zhao, J.H.; Liu, Y.; Wang, Z.; Zhang, Y.T.; Feng, N.P. Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int. J. Nanomed. 2012, 7, 2033–2043. [Google Scholar]
- Chen, Y.; Zhou, L.; Yuan, L.; Zhang, Z.H.; Liu, X.; Wu, Q. Formulation, characterization, and evaluation of in vitro skin permeation and in vivo pharmacodynamics of surface-charged tripterine-loaded nanostructured lipid carriers. Int. J. Nanomed. 2012, 7, 3023–3032. [Google Scholar]
- Zhang, K.; Lv, S.; Li, X.; Feng, Y.; Li, X.; Liu, L.; Li, S.; Li, Y. Preparation, characterization, and in vivo pharmacokinetics of nanostructured lipid carriers loaded with oleanolic acid and gentiopicrin. Int. J. Nanomed. 2013, 8, 3227–3239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.G.; Zhu, Q.L.; Zhou, Y.; Liu, Y.; Chen, W.L.; Yuan, Z.Q.; Yang, S.D.; Zhou, X.F.; Zhu, A.J.; Zhang, X.N. N-Succinyl-chitosan nanoparticles coupled with low-density lipoprotein for targeted osthole-loaded delivery to low-density lipoprotein receptor-rich tumors. Int. J. Nanomed. 2014, 9, 2919–2932. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Sheng, H.H.; Feng, N.P.; Wei, H.; Wang, Z.T.; Wang, C.H. Preparation of and rographolide-loaded solid lipid nanoparticles and their in vitro and in vivo evaluations: Characteristics, release, absorption, transports, pharmacokinetics, and antihyperlipidemic activity. J. Pharm. Sci. 2013, 102, 4414–4425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Zhang, Y.T.; Zhao, J.H.; Shen, L.N.; Shi, F.; Feng, N.P. Preparationand in vitro anti-tumor properties of toad venom extract-loaded solid lipid nanoparticles. Pharmazie 2013, 68, 653–660. [Google Scholar] [PubMed]
- Zhang, X.; Lü, S.; Han, J.; Sun, S.; Wang, L.; Li, Y. Preparation, characterization and in vivo distribution of solid lipid nanoparticles loaded with syringopicroside. Pharmazie 2011, 66, 404–407. [Google Scholar] [PubMed]
- Zhang, Y.L.; Zhang, Z.H.; Jiang, T.Y.; Ayman-Waddad; Jing, L.; Lv, H.X.; Zhou, J.P. Cell uptake of paclitaxel solid lipid nanoparticles modified by cell-penetrating peptides in A549 cells. Pharmazie 2013, 68, 47–53. [Google Scholar] [PubMed]
- Qi, H.; Li, L.; Huang, C.; Li, W.; Wu, C. Optimization and physicochemical characterization of thermosensitive poloxamer gel containing puerarin for ophthalmic use. Chem. Pharm. Bull. (Tokyo) 2006, 54, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chow, M.S.; Zuo, Z. Effect of sodium caprate on the oral absorptions of danshensu and salvianolic acid B. Int. J. Pharm. 2009, 379, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Li, Q.; Han, N.; Zhang, C.L.; Yin, J. Soft tissue contusion repairing effects of Hong Yao with different penetration enhancers. J. Ethnopharmacol. 2013, 148, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.F.; Yang, Z.L.; Luo, J.B.; Zhu, Q.H.; Zhao, H.N. Effects of cinnamene enhancers on transdermal delivery of ligustrazine hydrochloride. Eur. J. Pharm. Biopharm. 2007, 67, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Gao, J.; Hou, X.; Ding, B.; Zhang, W.; Gao, S.; Ding, X. Effects of penetration enhancers on Shuangwu traumatic formula: In vitro percutaneous absorption and in vivo pharmacodynamic evaluation of an herb medicine. Eur. J. Pharm. Biopharm. 2009, 73, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, Z.; Zhang, C.; Zhang, B. Gelucire44/14as a novel absorption enhancer for drugs with different hydrophilicities: In vitro and in vivo improvement on transcorneal permeation. J. Pharm. Sci. 2011, 100, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, D.; Yang, H.; Liu, X.; Luan, L.; Bai, J.; Cui, H. Effect of borneol on the distribution of danshensu to the eye in rabbit via oral administration. Curr. Eye Res. 2010, 35, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Luo, H.; Duan, J.; Hong, C.; Ma, P.; Li, G.; Zhang, T.; Wu, T.; Ji, G. Phytic acid enhances the oral absorption of isorhamnetin, quercetin, and kaempferol in total favones of Hippophae rhamnoides L. Fitoterapia 2014, 93, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, W.; Ou, S.; Guan, Y.; Chen, L.; Yang, M. Effects of penetration enhancers on in vitro percutaneous absorption and amount retained in skin of paeonol, dictamnine, fraxinellone and glycyrrhetinic acid in Liangfu cream. Zhongguo Zhong Yao Za Zhi 2009, 34, 1778–1782. [Google Scholar] [PubMed]
- Lin, X.; Lu, Z.; Xu, D.; Feng, Y.; Shen, L. Oral absorption enhancers of Ophiopogon japonicas polysaccharides. Zhongguo Zhong Yao Za Zhi 2009, 34, 1498–1502. [Google Scholar] [PubMed]
- Luo, M.F.; Shen, Q.; Zhang, T.; Xu, Y.H. Effect of Atractylodes Rhizome oil and other volatile oils on percutaneous absorption of baicalin. Zhong Yao Cai 2008, 31, 1721–1724. [Google Scholar] [PubMed]
- Sha, M.; Yin, L.F.; Xu, W.; Chen, Y.Z. Effects of 2-N-nonyl-1,3-dioxolane as an enhancer on transdermal absorption of Salvia miltiorrhiza gel. Zhongguo Zhong Yao Za Zhi 2007, 32, 487–489. [Google Scholar] [PubMed]
- Zhang, C.F.; Yang, Z.L.; Luo, J.B. Effects of d-limonene and l-limonene on transdermal absorption of ligustrazine hydrochloride. Yao Xue Xue Bao 2006, 41, 772–777. [Google Scholar] [PubMed]
- Zhou, W.; Zhu, X.X.; Yin, A.L.; Cai, B.C.; Wang, H.D.; Di, L.; Shan, J.J. Effect of various absorption enhancers based on tight junctions on the intestinal absorption of forsythoside A in Shuang-Huang-Lian, application to its antivirus activity. Pharmacogn. Mag. 2014, 10, 9–17. [Google Scholar] [PubMed]
- Liu, R.; Liu, Z.; Shu, L.; Zhang, C.; Zhang, B. Effect of three penetration enhancers on corneal permeability of mangiferin in vitro. Zhongguo Zhong Yao Za Zhi 2010, 35, 3131–3135. [Google Scholar] [PubMed]
- Shi, Z.H.; Xiong, F.L.; Huang, Z.J.; Xiong, D.K.; Zeng, Q.H. Effects of penetration enhancers on percutaneous permeability of geniposide in Xiaoer Niuhuang tuire cataplasms. Zhongguo Zhong Yao Za Zhi 2008, 33, 2061–2063. [Google Scholar] [PubMed]
- China Pharmacopoeia Committee, Pharmacopoeia of People’s Republic of China, 9th ed.; China Medical Science and Technology Press: Beijing, China, 2010; Volume 1.
- Zhou, W.; Shan, J.J.; Ju, W.Z.; Wang, S.C.; Meng, M.X.; Cai, B.C.; Di, L.Q. Simultaneous determination of twenty-six components of Flos Lonicerae Japonicae-Fructus Forsythiae herb couple using UPLC-ESI-MS/MS: Application to its preparations. Anal. Methods 2015, 7, 1425–1437. [Google Scholar] [CrossRef]
- Zhou, W.; Tam, K.Y.; Meng, M.; Shan, J.; Wang, S.; Ju, W.; Cai, B.; Di, L. Pharmacokinetics screening for multi-components absorbed in the rat plasma after oral administration of traditional Chinese medicine Flos Lonicerae Japonicae-Fructus Forsythiae herb couple by sequential negative and positive ionization ultra-high-performance liquid chromatography/tandem triple quadrupole mass spectrometric detection. J. Chromatogr. A 2015, 1376, 84–97. [Google Scholar] [PubMed]
- Zhou, W.; Tan, X.; Shan, J.; Wang, S.; Yin, A.; Cai, B.; Di, L. Study on the main components interaction from Flos Lonicerae and Fructus Forsythiae and their dissolution in vitro and intestinal absorption in rats. PLoS ONE 2014, 9, e109619. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Tan, X.; Shan, J.; Liu, T.; Cai, B.; Di, L. Effect of chito-oligosaccharide on the intestinal absorptions of phenylethanoid glycosides in Fructus Forsythiae extract. Phytomedicine 2014, 21, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Shan, J.; Tan, X.; Zou, J.; Yin, A.; Cai, B.; Di, L. Effect of chito-oligosaccharide on the oral absorptions of phenolic acids of Flos Lonicerae extract. Phytomedicine 2014, 21, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Qin, K.M.; Shan, J.J.; Ju, W.Z.; Liu, S.J.; Cai, B.C.; Di, L.Q. Improvement of intestinal absorption of forsythoside A in weeping forsythia extract by various absorption enhancers based on tight junctions. Phytomedicine 2012, 20, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Di, L.Q.; Wang, J.; Shan, J.J.; Liu, S.J.; Ju, W.Z.; Cai, B.C. Intestinal absorption of forsythoside A in in situ single-pass intestinal perfusion and in vitro Caco-2 cell models. Acta Pharmacol. Sin. 2012, 33, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; He, L.; Katsumi, H.; Sakane, T.; Fujita, T.; Yamamoto, A. Improvement of intestinal absorption of insulin and water-soluble macromolecular compounds by chitosan oligomers in rats. Int. J. Pharm. 2008, 359, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, H.; Zhu, X.; Shan, J.; Yin, A.; Cai, B.; Di, L. Improvement of intestinal absorption of forsythoside A and chlorogenic acid by different carboxymethyl chitosan and chito-oligosaccharide, application to Flos Lonicerae-Fructus Forsythiae herb couple preparations. PLoS ONE 2013, 8, e63348. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, W.; Wang, H.; Kuang, X.; Li, Q.; Wang, Y.; Xie, P.; Koike, K. A simple and rapid method to identify and quantitatively analyze triterpenoid saponins in Ardisia crenata using ultrafast liquid chromatography coupled with electrospray ionization quadrupole mass spectrometry. J. Pharm. Biomed. Anal. 2015, 102, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Schmitz, O.J. Comprehensive two-dimensional liquid chromatography tandem diode array detector (DAD) and accurate mass Q-TOF-MS for the analysis of flavonoids and iridoid glycosides in Hedyotis diffusa. Anal. Bioanal. Chem. 2015, 407, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, P.; Bocian, S.; Śliwka, K.; Buszewski, B. Simultaneous analysis of zolpidem and its metabolite in whole blood and oral fluid samples by SPE-LC/MS for clinical and forensic purposes. Adv. Med. Sci. 2015, 60, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, E.; Kojo, A.; Zhao, J.; Li, W.; Zhang, Y.; Wang, T.; Gao, X. Qualitative and quantitative analysis of Eclipta prostrata L. by LC/MS. Sci. World J. 2015, 2015, 980890. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Li, J.; Xu, N.; Wang, R.; Li, Z.; Yang, L.; Wang, Z. Pharmacokinetics, bioavailability, and metabolism of Notoginsenoside Fc in rats by liquid chromatography/electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2015, 109, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Liu, S.; Zheng, T.H.; Tao, Y.Y.; Liu, C.H. Comparative pharmacokinetics and tissue distribution profiles of lignin components in normal and hepatic fibrosis rats after oral administration of Fuzheng Huayu recipe. J. Ethnopharmacol. 2015, 166, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Zhou, L.; Xie, L.; Hao, K.; Rao, T.; Wang, Q.; Ye, W.; Fu, H.; Wang, X.; Wang, G.; et al. Development of a systematic approach to rapid classification and identification of notoginsenosides and metabolites in rat feces based on liquid chromatography coupled triple time-of-flight mass spectrometry. Anal. Chim. Acta 2015, 867, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Xu, X.; Zhu, T.; Shi, F.; Qin, K.; Cai, B. Screening and identification of multiple constituents and their metabolites of Fangji Huangqi Tang in rats by ultra-high performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry basing on coupling data processing techniques. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 985, 14–28. [Google Scholar]
- Su, S.; Duan, J.; Wang, P.; Liu, P.; Guo, J.; Shang, E.; Qian, D.; Tang, Y.; Tang, Z. Metabolomic study of biochemical changes in the plasma and urine of primary dysmenorrhea patients using UPLC-MS coupled with a pattern recognition approach. J. Proteome Res. 2013, 12, 852–865. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tang, Y.; Guo, J.; Shang, E.; Qian, Y.; Wang, L.; Zhang, L.; Liu, P.; Su, S.; Qian, D. Comparative metabolomics analysis on hematopoietic functions of herb pair Gui-Xiong by ultra-high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry and pattern recognition approach. J. Chromatogr. A 2014, 1346, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wei, H.; Zhang, F. Qualitative analysis and quality control of Traditional Chinese Medicine preparation Tanreqing injection by LC-TOF/MS and HPLC-DAD-ELSD. Anal. Methods 2013, 5, 6431–6440. [Google Scholar] [CrossRef]
- Ip, S.P.; Zhao, M.; Xian, Y.; Chen, M.; Zong, Y.; Tjong, Y.W.; Tsai, S.H.; Sung, J.J.; Bensoussan, A.; Berman, B.; et al. Quality assurance for Chinese herbal formulae: Standardization of IBS-20, a 20-herb preparation. Chin. Med. 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.R.; Yau, L.F.; Ho, H.M.; Chan, C.L.; Hu, P.; Liu, L.; Jiang, Z.H. A cellular lipidomic study on the Aβ-induced neurotoxicity and neuroprotective effects of EGCG by using UPLC/MS-based glycerolipids profiling and multivariate analysis. Mol. Biosyst. 2012, 8, 3208–3215. [Google Scholar] [CrossRef] [PubMed]
- Korecka, M.; Waligorska, T.; Figurski, M. Qualification of a surrogate matrix-based absolute quantification method for amyloid-β in human cerebrospinal fluid using 2D UPLC-tandem mass spectrometry. J. Alzheimers Dis. 2014, 41, 441–451. [Google Scholar] [PubMed]
- Hu, J.; Wu, Z.; Yan, J.; Pang, W.; Liang, D.; Xu, X. A promising approach for understanding the mechanism of Traditional Chinese Medicine by the aggregation morphology. J. Ethnopharmacol. 2009, 123, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.S.; Zhao, Y.; Yang, T.H.; Chen, J.; Xiong, C.M.; Ruan, J.L. Preparation of magnetic molecularly imprinted polymers for selective isolation and determination of kaempferol and protoapigenone in Macrothelypteris torresiana. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2014, 34, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.A.; Wang, Y.M.; Liang, Q.L.; Liu, Q.F. System Biology for Traditional Chinese Medicine; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Jonker, C.; Hamman, J.H.; Kotzé, A.F. Intestinal paracellular permeation enhancement with quaternised chitosan: In situ and in vitro evaluation. Int. J. Pharm. 2002, 238, 205–213. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Cai, B.; Shan, J.; Wang, S.; Di, L. Discovery and Current Status of Evaluation System of Bioavailability and Related Pharmaceutical Technologies for Traditional Chinese Medicines—Flos Lonicerae Japonicae—Fructus Forsythiae Herb Couples as an Example. Int. J. Mol. Sci. 2015, 16, 28812-28840. https://doi.org/10.3390/ijms161226132
Zhou W, Cai B, Shan J, Wang S, Di L. Discovery and Current Status of Evaluation System of Bioavailability and Related Pharmaceutical Technologies for Traditional Chinese Medicines—Flos Lonicerae Japonicae—Fructus Forsythiae Herb Couples as an Example. International Journal of Molecular Sciences. 2015; 16(12):28812-28840. https://doi.org/10.3390/ijms161226132
Chicago/Turabian StyleZhou, Wei, Baochang Cai, Jinjun Shan, Shouchuan Wang, and Liuqing Di. 2015. "Discovery and Current Status of Evaluation System of Bioavailability and Related Pharmaceutical Technologies for Traditional Chinese Medicines—Flos Lonicerae Japonicae—Fructus Forsythiae Herb Couples as an Example" International Journal of Molecular Sciences 16, no. 12: 28812-28840. https://doi.org/10.3390/ijms161226132
APA StyleZhou, W., Cai, B., Shan, J., Wang, S., & Di, L. (2015). Discovery and Current Status of Evaluation System of Bioavailability and Related Pharmaceutical Technologies for Traditional Chinese Medicines—Flos Lonicerae Japonicae—Fructus Forsythiae Herb Couples as an Example. International Journal of Molecular Sciences, 16(12), 28812-28840. https://doi.org/10.3390/ijms161226132






