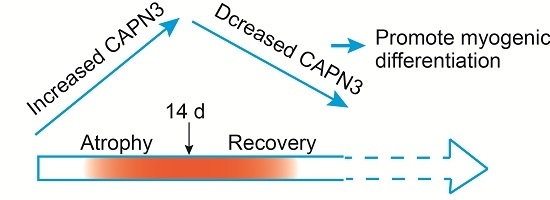
Calpain 3 Expression Pattern during Gastrocnemius Muscle Atrophy and Regeneration Following Sciatic Nerve Injury in Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. CAPN3 Is Expressed in the Gastrocnemius Muscle Following Sciatic Nerve Crush Injury
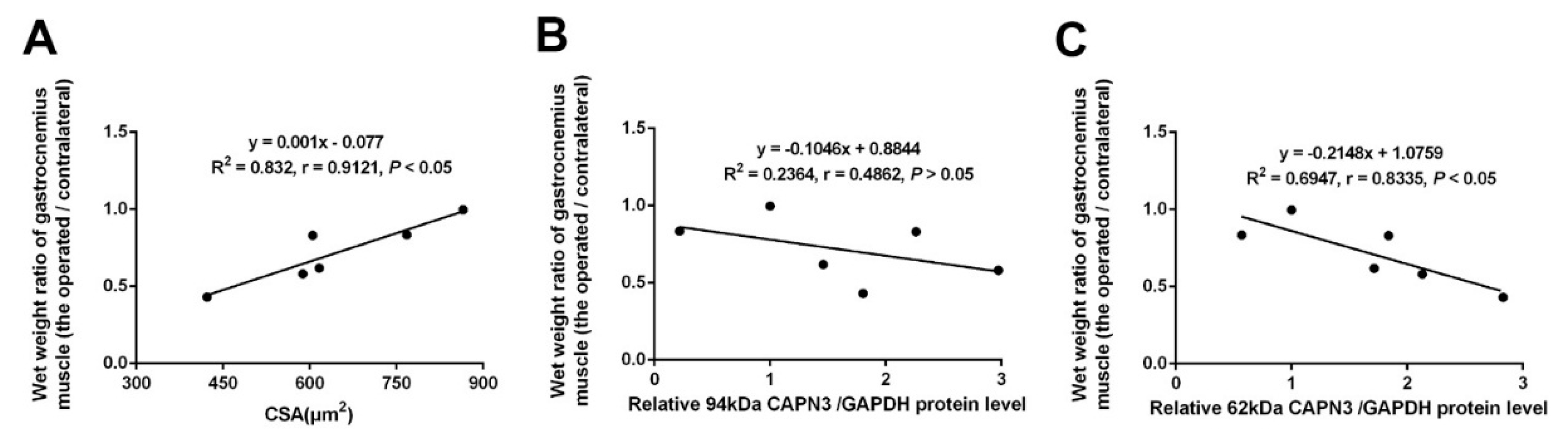
2.2. CAPN3 Expression Is Associated with Gastrocnemius Muscle Atrophy

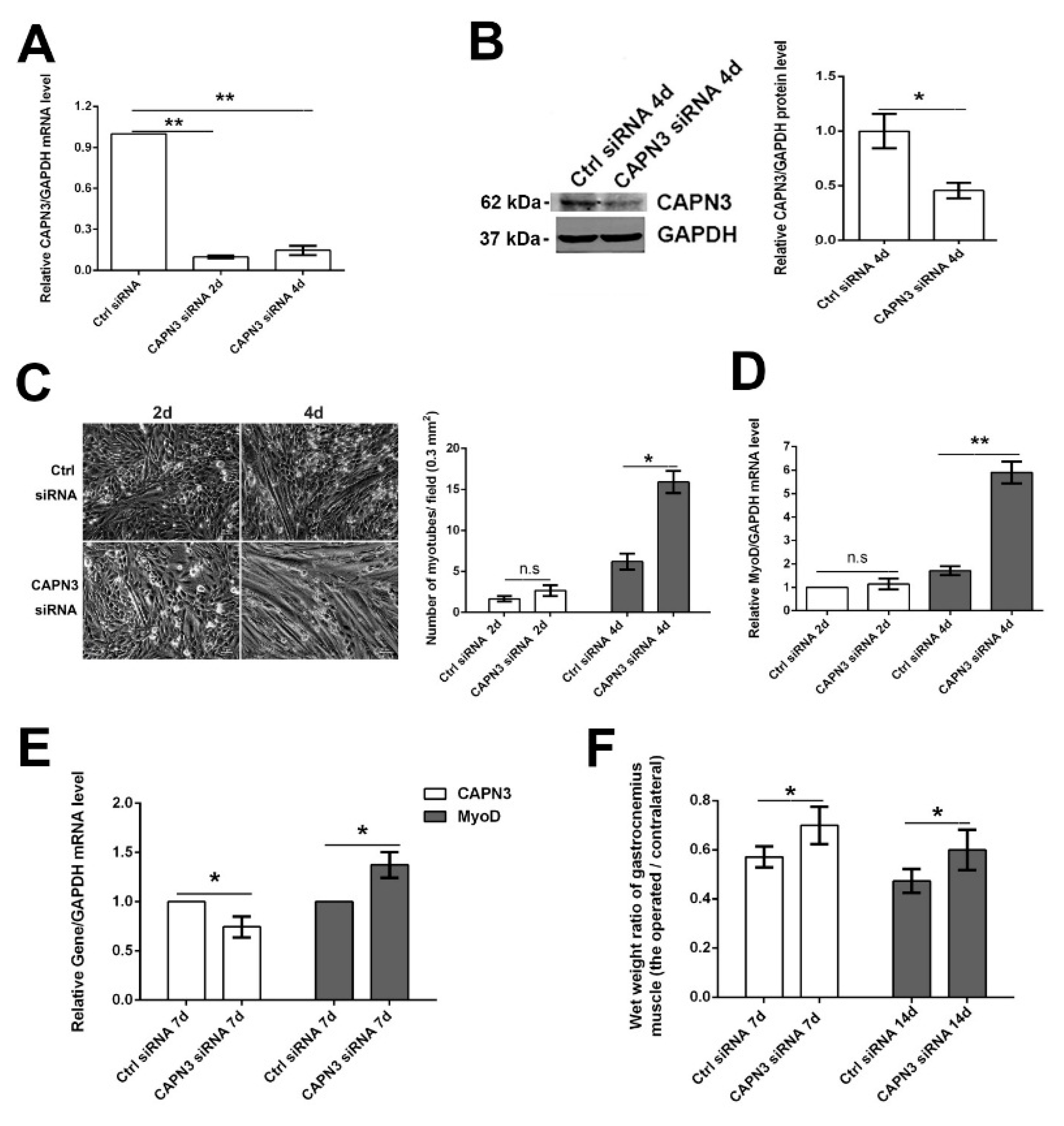
2.3. CAPN3 Deficiency Promotes L6 Cell Differentiation and Delays Skeletal Muscle Atrophy
3. Experimental Section
3.1. Animals and Surgical Procedures
3.2. Functional and Morphometric Analyses Following Sciatic Nerve Crush Injury
3.3. CAPN3 Short Interfering (si) RNA Knockdown
| Usage | Target | Sequence (5′ to 3′) |
|---|---|---|
| qRT-PCR | GAPDH sense | CCTTCATTGACCTCAACTACATG |
| GAPDH antisense | TCAAACTTGTGATCCAGGCG | |
| CAPN3 sense | CATTGTCCCCTCCACTTACG | |
| CAPN3 antisense | GCTCCTTGTTGCTGTTTGC | |
| MyoD sense | CGACTCTTCAGGCTTGGGTT | |
| MyoD antisense | CCAGGTCCTCAAAAAAGCG | |
| siRNA sequence | Ctrl sense | CCUACGCCACCAAUUUCGUTT |
| Ctrl antisense | ACGAAAUUGGUGGCGUAGGTT | |
| CAPN3 sense | CUGAAGACAAGGGUUCATT | |
| CAPN3 antisense | UGAACCCUUGUGUCUUCAGTT |
3.4. Real-Time (RT-) PCR and Western Blot Analysis
3.5. L6 Cell Culture, Differentiation and Myotube Calculation
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Otto, A.; Patel, K. Signalling and the control of skeletal muscle size. Exp. Cell Res. 2010, 316, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Jackman, R.W.; Kandarian, S.C. The molecular basis of skeletal muscle atrophy. Am. J. Physiol. Cell Physiol. 2004, 287, C834–C843. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Dai, G.; Hu, Z.; Wang, X.; Du, J.; Mitch, W.E. Regulation of muscle protein degradation: Coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J. Am. Soc. Nephrol. 2004, 15, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.; Huttenlocher, A.; Lauffenburger, D.A. Calpain proteases in cell adhesion and motility. Int. Rev. Cytol. 2005, 245, 1–16. [Google Scholar] [PubMed]
- Sorimachi, H.; Kimura, S.; Kinbara, K.; Kazama, J.; Takahashi, M.; Yajima, H.; Ishiura, S.; Sasagawa, N.; Nonaka, I.; Sugita, H.; et al. Structure and physiological functions of ubiquitous and tissue-specific calpain species. Muscle-specific calpain, p94, interacts with connectin/titin. Adv. Biophys. 1996, 33, 101–122. [Google Scholar] [CrossRef]
- Sorimachi, H.; Kinbara, K.; Kimura, S.; Takahashi, M.; Ishiura, S.; Sasagawa, N.; Sorimachi, N.; Shimada, H.; Tagawa, K.; Maruyama, K.; et al. Muscle-specific calpain, p94, responsible for limb girdle muscular dystrophy type 2a, associates with connectin through is 2, a p94-specific sequence. J. Biol. Chem. 1995, 270, 31158–31162. [Google Scholar] [PubMed]
- Sorimachi, H.; Ono, Y.; Suzuki, K. Skeletal muscle-specific calpain, p94, and connectin/titin: Their physiological functions and relationship to limb-girdle muscular dystrophy type 2a. Adv. Exp. Med. Biol. 2000, 481, 383–395. [Google Scholar] [PubMed]
- Ojima, K.; Kawabata, Y.; Nakao, H.; Nakao, K.; Doi, N.; Kitamura, F.; Ono, Y.; Hata, S.; Suzuki, H.; Kawahara, H.; et al. Dynamic distribution of muscle-specific calpain in mice has a key role in physical-stress adaptation and is impaired in muscular dystrophy. J. Clin. Investig. 2010, 120, 2672–2683. [Google Scholar] [CrossRef] [PubMed]
- Ojima, K.; Ono, Y.; Ottenheijm, C.; Hata, S.; Suzuki, H.; Granzier, H.; Sorimachi, H. Non-proteolytic functions of calpain-3 in sarcoplasmic reticulum in skeletal muscles. J. Mol. Biol. 2011, 407, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Ojima, K.; Ono, Y.; Hata, S.; Noguchi, S.; Nishino, I.; Sorimachi, H. Muscle-specific calpain-3 is phosphorylated in its unique insertion region for enrichment in a myofibril fraction. Genes Cells 2014, 19, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Laure, L.; Daniele, N.; Suel, L.; Marchand, S.; Aubert, S.; Bourg, N.; Roudaut, C.; Duguez, S.; Bartoli, M.; Richard, I. A new pathway encompassing calpain 3 and its newly identified substrate cardiac ankyrin repeat protein is involved in the regulation of the nuclear factor-κB pathway in skeletal muscle. FEBS J. 2010, 277, 4322–4337. [Google Scholar] [CrossRef] [PubMed]
- Moretti, D.; del Bello, B.; Cosci, E.; Biagioli, M.; Miracco, C.; Maellaro, E. Novel variants of muscle calpain 3 identified in human melanoma cells: Cisplatin-induced changes in vitro and differential expression in melanocytic lesions. Carcinogenesis 2009, 30, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Roperto, S.; De Tullio, R.; Raso, C.; Stifanese, R.; Russo, V.; Gaspari, M.; Borzacchiello, G.; Averna, M.; Paciello, O.; Cuda, G.; et al. Calpain3 is expressed in a proteolitically active form in papillomavirus-associated urothelial tumors of the urinary bladder in cattle. PLoS ONE 2010, 5, e10299. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Xu, M.; Zhao, P.; Liu, M. The mRNA alteration and correlation of calpains and foxos during gastrocnemius muscle atrophy induced by sciatic nerver injury and cardiotoxin injection. Chin. J. Tissue Eng. Res. 2012, 16, 1173–1179. [Google Scholar]
- Hussain, H.; Dudley, G.A.; Johnson, P. Effects of denervation on calpain and calpastatin in hamster skeletal muscles. Exp. Neurol. 1987, 97, 635–643. [Google Scholar] [CrossRef]
- Stockholm, D.; Herasse, M.; Marchand, S.; Praud, C.; Roudaut, C.; Richard, I.; Sebille, A.; Beckmann, J.S. Calpain 3 mRNA expression in mice after denervation and during muscle regeneration. Am. J. Physiol. Cell Physiol. 2001, 280, C1561–C1569. [Google Scholar] [PubMed]
- Yao, D.; Li, M.; Shen, D.; Ding, F.; Lu, S.; Zhao, Q.; Gu, X. Expression changes and bioinformatic analysis of wallerian degeneration after sciatic nerve injury in rat. Neurosci. Bull. 2013, 29, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Durbeej, M.; Sawatzki, S.M.; Barresi, R.; Schmainda, K.M.; Allamand, V.; Michele, D.E.; Campbell, K.P. Gene transfer establishes primacy of striated vs. Smooth muscle sarcoglycan complex in limb-girdle muscular dystrophy. Proc. Natil. Acad. Sci. USA 2003, 100, 8910–8915. [Google Scholar] [CrossRef] [PubMed]
- Stuelsatz, P.; Pouzoulet, F.; Lamarre, Y.; Dargelos, E.; Poussard, S.; Leibovitch, S.; Cottin, P.; Veschambre, P. Down-regulation of MyoD by Calpain 3 promotes generation of reserve cells in C2C12 myoblasts. J. Biol. Chem. 2010, 285, 12670–12683. [Google Scholar] [CrossRef] [PubMed]
- Joung, H.; Eom, G.H.; Choe, N.; Lee, H.M.; Ko, J.H.; Kwon, D.H.; Nam, Y.S.; Min, H.; Shin, S.; Kook, J.; et al. Ret finger protein mediates Pax7-induced ubiquitination of MyoD in skeletal muscle atrophy. Cell Signal. 2014, 26, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, D.; Shao, C.; Liu, J.; Ding, F.; Gu, X. Expression pattern of myostatin in gastrocnemius muscle of rats after sciatic nerve crush injury. Muscle Nerve 2007, 35, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.H.; Wang, P.; Yang, L.; Li, Y.; Liu, Y.; Liu, M. A potential indicator of denervated muscle atrophy: The ratio of myostatin to follistatin in peripheral blood. Genet. Mol. Res. 2011, 10, 3914–3923. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, J.; Ding, F.; Wang, X.; Liu, M.; Gu, X. Investigation of differentially expressed proteins in rat gastrocnemius muscle during denervation-reinnervation. J. Muscle Res. Cell Motil. 2006, 27, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Mir, L.M.; Bureau, M.F.; Gehl, J.; Rangara, R.; Rouy, D.; Caillaud, J.M.; Delaere, P.; Branellec, D.; Schwartz, B.; Scherman, D. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc. Natl. Acad. Sci. USA 1999, 96, 4262–4267. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.N.; Lau, P.; Crowther, L.M.; Cleasby, M.E.; Millard, S.; Leong, G.M.; Cooney, G.J.; Muscat, G.E. Rev-erb β regulates the Srebp-1c promoter and mRNA expression in skeletal muscle cells. Biochem. Biophys. Res. Commun. 2009, 388, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Nadar, V.C.; Kozielski, F.; Kozlowska, M.; Yu, W.; Baas, P.W. Kinesin-12, a mitotic microtubule-associated motor protein, impacts axonal growth, navigation, and branching. J. Neurosci. 2010, 30, 14896–14906. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Yan, Y.; Yao, J.; Liu, Y.; Zhao, J.; Liu, M. Calpain 3 Expression Pattern during Gastrocnemius Muscle Atrophy and Regeneration Following Sciatic Nerve Injury in Rats. Int. J. Mol. Sci. 2015, 16, 26927-26935. https://doi.org/10.3390/ijms161126003
Wu R, Yan Y, Yao J, Liu Y, Zhao J, Liu M. Calpain 3 Expression Pattern during Gastrocnemius Muscle Atrophy and Regeneration Following Sciatic Nerve Injury in Rats. International Journal of Molecular Sciences. 2015; 16(11):26927-26935. https://doi.org/10.3390/ijms161126003
Chicago/Turabian StyleWu, Ronghua, Yingying Yan, Jian Yao, Yan Liu, Jianmei Zhao, and Mei Liu. 2015. "Calpain 3 Expression Pattern during Gastrocnemius Muscle Atrophy and Regeneration Following Sciatic Nerve Injury in Rats" International Journal of Molecular Sciences 16, no. 11: 26927-26935. https://doi.org/10.3390/ijms161126003