Molecular Characterization of Endoplasmic Reticulum Oxidoreductin 1 from Bombyx mori
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening and Analysis of bEro1 cDNA
| Scientific Name | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B. mori | - | ||||||||||||
| A. mellifera | 57 | - | |||||||||||
| T. castaneum | 57 | 62 | - | ||||||||||
| A. aegypti | 52 | 54 | 61 | - | |||||||||
| D. melanogaster | 51 | 52 | 54 | 56 | - | ||||||||
| X. tropicalis | 46 | 44 | 46 | 43 | 45 | - | |||||||
| M. musculus | 46 | 45 | 46 | 44 | 45 | 69 | - | ||||||
| H. sapiens | 47 | 45 | 48 | 45 | 44 | 57 | 69 | - | |||||
| P. toglopytes | 47 | 45 | 47 | 46 | 45 | 58 | 57 | 58 | - | ||||
| B. taurus | 51 | 50 | 51 | 50 | 48 | 62 | 58 | 58 | 98 | - | |||
| G. gallus | 48 | 46 | 49 | 46 | 45 | 58 | 62 | 62 | 96 | 96 | - | ||
| D. rerio | 49 | 47 | 49 | 47 | 47 | 58 | 58 | 60 | 85 | 86 | 92 | - | |
| S. cerevisiae | 24 | 28 | 26 | 25 | 26 | 27 | 26 | 29 | 29 | 30 | 27 | 27 | - |

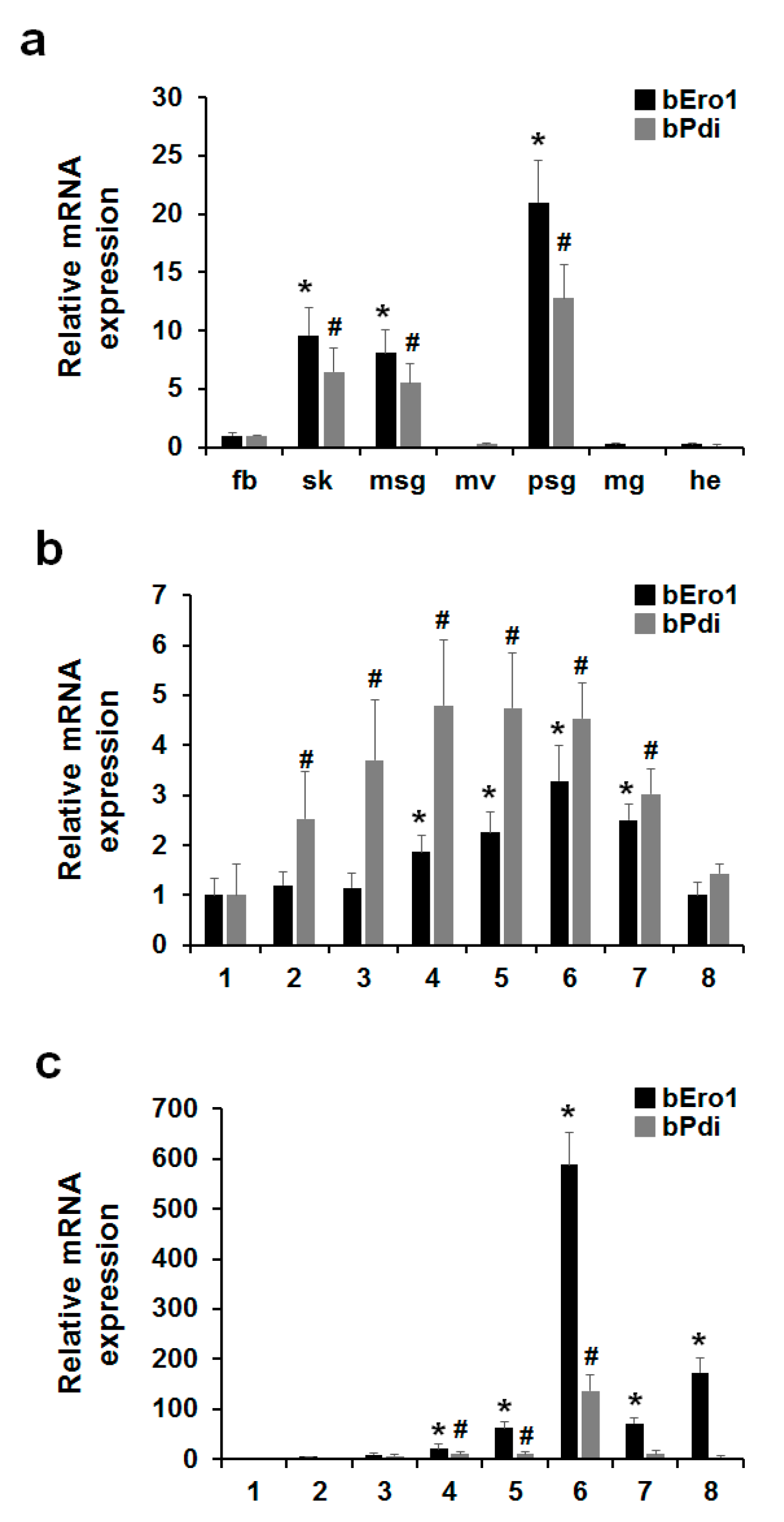
2.2. Tissue Distribution of bEro1 Expression

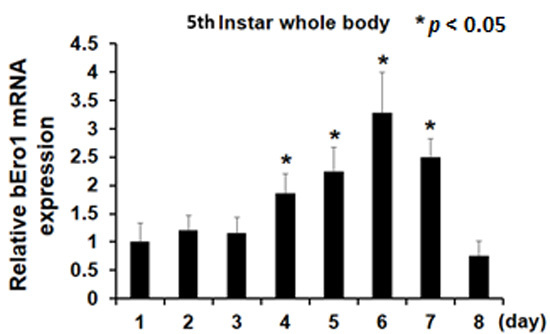
2.3. bEro1 Expression in Development Stages of 5th Instar Larvae
| Scientific Name | N-Terminal (CXXXXC Motif) | C-Terminal (CXXCXXC Motif) |
|---|---|---|
| A. mellifera | CHVQPC | CVGCDKC |
| T. castaneum | CHVEAC | CVGCDKC |
| A. aegypti | CHVEQC | CVGCDKC |
| D. melanogaster | CQVENC | CVGCDKC |
| X. tropicalis | CAVKPC | CVGCDKC |
| M. musculus | CAVKPC | CVGCDKC |
| H. sapiens | CHVEPC | CVGCDKC |
| P. toglopytes | CHVEPC | CVGCDKC |
| B. taurus | CHVEPC | CVGCDKC |
| G. gallus | CHVEPC | CVGCDKC |
| D. rerio | CHVEPC | CVGCDKC |
| B. mori | CHIKTC | CVGCDKC |
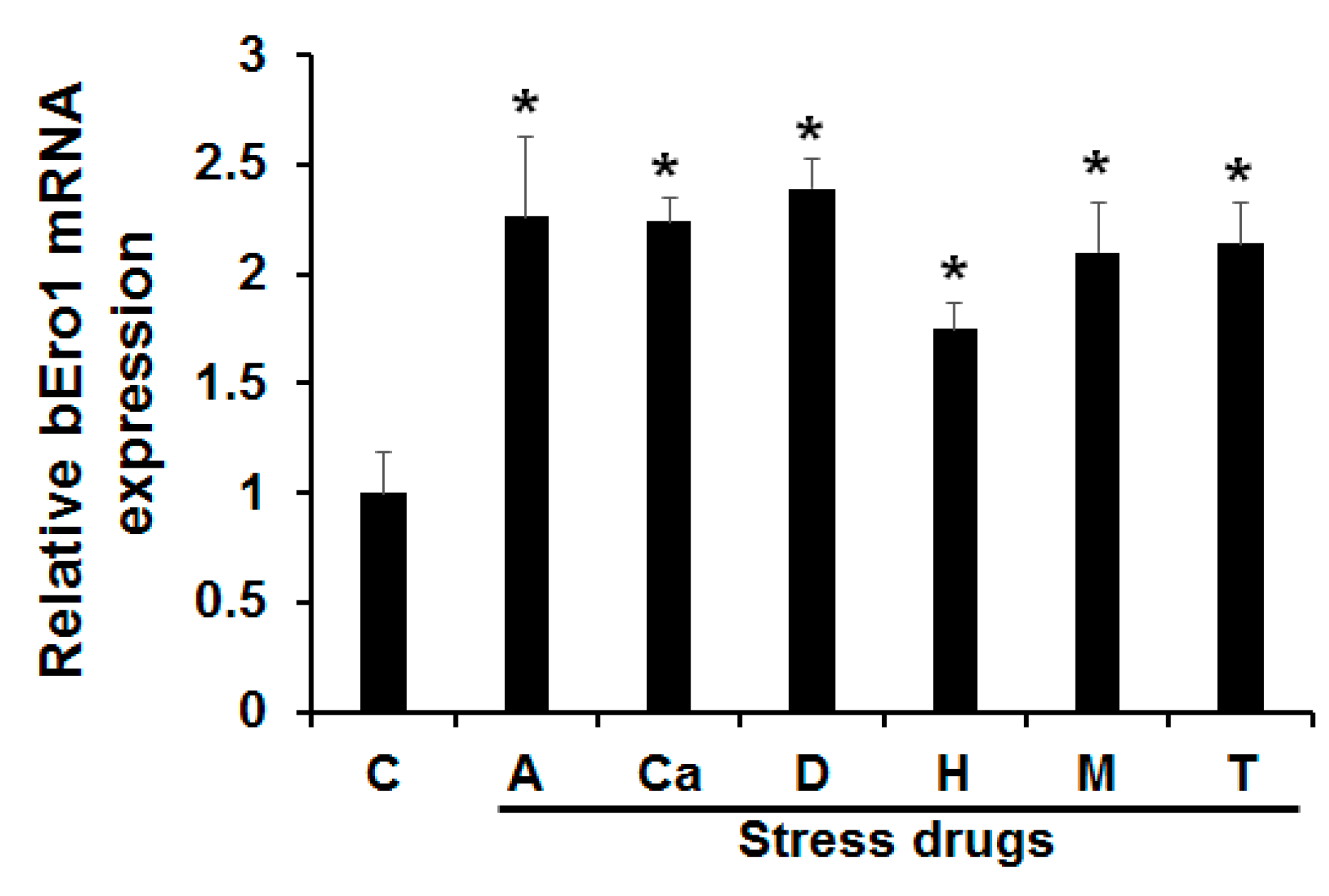
2.4. Induction of bEro1 during ER Stress
2.5. Relationship between bERO1 and bPDI
3. Experimental Section
3.1. Insects and Cells
3.2. RACE PCR Analysis
3.3. Reverse Transcription PCR
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sevier, C.S.; Kaiser, C.A. Formation and transfer of disulphide bonds in living cells. Nat. Rev. Mol. Cell Biol. 2002, 3, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Schroder, M. Engineering eukaryotic protein factories. Biotechnol. Lett. 2008, 30, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sevier, C.S.; Kaiser, C.A. Disulfide transfer between two conserved cysteine pairs imparts selectivity to protein oxidation by Ero1. Mol. Biol. Cell 2006, 17, 2256–2266. [Google Scholar] [CrossRef] [PubMed]
- Jessop, C.E.; Chakravarthi, S.; Watkins, R.H.; Bulleid, N.J. Oxidative protein folding in the mammalian endoplasmic reticulum. Biochem. Soc. Trans. 2004, 32, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.P.; Weissman, J.S. Oxidative protein folding in eukaryotes: Mechanisms and consequences. J. Cell Biol. 2004, 164, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Frand, A.R.; Kaiser, C.A. ERO1p oxidizes protein disulfide isomerase in a pathway for disulfide bond formation in the endoplasmic reticulum. Mol. Cell 1999, 4, 469–477. [Google Scholar] [CrossRef]
- Dias-Gunasekara, S.; Gubbens, J.; van Lith, M.; Dunne, C.; Williams, J.A.; Kataky, R.; Scoones, D.; Lapthorn, A.; Bulleid, N.J.; Benham, A.M. Tissue-specific expression and dimerization of the endoplasmic reticulum oxidoreductase Ero1beta. J. Biol. Chem. 2005, 280, 33066–33075. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Sideris, D.P.; Sevier, C.S.; Kaiser, C.A. Balanced Ero1 activation and inactivation establishes ER redox homeostasis. J. Cell Biol. 2012, 196, 713–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, B.; Gilbert, H.F. Protein disulfide isomerase. Biochim. Biophys. Acta 2004, 1699, 35–44. [Google Scholar] [CrossRef]
- Pollard, M.G.; Travers, K.J.; Weissman, J.S. ERO1p: A novel and ubiquitous protein with an essential role in oxidative protein folding in the endoplasmic reticulum. Mol. Cell 1998, 1, 171–182. [Google Scholar] [CrossRef]
- Takemori, Y.; Sakaguchi, A.; Matsuda, S.; Mizukami, Y.; Sakurai, H. Stress-induced transcription of the endoplasmic reticulum oxidoreductin gene ERO1 in the yeast Saccharomyces cerevisiae. Mol. Genet. Genom. 2006, 275, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Frand, A.R.; Kaiser, C.A. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol. Cell 1998, 1, 161–170. [Google Scholar] [CrossRef]
- Hoog, C. Isolation of a large number of novel mammalian genes by a differential cDNA library screening strategy. Nucleic Acids Res. 1991, 19, 6123–6127. [Google Scholar] [CrossRef] [PubMed]
- Goo, T.W.; Yun, E.Y.; Hwang, J.S.; Kang, S.W.; You, K.H.; Kwon, O.Y. Phylogenetic relationship of Bombyx mori protein disulfide isomerase. Z. Naturforschung. C J. Biosci. 2002, 57, 189–196. [Google Scholar] [CrossRef]
- Gross, E.; Kastner, D.B.; Kaiser, C.A.; Fass, D. Structure of ERO1p, source of disulfide bonds for oxidative protein folding in the cell. Cell 2004, 117, 601–610. [Google Scholar] [CrossRef]
- Hu, M.C.; Gong, H.Y.; Lin, G.H.; Hu, S.Y.; Chen, M.H.; Huang, S.J.; Liao, C.F.; Wu, J.L. XBP-1, a key regulator of unfolded protein response, activates transcription of IGF1 and Akt phosphorylation in zebrafish embryonic cell line. Biochem. Biophys. Res. Commun. 2007, 359, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Yang, H.J.; Lan, T.Y.; Wei, H.; Zhang, H.R.; Chen, M.; Fan, W.; Ma, Y.Y.; Zhong, B.X. Expression profiling and regulation of genes related to silkworm posterior silk gland development and fibroin synthesis. J. Proteome Res. 2011, 10, 3551–3564. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pareja, K.A.; Kaiser, C.A.; Sevier, C.S. Redox signaling via the molecular chaperone BiP protects cells against endoplasmic reticulum-derived oxidative stress. Elife 2014, 3, e03496. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hendershot, L.M. ER chaperone functions during normal and stress conditions. J. Chem. Neuroanatomy 2004, 28, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.; Sevier, C.S.; Heldman, N.; Vitu, E.; Bentzur, M.; Kaiser, C.A.; Thorpe, C.; Fass, D. Generating disulfides enzymatically: Reaction products and electron acceptors of the endoplasmic reticulum thiol oxidase ERO1p. Proc. Natl. Acad. Sci. USA 2006, 103, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Zito, E. ERO1: A protein disulfide oxidase and H2O2 producer. Free Radic. Biol. Med. 2015, 83, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.P.; Weissman, J.S. The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol. Cell 2002, 10, 983–994. [Google Scholar] [CrossRef]
- Zito, E.; Melo, E.P.; Yang, Y.; Wahlander, A.; Neubert, T.A.; Ron, D. Oxidative protein folding by an endoplasmic reticulum-localized peroxiredoxin. Mol. Cell 2010, 40, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Zito, E.; Chin, K.T.; Blais, J.; Harding, H.P.; Ron, D. ERO1-beta, a pancreas-specific disulfide oxidase, promotes insulin biogenesis and glucose homeostasis. J. Cell Biol. 2010, 188, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Zhang, Y.; Zeng, H.; Novoa, I.; Lu, P.D.; Calfon, M.; Sadri, N.; Yun, C.; Popko, B.; Paules, R.; et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 2003, 11, 619–633. [Google Scholar] [CrossRef]
- Baker, K.M.; Chakravarthi, S.; Langton, K.P.; Sheppard, A.M.; Lu, H.; Bulleid, N.J. Low reduction potential of Ero1alpha regulatory disulphides ensures tight control of substrate oxidation. EMBO J. 2008, 27, 2988–2997. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Inoue, S.; Mizuno, S. Hydrophobic interaction of P25, containing Asn-linked oligosaccharide chains, with the H-L complex of silk fibroin produced by Bombyx mori. Insect Biochem. Mol. Biol. 1999, 29, 269–276. [Google Scholar] [CrossRef]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Kikuchi, Y.; Takagi, T.; Kikuchi, A.; Oyama, F.; Shimura, K.; Mizuno, S. Primary structure of the silk fibroin light chain determined by cDNA sequencing and peptide analysis. J. Mol. Biol. 1989, 210, 127–139. [Google Scholar] [CrossRef]
- Tanaka, K.; Kajiyama, N.; Ishikura, K.; Waga, S.; Kikuchi, A.; Ohtomo, K.; Takagi, T.; Mizuno, S. Determination of the site of disulfide linkage between heavy and light chains of silk fibroin produced by Bombyx mori. Biochim. Biophys. Acta 1999, 1432, 92–103. [Google Scholar] [CrossRef]
- Mori, K.; Tanaka, K.; Kikuchi, Y.; Waga, M.; Waga, S.; Mizuno, S. Production of a chimeric fibroin light-chain polypeptide in a fibroin secretion-deficient naked pupa mutant of the silkworm Bombyx mori. J. Mol. Biol. 1995, 251, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Goo, T.W.; Yun, E.Y.; Hwang, J.S.; Kang, S.W. Gene Expression Profile of the Posterior Silk Glands of the Silkworm, Bombyx mori L. Korean J. Genet. 2007, 29, 193–201. [Google Scholar]
- Wang, W.; Swevers, L.; Iatrou, K. Mariner (Mos1) transposase and genomic integration of foreign gene sequences in Bombyx mori cells. Insect Mol. Biol. 2000, 9, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Goo, T.W.; Yun, E.Y.; Hwang, J.S.; Kang, S.W.; Park, S.; You, K.H.; Kwon, O.Y. Molecular characterization of a Bombyx mori protein disulfide isomerase (bPDI). Cell Stress Chaperones 2002, 7, 118–125. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, M.; Ryou, H.-J.; Yun, E.-Y.; Goo, T.-W. Molecular Characterization of Endoplasmic Reticulum Oxidoreductin 1 from Bombyx mori. Int. J. Mol. Sci. 2015, 16, 26520-26529. https://doi.org/10.3390/ijms161125977
Seo M, Ryou H-J, Yun E-Y, Goo T-W. Molecular Characterization of Endoplasmic Reticulum Oxidoreductin 1 from Bombyx mori. International Journal of Molecular Sciences. 2015; 16(11):26520-26529. https://doi.org/10.3390/ijms161125977
Chicago/Turabian StyleSeo, Minchul, Hee-Joo Ryou, Eun-Young Yun, and Tae-Won Goo. 2015. "Molecular Characterization of Endoplasmic Reticulum Oxidoreductin 1 from Bombyx mori" International Journal of Molecular Sciences 16, no. 11: 26520-26529. https://doi.org/10.3390/ijms161125977