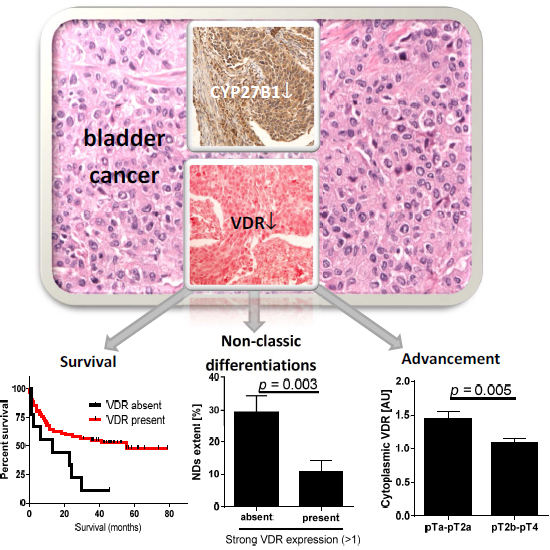
Expression of Vitamin D Receptor (VDR) Positively Correlates with Survival of Urothelial Bladder Cancer Patients
Abstract
:1. Introduction
2. Results
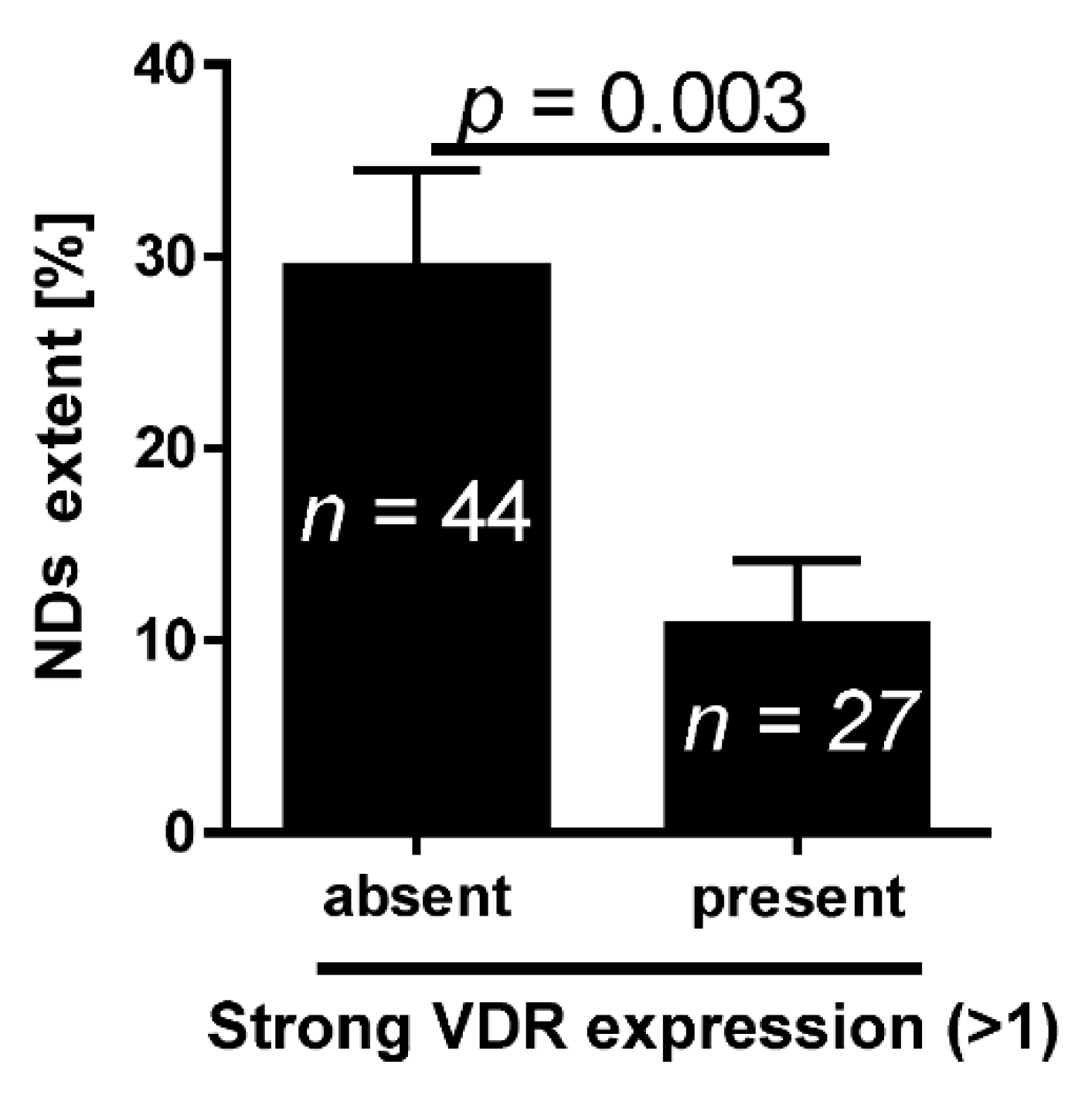
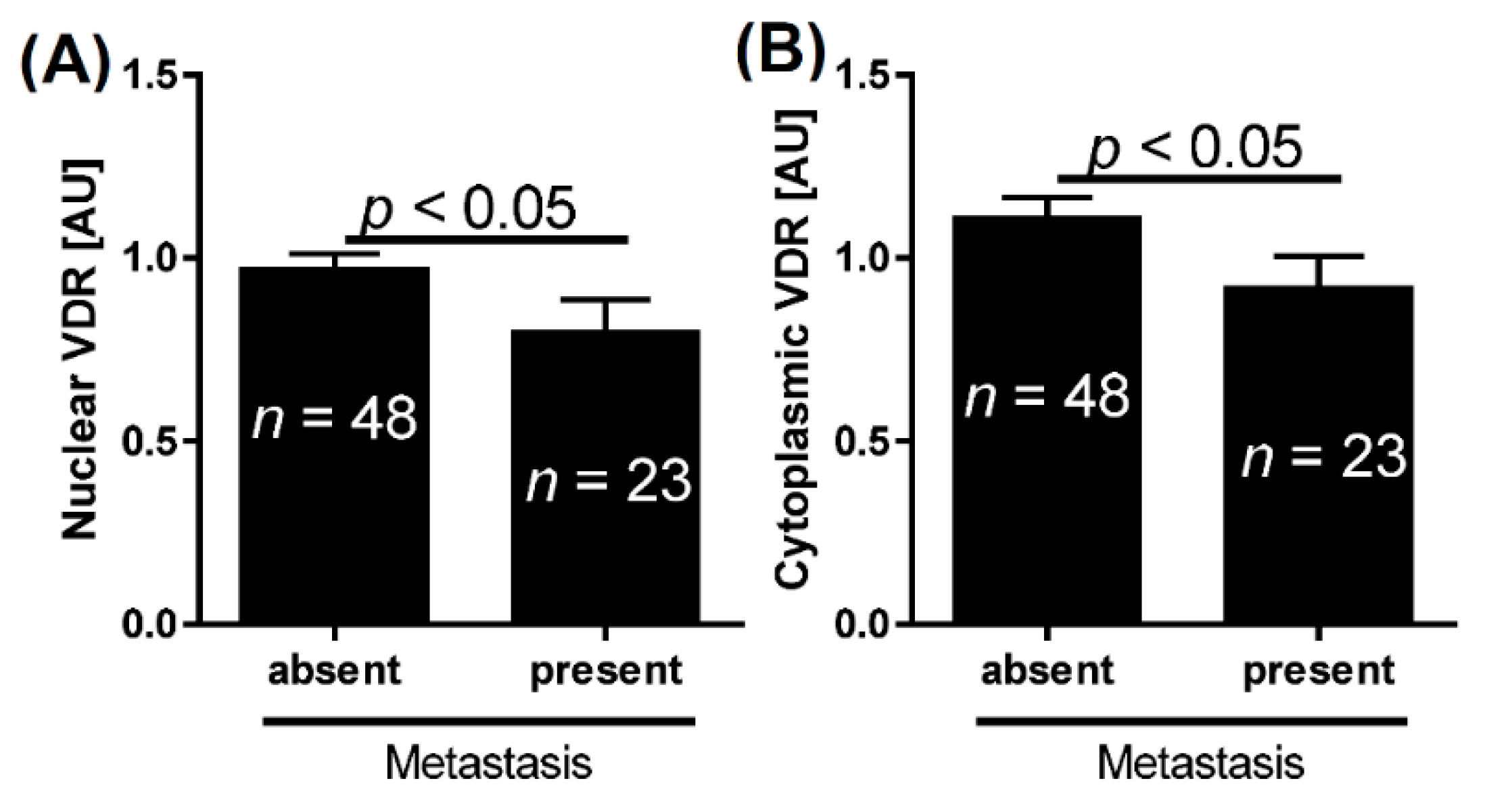
2.1. VDR and Bladder Cancer

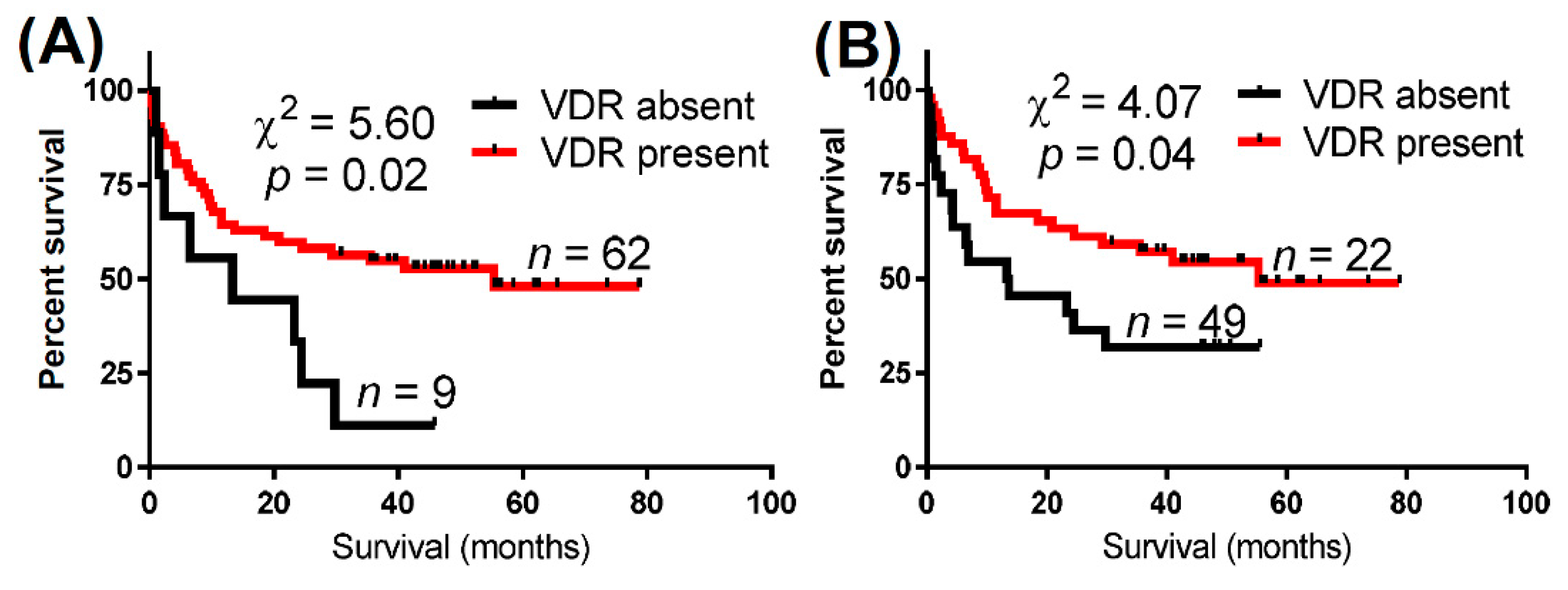
| VDR Expression | Total Cases (n) | Deaths (n) | Median Overall Survival (Months) | Log-Rank (Mantel–Cox) Test | Gehan–Breslow–Wilcoxon Test | Hazard Ratio (95% CI) | |
|---|---|---|---|---|---|---|---|
| χ2 (p Value) | χ2 (p Value) | ||||||
| Nuclear VDR | Absent | 9 | 8 | 13.3 | 5.60 (0.02) | 4.00 (<0.05) | 2.47 |
| Present | 62 | 30 | 55.3 | (0.85–7.22) | |||
| Cytoplasmic VDR | Absent | 22 | 15 | 13.5 | 4.07 (0.04) | 4.63 (0.03) | 1.92 |
| Present | 49 | 23 | 55.3 | (0.53–4.00) | |||
| Either nuclear or cytoplasmic VDR | Absent | 9 | 8 | 13.5 | 5.63 (0.02) | 4.00 (<0.05) | 2.47 |
| Present | 62 | 30 | 55.3 | (0.85–7.22) | |||
| Adjustment | χ2 | p Value | Variable | b | SE | p Value | Exp(b) | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|
| Nuclear VDR | |||||||||
| Age and gender | 9.45 | 0.024 | Nuclear VDR | −0.85 | 0.40 | 0.04 | 0.43 | 0.20–0.94 | |
| Age | 0.04 | 0.02 | 0.03 | 1.05 | 1.01–1.09 | ||||
| Gender * | 0.10 | 0.43 | 0.81 | 1.10 | 0.48–2.56 | ||||
| Age, gender and advancement | 17.13 | 0.002 | Nuclear VDR | −1.10 | 0.41 | 0.01 | 0.34 | 0.15–0.75 | |
| Age | 0.04 | 0.02 | 0.04 | 1.05 | 1.00–1.09 | ||||
| Gender * | 0.10 | 0.43 | 0.81 | 1.10 | 0.48–2.56 | ||||
| Advancement ** | 1.12 | 0.45 | 0.01 | 3.09 | 1.27–7.49 | ||||
| Age, gender and metastases | 14.24 | 0.01 | Nuclear VDR | −0.56 | 0.47 | 0.23 | 0.60 | 0.23–1.42 | |
| Age | 0.05 | 0.02 | 0.03 | 1.05 | 1.00–1.10 | ||||
| Gender * | −0.27 | 0.49 | 0.58 | 0.76 | 0.30–2.00 | ||||
| Metastases *** | 0.98 | 0.37 | 0.01 | 2.67 | 1.29–5.51 | ||||
| Age, gender, advancement, the presence of metastases | 17.22 | 0.004 | Nuclear VDR | −0.79 | 0.50 | 0.11 | 0.45 | 0.17–1.17 | |
| Age | 0.05 | 0.02 | 0.04 | 1.05 | 1.00–1.10 | ||||
| Gender * | −0.12 | 0.50 | 0.81 | 0.89 | 0.34–2.32 | ||||
| Advancement ** | 0.81 | 0.49 | 0.10 | 2.24 | 0.86–5.87 | ||||
| Metastasis *** | 0.68 | 0.40 | 0.09 | 1.97 | 0.89–4.33 | ||||
| Cytoplasmic VDR | |||||||||
| Age and gender | 9.95 | 0.02 | Cytoplasmic VDR | −0.72 | 0.34 | 0.03 | 0.49 | 0.25–0.94 | |
| Age | 0.05 | 0.02 | 0.02 | 1.05 | 1.01–1.09 | ||||
| Gender * | 0.10 | 0.43 | 0.81 | 1.11 | 0.48–2.58 | ||||
| Age, gender and advancement | 15.98 | 0.003 | Cytoplasmic VDR | −0.75 | 0.34 | 0.03 | 0.47 | 0.24–0.92 | |
| Age | 0.05 | 0.02 | 0.03 | 1.05 | 1.00–1.09 | ||||
| Gender * | 0.16 | 0.43 | 0.71 | 1.17 | 0.50–2.73 | ||||
| Advancement ** | 0.99 | 0.45 | 0.03 | 2.70 | 1.13–6.46 | ||||
| Age, gender and metastases | 17.13 | 0.002 | Cytoplasmic VDR | −0.43 | 0.37 | 0.25 | 0.65 | 0.32–1.34 | |
| Age | 0.05 | 0.02 | 0.02 | 1.05 | 1.01–1.10 | ||||
| Gender * | −0.26 | 0.49 | 0.59 | 0.77 | 0.30–2.00 | ||||
| Metastases *** | 0.91 | 0.38 | 0.02 | 2.50 | 1.18–5.26 | ||||
| Age, gender, advancement, the presence of metastases | 16.72 | 0.005 | Cytoplasmic VDR | −0.52 | 0.38 | 0.17 | 0.59 | 0.28–1.25 | |
| Age | 0.05 | 0.02 | 0.04 | 1.05 | 1.00–1.10 | ||||
| Gender * | −0.11 | 0.50 | 0.82 | 0.89 | 0.34–2.36 | ||||
| Advancement ** | 0.72 | 0.48 | 0.13 | 2.05 | 0.81–5.22 | ||||
| Metastasis *** | 0.66 | 0.40 | 0.10 | 1.94 | 0.89–4.26 | ||||
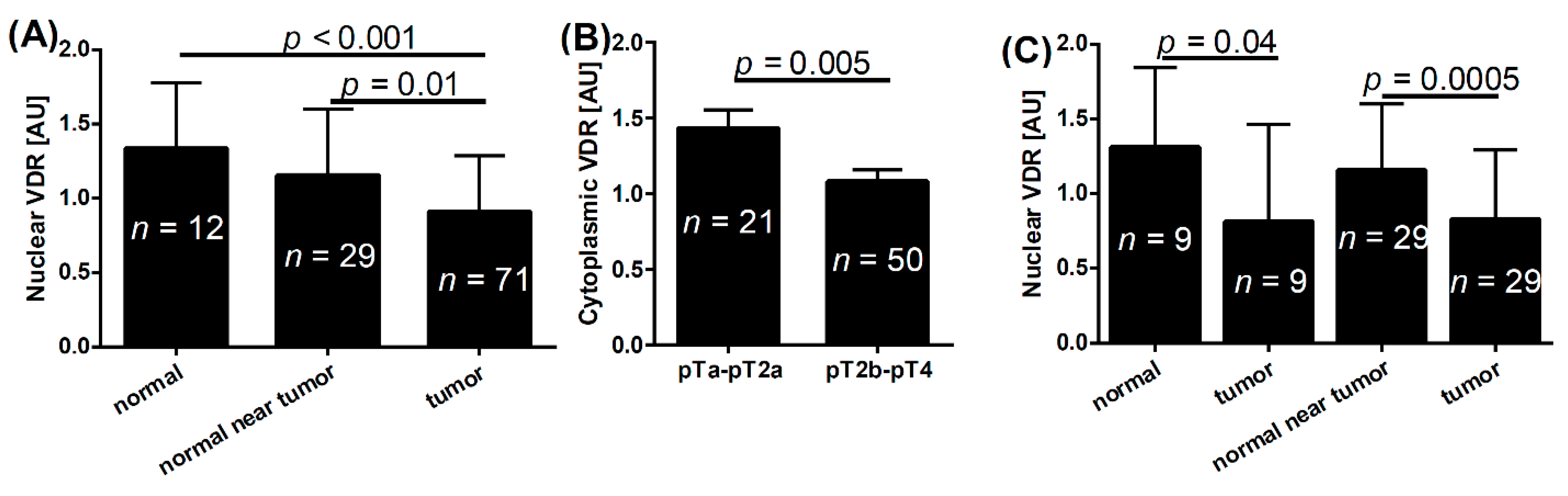
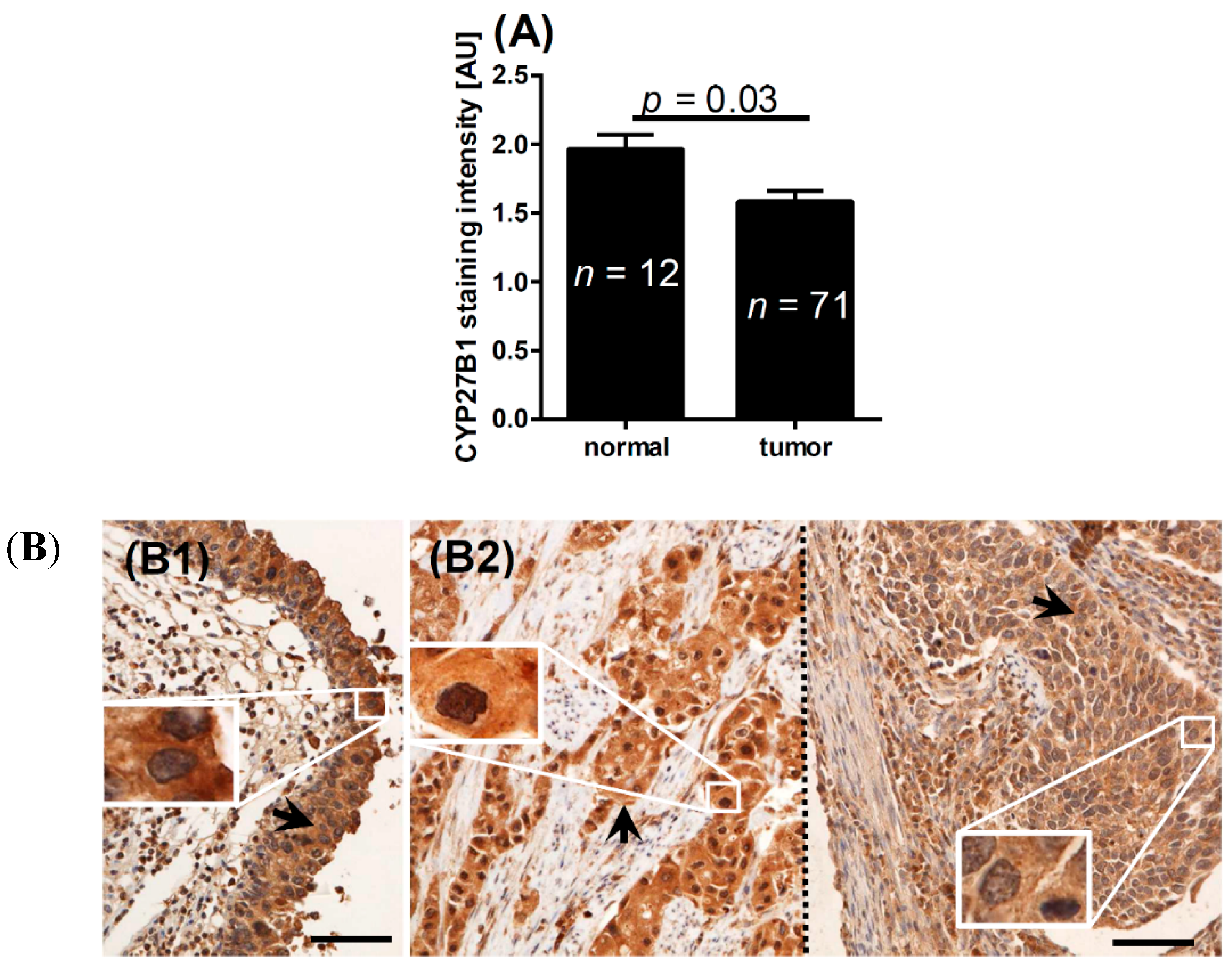
2.2. CYP2B1 and Bladder Cancer
| Feature | Nuclear VDR | Cytoplasmic VDR | CYP27B1 | ||||
|---|---|---|---|---|---|---|---|
| Mean (AU) | p Value | Mean (AU) | p Value | Mean (AU) | p Value | ||
| Normal epithelium | 1.3 | <0.001 * | 1.0 | 0.2 * | 2.0 | 0.03 * | |
| Normal epithelium near tumor | 1.2 | 0.01 * | 1.1 | 0.5 * | 1.9 | 0.1 * | |
| Tumor | 0.9 | – | 1.1 | 1.6 | – | ||
| pT | pTa-pT2a | 0.8 | 0.3 | 1.4 | 0.005 | 1.6 | 0.06 |
| pT2b-pT4 | 0.7 | 1.0 | 1.8 | ||||
| Metastasis | absent | 1.0 | <0.05 | 1.1 | <0.05 | 1.6 | 0.1 |
| present | 0.8 | 0.9 | 1.8 | ||||
| Tumor grade | high | 0.9 | 0.3 | 0.8 | 0.2 | 1.7 | 0.07 |
| low | 0.9 | 1.0 | 1.5 | ||||
| Mitosis | <10 mitoses per 1000 tumor cells | 1.1 | 0.08 | 1.0 | 0.2 | 1.5 | 0.2 |
| >10 mitoses per 1000 tumor cells | 0.9 | 0.8 | 1.7 | ||||
3. Discussion
4. Experimental Section
4.1. Patients and Pathological Morphological Assessment
| Feature | Number of Patients | |
|---|---|---|
| Gender | female | 13 |
| males | 58 | |
| pT a | a | 5 |
| is | 1 | |
| 1 | 12 | |
| 2a | 3 | |
| 2b | 14 | |
| 3 | 24 | |
| 4 | 12 | |
| NDs b | Absent | 32 |
| Present | 39 | |
| Grade | Low grade | 19 |
| High grade | 52 | |
| Metastases | Absent | 44 |
| Present | 27 | |
| Second tumor | Absent | 62 |
| Present | 9 | |
| Concomitant carcinoma in situ | Absent | 64 |
| Present | 7 | |
| Survival | Alive | 33 |
| Death | 38 | |
4.2. Immunohistochemical Staining and Evaluation of the VDR

4.3. Evaluation of the NDs
4.4. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lopez-Beltran, A.; Sauter, G.; Gasser, T.; Hartmann, A.; Schmitz-Dräger, B.J.; Helpap, B.; Ayala, A.G.; Tamboli, P.; Knowles, M.A.; Sidransky, D.; et al. Tumours of the urinary system. Infiltrating urothelial carcinoma. In WHO Classification of Tumours. Pathology and Genetics. Pathology and Genetics of Tumors of the Urinary System and Male Genital Organs; Eble, J.N., Sauter, G., Epstein, J.I., Sesterhenn, I.A., Eds.; IARC Press: Lyon, France, 2004; pp. 93–109. [Google Scholar]
- Domanowska, E.; Jozwicki, W.; Domaniewski, J.; Golda, R.; Skok, Z.; Wisniewska, H.; Sujkowska, R.; Wolski, Z.; Jozwicka, G. Muscle-invasive urothelial cell carcinoma of the human bladder: Multidirectional differentiation and ability to metastasize. Hum. Pathol. 2007, 38, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Madersbacher, S.; Hochreiter, W.; Burkhard, F.; Thalmann, G.N.; Danuser, H.; Markwalder, R.; Studer, U.E. Radical cystectomy for bladder cancer today—A homogeneous series without neoadjuvant therapy. J. Clin. Oncol. 2003, 21, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Niegisch, G.; Lorch, A.; Droller, M.J.; Lavery, H.J.; Stensland, K.D.; Albers, P. Neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer: Which patients benefit? Eur. Urol. 2012, 64, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Boorjian, S.A.; Kim, S.P.; Cheville, J.C.; Thapa, P.; Tarrel, R.; Dronca, R.; Costello, B.; Frank, I. Eligibility for neoadjuvant/adjuvant cisplatin-based chemotherapy among radical cystectomy patients. BJU Int. 2014, 113, E17–E21. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Gröschel, C.; Tennakoon, S.; Kállay, E. Cytochrome P450 vitamin D hydroxylases in inflammation and cancer. Adv. Pharmacol. 2015, 74, 413–458. [Google Scholar] [PubMed]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Cieslak, K.; Feingold, J.; Antonius, D.; Walsh-Messinger, J.; Dracxler, R.; Rosedale, M.; Aujero, N.; Keefe, D.; Goetz, D.; Goetz, R.; et al. Low vitamin D levels predict clinical features of schizophrenia. Schizophr. Res. 2014, 159, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Koplin, J.J.; Suaini, N.H.; Vuillermin, P.; Ellis, J.A.; Panjari, M.; Ponsonby, A.L.; Peters, R.L.; Matheson, M.C.; Martino, D.; Dang, T.; et al. Polymorphisms affecting vitamin D-binding protein modify the relationship between serum vitamin D (25[OH]D3) and food allergy. J. Allergy. Clin. Immunol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.; Penna-Martinez, M.; Klahold, E.; Kärger, D.; Shoghi, F.; Kahles, H.; Bayer, M.; Hintermann, E.; Pfeilschifter, J.M.; Badenhoop, K.; et al. Influence of the vitamin D plasma level and vitamin D-related genetic polymorphisms on the immune status of patients with type 1 diabetes: A pilot study. Clin. Exp. Immunol. 2013, 171, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.; Kim, S.Y.; Jeong, B.H.; Chang, B.; Shin, S.J.; Koh, W.J. Severe vitamin D deficiency is associated with non-tuberculous mycobacterial lung disease: A case-control study. Respirology 2013, 18, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Maalmi, H.; Ordóñez-Mena, J.M.; Schöttker, B.; Brenner, H. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: Systematic review and meta-analysis of prospective cohort studies. Eur. J. Cancer 2014, 50, 1510–1521. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.J.; Byun, S.S.; Lee, S.E.; Hong, S.K.; Jeong, C.W.; Choi, W.S.; Kim, D.; Kim, H.J.; Myung, S.C. Genetic variants in the CYP24A1 gene are associated with prostate cancer risk and aggressiveness in a Korean study population. Prostate Cancer Prostatic Dis. 2014, 17, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Agic, A.; Xu, H.; Altgassen, C.; Noack, F.; Wolfler, M.M.; Diedrich, K.; Friedrich, M.; Taylor, R.N.; Hornung, D. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1α-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod. Sci. 2007, 14, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Jochymski, C.; Janjetovic, Z.; Jozwicki, W.; Tuckey, R.C.; Slominski, A.T. CYP24A1 expression inversely correlates with melanoma progression: Clinic-pathological studies. Int. J. Mol. Sci. 2014, 15, 19000–19017. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Jozwicki, W.; Jochymski, C.; Slominski, A.T. Decreased expression of CYP27B1 correlates with the increased aggressiveness of ovarian carcinomas. Oncol. Rep. 2015, 33, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Jozwicki, W.; Slominski, A.T. Decreased VDR expression in cutaneous melanomas as marker of tumor progression: New data and analyses. Anticancer Res. 2014, 34, 2735–2743. [Google Scholar] [PubMed]
- Clinckspoor, I.; Verlinden, L.; Overbergh, L.; Korch, C.; Bouillon, R.; Mathieu, C.; Verstuyf, A.; Decallonne, B. 1,25-dihydroxyvitamin D3 and a superagonistic analog in combination with paclitaxel or suberoylanilide hydroxamic acid have potent antiproliferative effects on anaplastic thyroid cancer. J. Steroid Biochem. Mol. Biol. 2012, 124, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.F.; Mendez-Pertuz, M.; Munoz, A.; Silverman, D.T.; Allory, Y.; Kogevinas, M.; Lloreta, J.; Rothman, N.; Carrato, A.; Rivas del Fresno, M.; et al. Plasma 25-hydroxyvitamin D3 and bladder cancer risk according to tumor stage and FGFR3 status: A mechanism-based epidemiological study. J. Natl. Cancer Inst. 2012, 104, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, Q.; Yu, Y.; Yang, W.; Shi, F.; Qu, Y. Association of vitamin C, vitamin D, vitamin E and risk of bladder cancer: A dose–response meta-analysis. Sci. Rep. 2015, 5, 9599. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Huang, J.L.; Qiu, M.X.; Ma, Z.W. Impact of serum vitamin D level on risk of bladder cancer: A systemic review and meta-analysis. Tumour Biol. 2015, 36, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Weinstein, S.J.; Mannisto, S.; Snyder, K.; Horst, R.L.; Virtamo, J.; Albanes, D. Serum vitamin D and risk of bladder cancer. Cancer Res. 2010, 70, 9218–9223. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, Z.; Tuckey, R.C.; Nguyen, M.N.; Thorpe, E.M., Jr.; Slominski, A.T. 20,23-dihydroxyvitamin D3, novel p450scc product, stimulates differentiation and inhibits proliferation and NF-κB activity in human keratinocytes. J. Cell. Physiol. 2010, 223, 36–48. [Google Scholar] [PubMed]
- Slominski, A.T.; Janjetovic, Z.; Kim, T.K.; Wright, A.C.; Grese, L.N.; Riney, S.J.; Nguyen, M.N.; Tuckey, R.C. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012, 32, 3733–3742. [Google Scholar] [PubMed]
- Tieu, E.W.; Tang, E.K.; Chen, J.; Li, W.; Nguyen, M.N.; Janjetovic, Z.; Slominski, A.; Tuckey, R.C. Rat CYP24A1 acts on 20-hydroxyvitamin D3 producing hydroxylated products with increased biological activity. Biochem. Pharmacol. 2012, 84, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Slominski, A.; Tuckey, R.C.; Janjetovic, Z.; Kulkarni, A.; Chen, J.; Postlethwaite, A.E.; Miller, D.; Li, W. 20-Hydroxyvitamin D3 inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012, 32, 739–746. [Google Scholar] [PubMed]
- Alibhai, S.M.; Mohamedali, H.Z.; Gulamhusein, H.; Panju, A.H.; Breunis, H.; Timilshina, N.; Fleshner, N.; Krahn, M.D.; Naglie, G.; Tannock, I.F.; et al. Changes in bone mineral density in men starting androgen deprivation therapy and the protective role of vitamin D. Osteoporos. Int. 2013, 24, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Dueregger, A.; Heidegger, I.; Ofer, P.; Perktold, B.; Ramoner, R.; Klocker, H.; Eder, I.E. The use of dietary supplements to alleviate androgen deprivation therapy side effects during prostate cancer treatment. Nutrients 2015, 6, 4491–4519. [Google Scholar] [CrossRef] [PubMed]
- Zeichner, S.B.; Koru-Sengul, T.; Shah, N.; Liu, Q.; Markward, N.J.; Montero, A.J.; Gluck, S.; Silva, O.; Ahn, E.R. Improved clinical outcomes associated with vitamin D supplementation during adjuvant chemotherapy in patients with HER2+ nonmetastatic breast cancer. Clin. Breast Cancer 2015, 15, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Jozwicki, W.; Janjetovic, Z.; Slominski, A.T. Expression of vitamin D receptor decreases during progression of pigmented skin lesions. Hum. Pathol. 2011, 42, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Jozwicki, W.; Janjetovic, Z.; Slominski, A.T. Expression of the vitamin D-activating enzyme 1α-hydroxylase (CYP27B1) decreases during melanoma progression. Hum. Pathol. 2013, 44, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Tokumoto, M.; Tsuruya, K.; Fukuda, K.; Kanai, H.; Kuroki, S.; Hirakata, H. Reduced p21, p27 and vitamin D receptor in the nodular hyperplasia in patients with advanced secondary hyperparathyroidism. Kidney Int. 2002, 62, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Menezes, R.J.; Cheney, R.T.; Husain, A.; Tretiakova, M.; Loewen, G.; Johnson, C.S.; Jayaprakash, V.; Moysich, K.B.; Salgia, R.; Reid, M.E. Vitamin D receptor expression in normal, premalignant, and malignant human lung tissue. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Meyer, M.B. The vitamin D receptor: New paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D3. Endocrinol. Dis. Clin. N. Am. 2010, 39, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Lee, S.M.; Meyer, M.B. Regulation of gene expression by 1,25-dihydroxyvitamin D3 in bone cells: exploiting new approaches and defining new mechanisms. BoneKEy Rep. 2014, 3, 428. [Google Scholar] [CrossRef] [PubMed]
- Iglar, P.J.; Hogan, K.J. Vitamin D status and surgical outcomes: A systematic review. Patient Saf. Surg. 2015, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Turan, A.; Hesler, B.D.; You, J.; Saager, L.; Grady, M.; Komatsu, R.; Kurz, A.; Sessler, D.I. The association of serum vitamin D concentration with serious complications after noncardiac surgery. Anesth. Analg. 2014, 119, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Kim, T.K.; Li, W.; Yi, A.K; Postlethwaite, A.; Tuckey, R.C. The physiological role of CYP11A1 in vitamin D regulation of epidermal functions. J. Steroid Biochem. Mol. Biol. 2014, 144PA, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.-K.; Shehabi, H.Z.; Semak, I.; Tang, E.K.; Nguyen, M.N.; Benson, H.A.; Korik, E.; Janjetovic, Z.; Chen, J.; et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012, 26, 3901–3915. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Li, W.; Kim, T.; Semak, I.; Wang, J.; Zjawiony, J.; Tuckey, R.C. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid Biochem. Mol. Biol. 2015, 151, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Janjetovic, Z.; Kim, T.-K.; Wasilewski, P.; Rosas, S.; Hanna, S.; Sayre, R.M.; Dowdy, J.C.; Li, W.; Tuckey, R.C. Novel non-calcemic secosteroids that are produced by human epidermal keratinocytes protect against solar radiation. J. Steroid Biochem. Mol. Biol. 2015, 148, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.-K.; Janjetovic, Z.; Tuckey, R.C.; Bieniek, R.; Yue, Y.; Li, W.; Chen, J.; Miller, D.; Chen, T.; et al. 20-Hydroxyvitamin D2 is a non-calcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am. J. Physiol. Cell Physiol. 2011, 300, C526–C541. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Kim, T.-K.; Takeda, Y.; Janjetovic, Z.; Brożyna, A.A.; Skobowiat, C.; Wang, J.; Postlethwite, A.; Li, W.; Tuckey, R.C.; et al. RORα and RORγ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxy-vitamin D. FASEB J. 2014, 28, 2775–2789. [Google Scholar] [CrossRef] [PubMed]
- Sahin, M.O.; Canda, A.E.; Yorukoglu, K.; Mungan, M.U.; Sade, M.; Kirkali, Z. 1,25 Dihydroxyvitamin D3 receptor expression in superficial transitional cell carcinoma of the bladder: A possible prognostic factor? Eur. Urol. 2005, 47, 52–57. [Google Scholar] [PubMed]
- Zhou, Z.; Xia, Y.; Bandla, S.; Zakharov, V.; Wu, S.; Peters, J.; Godfrey, T.E.; Sun, J. Vitamin D receptor is highly expressed in precancerous lesions and esophageal adenocarcinoma with significant sex difference. Hum. Pathol. 2014, 45, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Jozwicki, W.; Brożyna, A.A.; Siekiera, J. Expression of OCT4a: The first step to the next stage of urothelial bladder cancer progression. Int. J. Mol. Sci. 2014, 15, 16069–16082. [Google Scholar] [CrossRef] [PubMed]
- Jozwicki, W.; Brożyna, A.A.; Siekiera, J.; Slominski, A.T. Expression of RCAS1 correlates with urothelial bladder cancer malignancy. Int. J. Mol. Sci. 2015, 16, 3783–3803. [Google Scholar] [CrossRef] [PubMed]
- Jóźwicki, W.; Skok, Z.; Brożyna, A.; Siekiera, J.; Wolski, Z.; Domaniewski, J. Prognostic and diagnostic implications of histological differentiation in invasive urothelial cell carcinoma of the bladder: Variant or non-classic differentiation number. Cent. Eur. J. Urol. 2010, 63, 112–116. [Google Scholar] [CrossRef]
- Gandini, S.; Gnagnarella, P.; Serrano, D.; Pasquali, E.; Raimondi, S. Vitamin D receptor polymorphisms and cancer. Adv. Exp. Med. Biol. 2014, 810, 69–105. [Google Scholar] [PubMed]
- Cartwright, R.; Mangera, A.; Tikkinen, K.A.; Rajan, P.; Pesonen, J.; Kirby, A.C.; Thiagamoorthy, G.; Ambrose, C.; Gonzalez-Maffe, J.; Bennett, P.R.; et al. Systematic review and meta-analysis of candidate gene association studies of lower urinary tract symptoms in men. Eur. Urol. 2014, 66, 752–768. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.D.; Manchanda, P.K.; Bhat, S.; Bid, H.K. Association of vitamin-D receptor (Fok-I) gene polymorphism with bladder cancer in an Indian population. BJU Int. 2007, 99, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, C.; Morelli, A.; Adorini, L.; Ferruzzi, P.; Luconi, M.; Vannelli, G.B.; Marini, M.; Gelmini, S.; Fibbi, B.; Donati, S.; et al. Human bladder as a novel target for vitamin D receptor ligands. J. Clin. Endocrinol. Metab. 2005, 90, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Adorini, L.; Penna, G.; Amuchastegui, S.; Cossetti, C.; Aquilano, F.; Mariani, R.; Fibbi, B.; Morelli, A.; Uskokovic, M.; Colli, E.; et al. Inhibition of prostate growth and inflammation by the vitamin D receptor agonist BXL-628 (elocalcitol). J. Steroid Biochem. Mol. Biol. 2007, 103, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hu, Q.; Luo, W.; Pratt, R.N.; Glenn, S.T.; Liu, S.; Trump, D.L.; Johnson, C.S. 1α,25(OH)2D3 differentially regulates miRNA expression in human bladder cancer cells. J. Steroid Biochem. Mol. Biol. 2015, 148, 166–171. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jóźwicki, W.; Brożyna, A.A.; Siekiera, J.; Slominski, A.T. Expression of Vitamin D Receptor (VDR) Positively Correlates with Survival of Urothelial Bladder Cancer Patients. Int. J. Mol. Sci. 2015, 16, 24369-24386. https://doi.org/10.3390/ijms161024369
Jóźwicki W, Brożyna AA, Siekiera J, Slominski AT. Expression of Vitamin D Receptor (VDR) Positively Correlates with Survival of Urothelial Bladder Cancer Patients. International Journal of Molecular Sciences. 2015; 16(10):24369-24386. https://doi.org/10.3390/ijms161024369
Chicago/Turabian StyleJóźwicki, Wojciech, Anna A. Brożyna, Jerzy Siekiera, and Andrzej T. Slominski. 2015. "Expression of Vitamin D Receptor (VDR) Positively Correlates with Survival of Urothelial Bladder Cancer Patients" International Journal of Molecular Sciences 16, no. 10: 24369-24386. https://doi.org/10.3390/ijms161024369
APA StyleJóźwicki, W., Brożyna, A. A., Siekiera, J., & Slominski, A. T. (2015). Expression of Vitamin D Receptor (VDR) Positively Correlates with Survival of Urothelial Bladder Cancer Patients. International Journal of Molecular Sciences, 16(10), 24369-24386. https://doi.org/10.3390/ijms161024369