Enhancement of Chaperone Activity of Plant-Specific Thioredoxin through γ-Ray Mediated Conformational Change
Abstract
:1. Introduction
2. Results
2.1. Influence of γ Irradiation on Enzymatic Functions of AtTDX
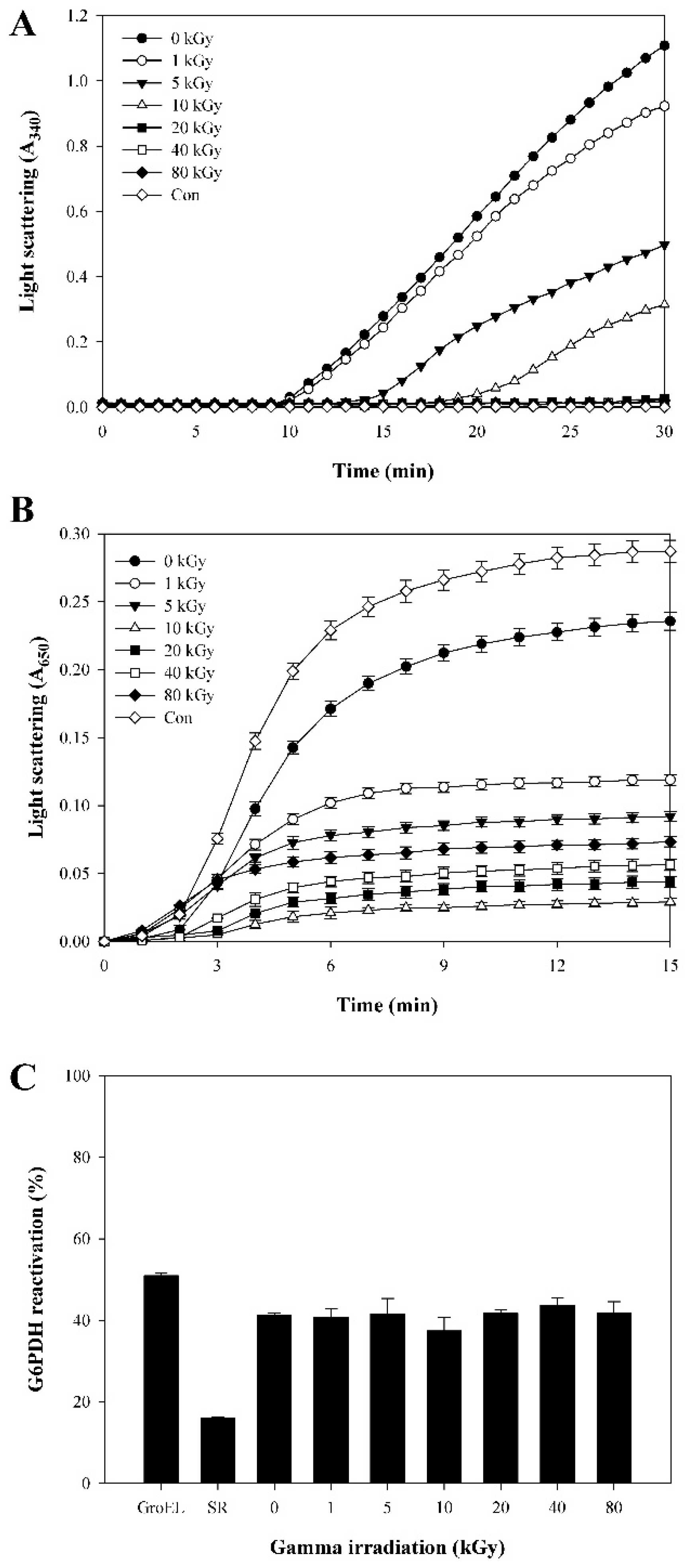
2.2. Relationship between Structural Changes and Functions in γ-Irradiated AtTDX


2.3. Effect of γ-Irradiation on Hydrophobicity and Secondary Structure of AtTDX


3. Discussion
4. Experimental Section
4.1. Materials
4.2. Expression and Purification of Recombinant AtTDX Protein
4.3. γ-Ray Irradiation
4.4. PAGE and SEC
4.5. Thioredoxin Activity Assay
4.6. Molecular Chaperone Activity Assay
4.7. Measurement of Hydrophobicity
4.8. CD Spectroscopy
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buxton, G.V. Radiation Chemistry. Principles and Applications; Farhataziz, Rodgers, M.A.J., Eds.; Verlag Chemie Publishers: Weinheim, Germany, 1987. [Google Scholar]
- Spinks, J.W.T.; Woods, R.J. An Introduction to Radiation Chemistry, 3rd ed.; Wiley-Interscience Publication: New York, NY, USA, 1990. [Google Scholar]
- Le Caër, S. Water radiolysis: Influence of oxide surfaces on H2 production under ionizing radiation. Water 2011, 3, 235–253. [Google Scholar] [CrossRef]
- Lee, M.H.; Moon, Y.R.; Bai, H.-W.; Kim, U.-J.; Lee, I.-C.; Kim, T.H.; Chung, B.Y. Electron paramagnetic resonance investigation of different plant organs after γ irradiation. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 237–240. [Google Scholar] [CrossRef]
- Kempner, E.S. Effects of high-energy electrons and γ rays directly on protein molecules. J. Pharm. Sci. 2001, 90, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Schuessler, H.; Schilling, K. Oxygen effect in the radiolysis of proteins. Part 2. Bovine serum albumin. Int. J. Radiat. Biol. 1984, 45, 267–281. [Google Scholar] [CrossRef]
- Davies, K.J.; Delsignore, M.E. Protein damage and degradation by oxygen radicals. III. Modification of secondary and tertiary structure. J. Biol. Chem. 1987, 262, 9908–9913. [Google Scholar] [PubMed]
- Filali-Mouhim, A.; Audette, M.; St-Louis, M.; Thauvette, L.; Denoroy, L.; Penin, F.; Chen, X.; Rouleau, N.; le Caer, J.-P.; Rossier, J.; et al. Lysozyme fragmentation induced by γ-radiolysis. Int. J. Radiat. Biol. 1997, 72, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Song, K.B. Effect of γ-irradiation on the molecular properties of bovine serum albumin and β-lactoglobulin. BMB Rep. 2000, 33, 133–137. [Google Scholar]
- Lee, S.L.; Lee, M.S.; Song, K.B. Effect of γ-irradiation on the physicochemical properties of gluten films. Food Chem. 2005, 92, 621–625. [Google Scholar] [CrossRef]
- An, B.C.; Lee, S.S.; Lee, J.T.; Hong, S.H.; Wi, S.G.; Chung, B.Y. Engineering of 2-Cys peroxiredoxin for enhanced stress-tolerance. Mol. Cells 2011, 32, 257–264. [Google Scholar] [CrossRef] [PubMed]
- An, B.C.; Lee, S.S.; Wi, S.G.; Bai, H.-W.; Lee, S.Y.; Chung, B.Y. Improvement of chaperone activity of 2-Cys peroxiredoxin using γ ray. J. Radiat. Res. 2011, 52, 694–700. [Google Scholar] [CrossRef] [PubMed]
- An, B.C.; Lee, S.S.; Lee, J.T.; Park, C.-H.; Lee, S.Y.; Chung, B.Y. Change in the enzymatic dual function of the peroxiredoxin protein by γ irradiation. Radiat. Phys. Chem. 2012, 81, 1136–1140. [Google Scholar] [CrossRef]
- Hong, S.H.; An, B.C.; Lee, S.S.; Lee, J.T.; Cho, J.-H.; Jung, H.S.; Chung, B.Y. Improvement of chaperone activity of 2-Cys peroxiredoxin using electron beam. Radiat. Phys. Chem. 2012, 81, 1020–1024. [Google Scholar] [CrossRef]
- Vignols, F.; Mouaheb, N.; Thomas, D.; Meyer, Y. Redox control of Hsp70-Co-chaperone interaction revealed by expression of a thioredoxin-like Arabidopsis protein. J. Biol. Chem. 2003, 278, 4516–4523. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Lee, S.S.; Jang, H.H.; Lee, Y.M.; Park, J.H.; Park, S.-C.; Moon, J.C.; Park, S.K.; Kim, S.Y.; Lee, S.Y.; et al. Heat-shock dependent oligomeric status alters the function of a plant-specific thioredoxin-like protein, AtTDX. Proc. Natl. Acad. Sci. USA 2009, 106, 5978–5983. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Park, S.-K.; Lee, S.Y. Cellular localization of a plant-specific thioredoxin-like protein, Arabidopsis thaliana tetratricopeptide domain-containing thioredoxin (AtTDX). J. Korean Soc. Appl. Biol. Chem. 2011, 54, 544–550. [Google Scholar]
- Hansen, J.E.; Gafni, A. Thermal switching between enhanced and arrested reactivation of bacterial glucose-6-phosphate dehydrogenase assisted by GroEL in the absence of ATP. J. Biol. Chem. 1993, 268, 21632–21636. [Google Scholar] [PubMed]
- Prapapanich, V.; Chen, S.; Toran, E.J.; Rimerman, R.A.; Smith, D.F. Mutational analysis of the hsp70-interacting protein Hip. Mol. Cell. Biol. 1996, 16, 6200–6207. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.K.; Kaur, H.; Kumar, G.S.; Kester, K. Interaction of 1,1′-bi(4-anilino)naphthalene-5,5′-disulfonic acid with alpha-crystallin. J. Biol. Chem. 1998, 273, 8965–8970. [Google Scholar] [CrossRef] [PubMed]
- Das, K.P.; Surewicz, W.K. Temperature-induced exposure of hydrophobic surfaces and its effect on the chaperone activity of alpha-crystallin. FEBS Lett. 1995, 369, 321–325. [Google Scholar] [CrossRef]
- Le Maire, M.; Thauvette, L.; de Foresta, B.; Viel, A.; Beauregard, G.; Potier, M. Effects of ionizing radiations on proteins. Evidence of non-random fragmentations and a caution in the use of the method for determination of molecular mass. Biochem. J. 1990, 267, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Song, K.B. Effect of γ-irradiation on the molecular properties of ovalbumin and ovomucoid and protection by ascorbic acid. Food Chem. 2001, 74, 479–483. [Google Scholar] [CrossRef]
- Lee, Y.; Song, K.B. Effect of γ-irradiation on the molecular properties of myoglobin. BMB Rep. 2002, 35, 590–594. [Google Scholar]
- Garrison, W.M. Reaction mechanisms in the radiolysis of peptides, polypeptides, and proteins. Chem. Rev. 1987, 87, 381–398. [Google Scholar] [CrossRef]
- Kempner, E.S. Damage to proteins due to the direct action of ionizing radiation. Q. Rev. Biophys. 1993, 26, 27–48. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Aspects of radiation chemistry. In Wholesomeness of Irradiated Food: Report of a Joint FAO/IAEA/WHO Expert Committee; World Health Organization Technical Report Series 659; WHO: Geneva, Switzerland, 1981; pp. 13–15. [Google Scholar]
- Moon, J.C.; Hah, Y.-S.; Kim, W.Y.; Jung, B.G.; Jang, H.H.; Lee, J.R.; Kim, S.Y.; Lee, Y.M.; Jeon, M.G.; Kim, C.W.; et al. Oxidative stress-dependent structural and functional switching of a human 2-Cys peroxiredoxin isotype II that enhances HeLa cell resistance to H2O2-induced cell death. J. Biol. Chem. 2005, 280, 28775–28784. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.-H.; Kim, M.S.; Chung, N.; Kim, J.-S. Expression and characterization of a novel 2-deoxyribose-5-phosphate aldolase from Haemophilus influenzae Rd KW20. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 655–660. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bai, H.-W.; Hong, S.H.; Park, C.-H.; Jang, D.M.; Kim, T.H.; Chung, B.Y. Degradation of limonene by γ radiation for improving bioethanol production. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 1–4. [Google Scholar] [CrossRef]
- Holmgren, A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J. Biol. Chem. 1979, 254, 9627–9632. [Google Scholar] [PubMed]
- Yang, J.T.; Wu, C.S.; Martinez, H.M. Calculation of protein conformation from circular dichroism. Methods Enzymol. 1986, 130, 208–269. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.S.; Jung, H.S.; Park, S.-K.; Lee, E.M.; Singh, S.; Lee, Y.; Lee, K.O.; Lee, S.Y.; Chung, B.Y. Enhancement of Chaperone Activity of Plant-Specific Thioredoxin through γ-Ray Mediated Conformational Change. Int. J. Mol. Sci. 2015, 16, 27302-27312. https://doi.org/10.3390/ijms161126019
Lee SS, Jung HS, Park S-K, Lee EM, Singh S, Lee Y, Lee KO, Lee SY, Chung BY. Enhancement of Chaperone Activity of Plant-Specific Thioredoxin through γ-Ray Mediated Conformational Change. International Journal of Molecular Sciences. 2015; 16(11):27302-27312. https://doi.org/10.3390/ijms161126019
Chicago/Turabian StyleLee, Seung Sik, Hyun Suk Jung, Soo-Kwon Park, Eun Mi Lee, Sudhir Singh, Yuno Lee, Kyun Oh Lee, Sang Yeol Lee, and Byung Yeoup Chung. 2015. "Enhancement of Chaperone Activity of Plant-Specific Thioredoxin through γ-Ray Mediated Conformational Change" International Journal of Molecular Sciences 16, no. 11: 27302-27312. https://doi.org/10.3390/ijms161126019




