Mycophenolate Mofetil Modulates Differentiation of Th1/Th2 and the Secretion of Cytokines in an Active Crohn’s Disease Mouse Model
Abstract
:1. Introduction
2. Results
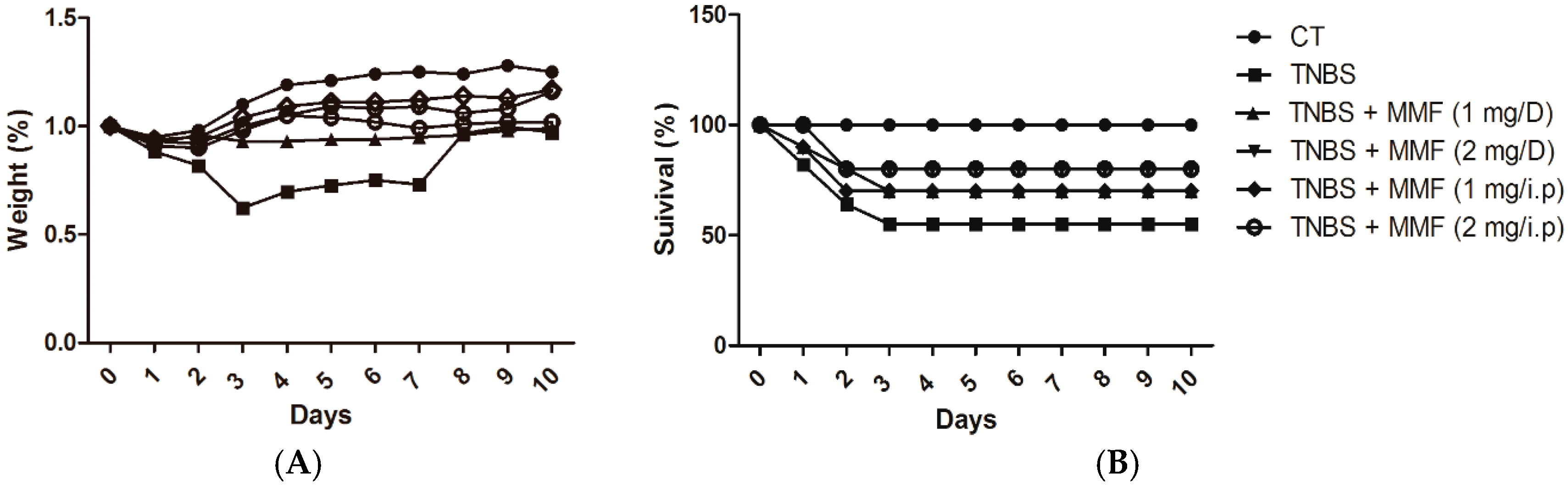
2.1. MMF Ameliorates the Development of TNBS-Induced Colitis in Mice
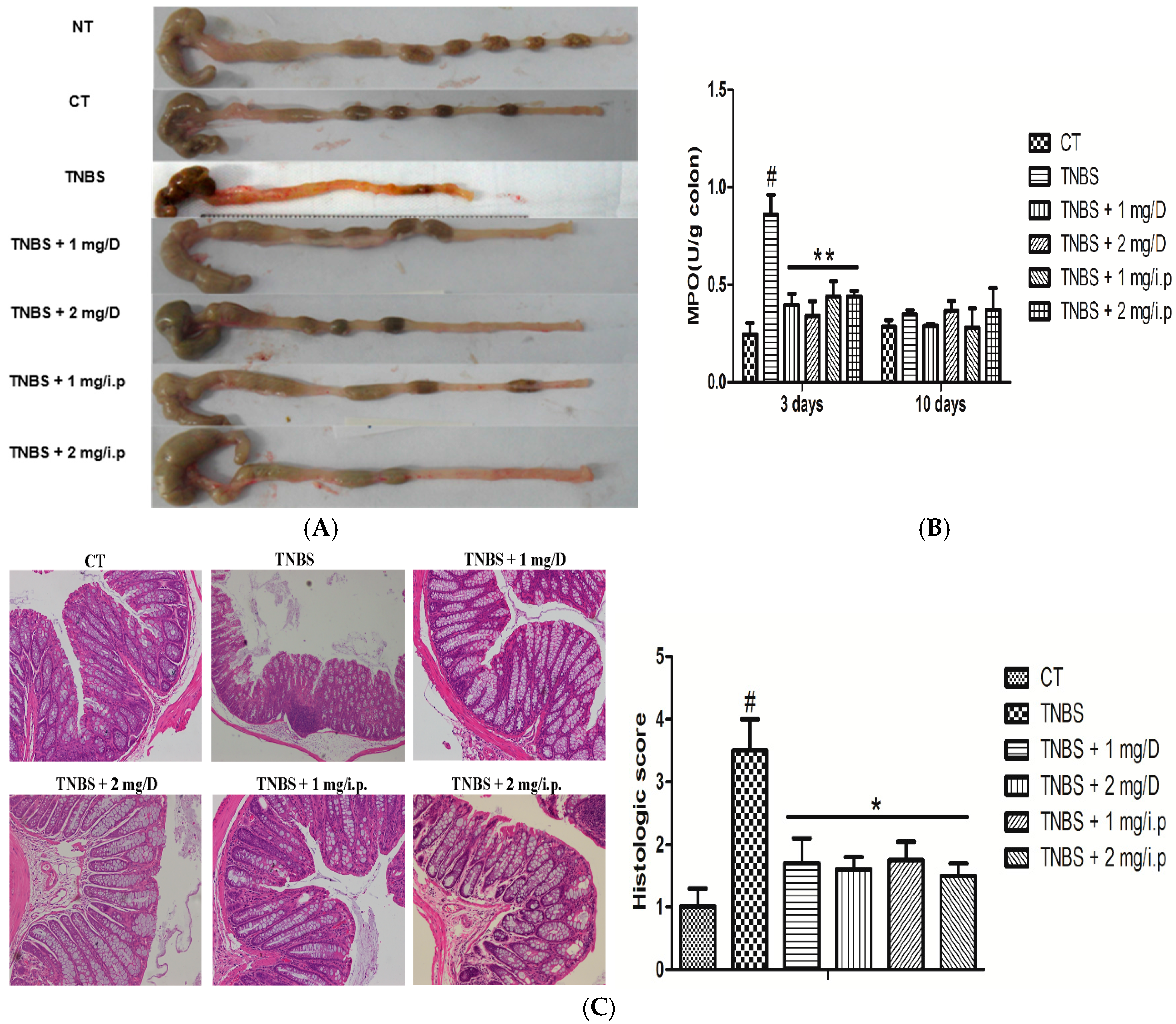
2.2. Histologic Changes and Inflammation Score
2.3. MMF Reduced the Production of Pro-Inflammatory Cytokines in the Serum and Colon Tissues of Mice with TNBS-Induced Colitis


2.4. MMF Affects the Differentiation of Splenic Th1/Th2 Cells in TNBS-Induced Colitis or in Vitro

3. Discussion
4. Materials and Methods
4.1. Animals and Induction of CD
4.2. Clinical and Histological Analysis
4.3. Isolation and Culture of Splenocytes
4.4. Real-Time PCR
| Gene | Sequence |
|---|---|
| β-actin sense | 5′-CCAACCGTGAAAAGATGACC-3′ |
| β-actin antisense | 5′-CAGTAATCTCCTTCTGCATCC-3′ |
| TNF-α sense | 5′-CTGTGAAGGGAATGGGTGTT-3′ |
| TNF-α antisense | 5′-CAGGGAAGAATCTGGAAAGGTC-3′ |
| IL-6 sense | 5′-TATGAATAAGGCTGCTATGAA-3′ |
| IL-6 antisense | 5′-TGGTAAGGATGTGGAGAA-3′ |
| IL-10 sense | 5′-GCTCTTACTGACTGGCATGAG-3′ |
| IL-10 antisense | 5′-CGCAGCTCTAGGAGCATGTG-3′ |
| IFN-γ sense | 5′-TGAGACAATGAACGCTAC-3′ |
| IFN-γ antisense | 5′-TTCCACATCTATGCCACT-3′ |
| IL-1β sense | 5′-CTCGGCCAAGACAGGTCGCTC-3′ |
| IL-1β antisense | 5′-CCCCCACACGTTGACAGCTAGG-3′ |
| IL-12/P40 sense | 5′-TCGCAGCAAAGCAAGGTAAG-3′ |
| IL-12/P40 antisense | 5′-TGGTCTGAGGTCCAGGTGAT-3′ |
4.5. ELISA Analysis
4.6. Flow Cytometry Analysis
4.7. Statistical Analysis
Supplementary Material
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fiocchi, C. Inflammatory bowel disease: Etiology and pathogenesis. Gastroenterology 1998, 115, 182–205. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Vermeire, S.; van Assche, G. Biological therapies for inflammatory bowel diseases. Gastroenterology 2009, 136, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.; Mannon, P. The fundamental basis of inflammatory bowel disease. J. Clin. Investig. 2007, 117, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Matricon, J.; Barnich, N.; Ardid, D. Immunopathogenesis of inflammatory bowel disease. Self Nonself 2010, 1, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.L.; Lewis, S.A.; Tavernini, M.M.; Hibbard, J.; Fedorak, R.N. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology 1997, 113, 151–159. [Google Scholar] [CrossRef]
- Van Deventer, S.; Elson, C.; Fedorak, R. Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn’s disease. Crohn’s disease study group. Gastroenterology 1997, 113, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Fuss, I.J. Is the Th1/Th2 paradigm of immune regulation applicable to IBD? Inflamm. Bowel Dis. 2008, 14, S110–S112. [Google Scholar] [CrossRef] [PubMed]
- Cominelli, F. Cytokine-based therapies for Crohn’s disease—New paradigms. N. Engl. J. Med. 2004, 351, 2045–2048. [Google Scholar] [CrossRef] [PubMed]
- Ensley, R.D.; Bristow, M.R.; Olsen, S.L.; Taylor, D.O.; Hammond, E.H.; O’Connell, J.B.; Dunn, D.; Osburn, L.; Jones, K.W.; Kauffman, R.S. The use of mycophenolate mofetil (rs-61443) in human heart transplant recipients. Transplantation 1993, 56, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Behrend, M. A review of clinical experience with the novel immunosuppressive drug mycophenolate mofetil in renal transplantation. Clin. Nephrol. 1996, 45, 336–341. [Google Scholar] [PubMed]
- Kobashigawa, J.; Miller, L.; Renlund, D.; Mentzer, R.; Alderman, E.; Bourge, R.; Costanzo, M.; Eisen, H.; Dureau, G.; Ratkovec, R. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients1. Transplantation 1998, 66, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Dooley, M.A.; Cosio, F.G.; Nachman, P.H.; Falkenhain, M.E.; Hogan, S.L.; Falk, R.J.; Hebert, L.A. Mycophenolate mofetil therapy in lupus nephritis clinical observations. J. Am. Soc. Nephrol. 1999, 10, 833–839. [Google Scholar] [PubMed]
- Nousari, H.C.; Sragovich, A.; Kimyai-Asadi, A.; Orlinsky, D.; Anhalt, G.J. Mycophenolate mofetil in autoimmune and inflammatory skin disorders. J. Am. Acad. Dermatol. 1999, 40, 265–268. [Google Scholar] [CrossRef]
- Behrend, M. Adverse gastrointestinal effects of mycophenolate mofetil. Drug Saf. 2001, 24, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.; Ferrebuz, A.; MacGregor, E.G.; Rodriguez-Iturbe, B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J. Am. Soc. Nephrol. 2006, 17, S218–S225. [Google Scholar] [CrossRef] [PubMed]
- Doukaki, S.; Platamone, A.; Alaimo, R.; Bongiorno, M.R. Mycophenolate mofetil and enteric-coated mycophenolate sodium in the treatment of pemphigus vulgaris and pemphigus foliaceus. J. Dermatol. Treat. 2014, 26, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Maripuri, S.; Kasiske, B.L. The role of mycophenolate mofetil in kidney transplantation revisited. Transplant. Rev. 2014, 28, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Daudén, E.; Pedraz, J.; Álvarez-Ruiz, S.; García-Río, I.; Sánchez-Peinado, C.; Oñate, M.-J.; García-Diez, A. Therapeutic drug monitoring of mycophenolic acid in patients with psoriasis. Eur. J. Dermatol. 2010, 20, 321–322. [Google Scholar] [PubMed]
- Rogler, G.; Andus, T. Cytokines in inflammatory bowel disease. World J. Surg. 1998, 22, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Van Dieren, J.M.; Kuipers, E.J.; Samsom, J.N.; Nieuwenhuis, E.E.; van der Woude, C.J. Revisiting the immunomodulators tacrolimus, methotrexate, and mycophenolate mofetil: Their mechanisms of action and role in the treatment of IBD. Inflamm. Bowel Dis. 2006, 12, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Orth, T.; Peters, M.; Schlaak, J.; Krummenauer, F.; Wanitschke, R.; Mayet, W.; Galle, P.; Neurath, M. Mycophenolate mofetil versus azathioprine in patients with chronic active ulcerative colitis: A 12-month pilot study. Am. J. Gastroenterol. 2000, 95, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Fulton, B.; Markham, A. Mycophenolate mofetil. Drugs 1996, 51, 278–298. [Google Scholar] [CrossRef] [PubMed]
- Fellermann, K.; Steffen, M.; Stein, J.; Raedler, A.; Hämling, J.; Ludwig, D.; Loeschke, K.; Stange, E. Mycophenolate mofetil: Lack of efficacy in chronic active inflammatory bowel disease. Aliment. Pharmacol. Ther. 2000, 14, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-J.; Zhang, H.-T.; Li, Y.-P.; Wei, Q.; Li, H.; Yang, Y.; Lu, Y. Safety of mycophenolate mofetil versus azathioprine in renal transplantation: A systematic review. Transplant. Proc. 2004, 36, 2068–2070. [Google Scholar] [CrossRef] [PubMed]
- Van Leuven, S.I.; Kastelein, J.J.; Allison, A.C.; Hayden, M.R.; Stroes, E.S. Mycophenolate mofetil (MMF): Firing at the atherosclerotic plaque from different angles? Cardiovasc. Res. 2006, 69, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Hassard, P.V.; Vasiliauskas, E.A.; Kam, L.Y.; Targan, S.R.; Abreu, M.T. Efficacy of mycophenolate mofetil in patients failing 6-mercaptopurine or azathioprine therapy for Crohn’s disease. Inflamm. Bowel Dis. 2000, 6, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.J.; Blumberg, R.S. The immunology of mucosal models of inflammation 1. Annu. Rev. Immunol. 2002, 20, 495–549. [Google Scholar] [CrossRef] [PubMed]
- Belle, A.; Baumann, C.; Bigard, M.A.; Zallot, C.; Gizard, E.; Gueant, J.L.; Bronowicki, J.P.; Peyrin-Biroulet, L. Impact of immunosuppressive therapy on hepatitis B vaccination in inflammatory bowel diseases. Eur. J. Gastroenterol. Hepatol. 2015, 27, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Marocchi, M.; Sartini, A.; Roda, E. Cytokine networks in ulcerative colitis. Ulcers 2011, 2011, 1–5. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.-S.; Kim, E.-Y.; Park, H.-J.; Chang, C.-Y.; Park, K.-S.; Jung, D.-Y.; Kwon, C.-H.; Joh, J.-W.; Kim, S.-J. Mycophenolate mofetil promotes down-regulation of expanded B cells and production of TNF-α in an experimental murine model of colitis. Cytokine 2008, 44, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, E.; Varela, N.; Sheibanie, A.F.; Chorny, A.; Ganea, D.; Delgado, M. Cortistatin, an antiinflammatory peptide with therapeutic action in inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2006, 103, 4228–4233. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, K.; Uchida, H.; Harada, Y.; Li, X.; Yanagisawa, R.; Takano, H.; Hayashi, H.; Taneda, S.; Mori, Y.; Yoshino, S. Effect of methotrexate on Th1 and Th2 immune responses in mice. J. Pharm. Pharmacol. 2003, 55, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Miroux, C.; Morales, O.; Ghazal, K.; Othman, S.B.; de Launoit, Y.; Pancré, V.; Conti, F.; Delhem, N. In vitro effects of cyclosporine a and tacrolimus on regulatory T-cell proliferation and function. Transplantation 2012, 94, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Qiao, Y.; Zhang, D.; Wang, W.-J.; Liu, J.; Liu, T.-Z.; Ma, T.-H. Remarkably decreased CD11b positive splenocytes in aquaporin 3-null mice. Chem. Res. Chin. Univ. 2010, 26, 217–220. [Google Scholar]
- Best, W.R.; Becktel, J.M.; Singleton, J.W.; Kern, F. Development of a crohn’s disease activity index. Gastroenterology 1976, 70, 439–444. [Google Scholar] [PubMed]
- Feldman, M.; Friedman, L.S.; Brandt, L.J. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management; Elsevier Health Sciences: Philadelphia, PA, USA, 2015. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Q.-K.; Liu, J.-X.; Li, S.-N.; Gao, Y.-J.; Lv, Y.; Xu, Z.-P.; Huang, B.-X.; Xu, S.-Y.; Yang, D.-X.; Zeng, Y.-L.; et al. Mycophenolate Mofetil Modulates Differentiation of Th1/Th2 and the Secretion of Cytokines in an Active Crohn’s Disease Mouse Model. Int. J. Mol. Sci. 2015, 16, 26654-26666. https://doi.org/10.3390/ijms161125985
Lv Q-K, Liu J-X, Li S-N, Gao Y-J, Lv Y, Xu Z-P, Huang B-X, Xu S-Y, Yang D-X, Zeng Y-L, et al. Mycophenolate Mofetil Modulates Differentiation of Th1/Th2 and the Secretion of Cytokines in an Active Crohn’s Disease Mouse Model. International Journal of Molecular Sciences. 2015; 16(11):26654-26666. https://doi.org/10.3390/ijms161125985
Chicago/Turabian StyleLv, Qing-Kang, Ju-Xiong Liu, Su-Nan Li, Ying-Jie Gao, Yan Lv, Zi-Peng Xu, Bing-Xu Huang, Shi-Yao Xu, Dong-Xue Yang, Ya-Long Zeng, and et al. 2015. "Mycophenolate Mofetil Modulates Differentiation of Th1/Th2 and the Secretion of Cytokines in an Active Crohn’s Disease Mouse Model" International Journal of Molecular Sciences 16, no. 11: 26654-26666. https://doi.org/10.3390/ijms161125985





