A Heavy Metal-Associated Protein (AcHMA1) from the Halophyte, Atriplex canescens (Pursh) Nutt., Confers Tolerance to Iron and Other Abiotic Stresses When Expressed in Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sequence Characterization and Deduced Amino Acid Sequence Comparison
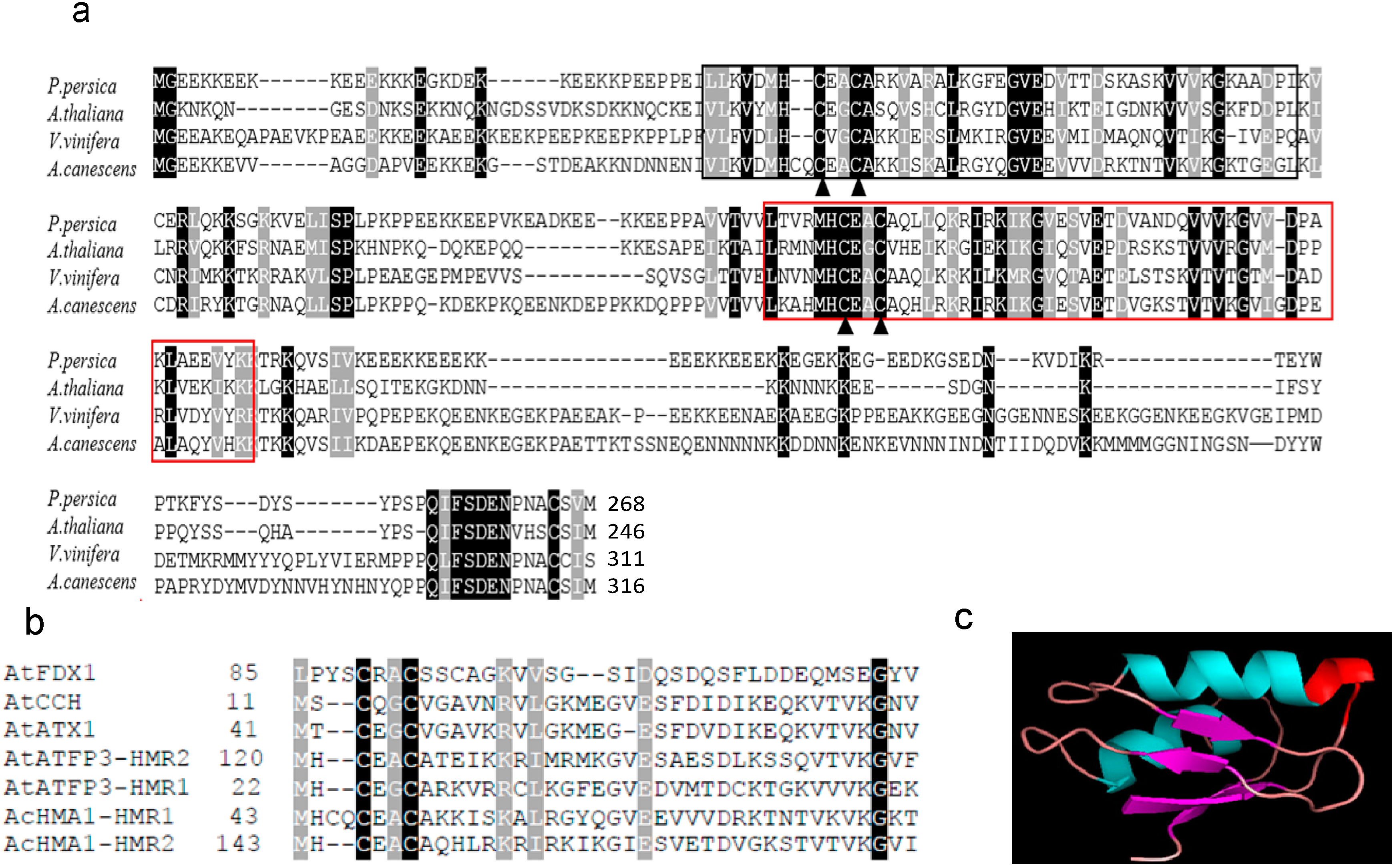
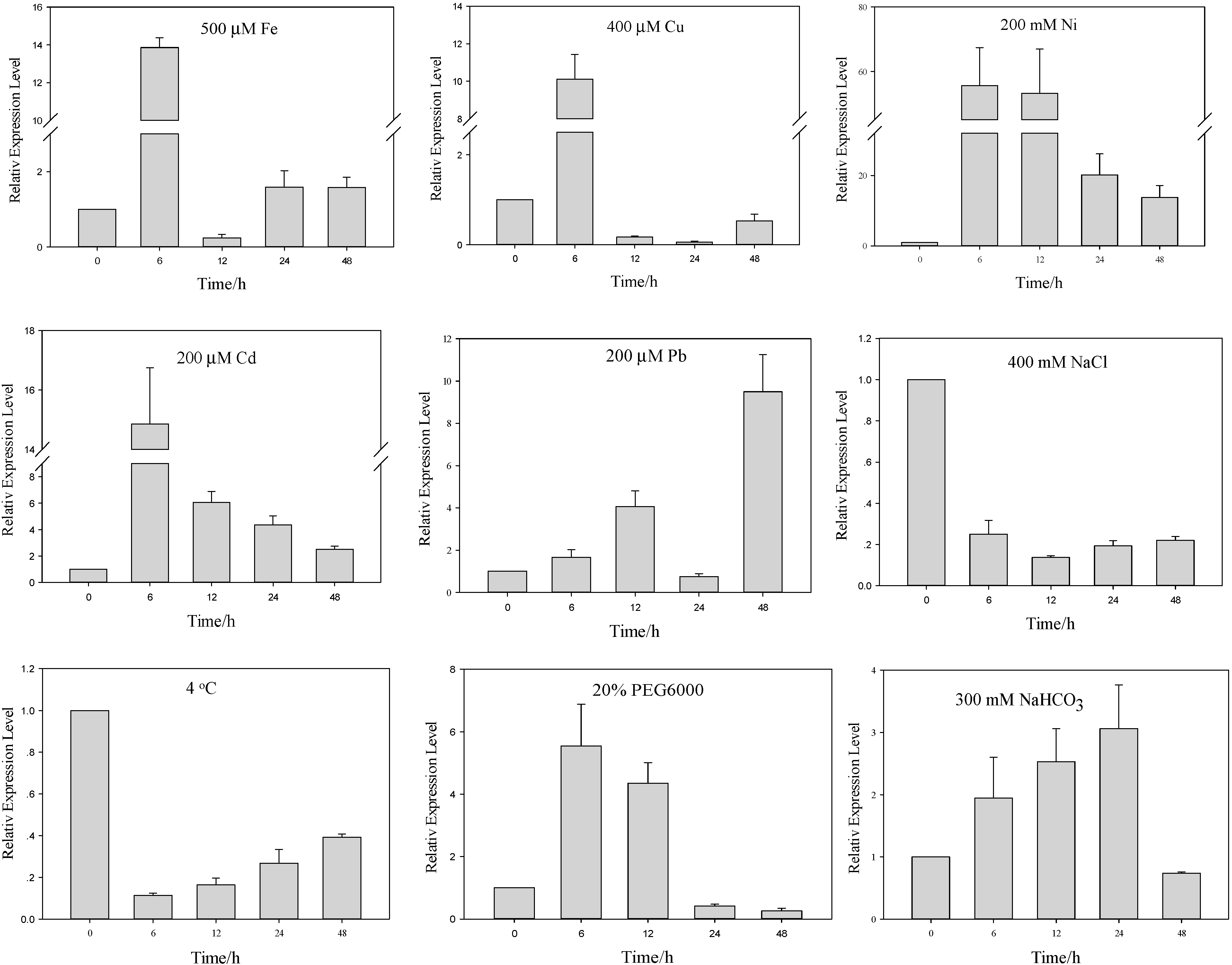
2.2. Differential Regulation of AcHMA1 Expression in Response to Various Abiotic Stresses
2.3. Overexpression of AcHMA1 Improves Iron Tolerance in Yeast Cells
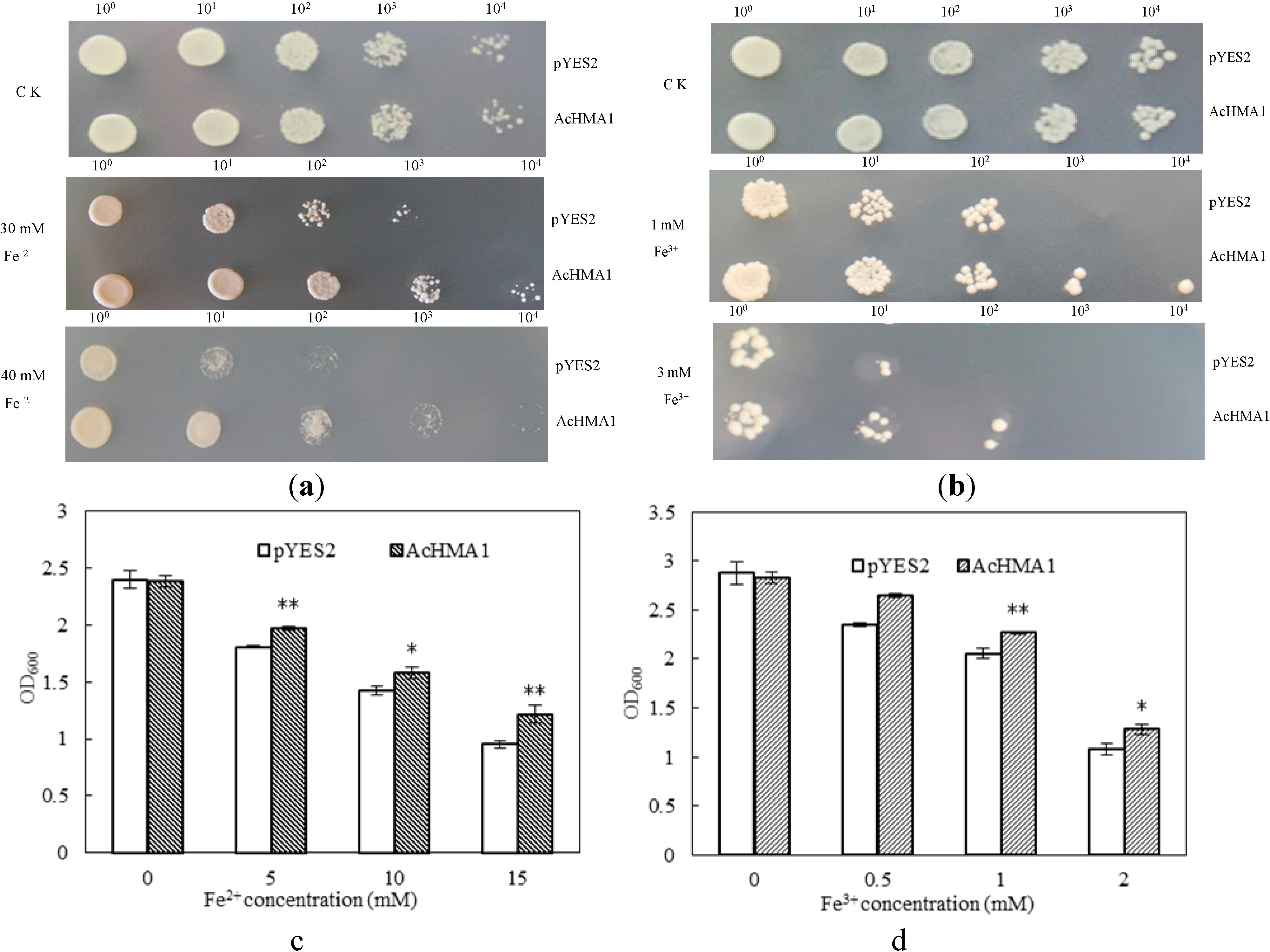
2.4. Response of the Transgenic Yeast Cells Expressing AcHMA1 to Abiotic Stresses

2.5. AcHMA1 Protein Localization to the Plasma Membrane of Plant Cells

3. Experimental Section
3.1. Cloning and Sequence Analysis of the AcHMA1 Gene from A. canescens
3.2. Plant Abiotic Stress Treatments to Study AcHMA1 Gene Regulation
3.3. RNA Isolation and Quantitative RT-PCR Assay
3.4. Yeast Cultures and Transformation
3.5. Stress Tolerance Analysis
3.6. Subcellular Localization of AcHMA1 in N. benthamiana
3.7. Statistical Analysis
3.8. Accession Numbers
3.9. Discussion
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clemens, S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta 2001, 212, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Grotz, N.; Guerinot, M.L. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim. Biophys. Acta 2006, 1763, 595–608. [Google Scholar] [CrossRef]
- Vulpe, C.D.; Packman, S. Cellular copper transport. Annu. Rev. Nutr. 1995, 15, 293–322. [Google Scholar] [CrossRef] [PubMed]
- Goyer, R.A. Toxic and essential metal interactions. Annu. Rev. Nutr. 1997, 17, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Peña, M.M.O.; Koch, K.A.; Thiele, D.J. Dynamic regulation of copper uptake and detoxification genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 1998, 18, 2514–2523. [Google Scholar]
- Hall, J.L.; Williams, L.E. Transition metal transporters in plants. J. Exp. Bot. 2003, 54, 2601–2613. [Google Scholar] [CrossRef] [PubMed]
- Hung, I.H.; Casareno, R.L.B.; Labesse, G.; Mathews, F.S.; Gitlin, J.D. HAH1 is a copper-binding protein with distinct amino acid residues mediating copper homeostasis and antioxidant defense. J. Biol. Chem. 1998, 273, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Dykema, P.; Sipes, P.; Marie, A.; Biermann, B.; Crowell, D.; Randall, S. A new class of proteins capable of binding transition metals. Plant Mol. Biol. 1999, 41, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Bull, P.C.; Thomas, G.R.; Rommens, J.M.; Forbes, J.R.; Cox, D.W. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat. Genet. 1993, 5, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yamaguchi, Y.; Koizumi, N.; Sano, H. Functional characterization of a heavy metal binding protein CdI19 from Arabidopsis. Plant J. 2002, 32, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Morby, A.P.; Parkhill, J.; Lee, B.T.O.; Brown, N.L.; Rouch, D.A.; Camakaris, J.; Williams, T. Bacterial resistances to mercury and copper. J. Cell. Biochem. 1991, 46, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-C.; Lee, W.-C.; Guo, W.-Y.; Pan, S.-M.; Chen, L.-J.; Li, H.-M.; Jinn, T.-L. A copper chaperone for superoxide dismutase that confers three types of copper/zinc superoxide dismutase activity in arabidopsis. Plant Physiol. 2005, 139, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.P. Transport and detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2002, 133, 689–693. [Google Scholar]
- Riger, C.J.; Fernandes, P.N.; Vilela, L.F.; Mielniczki-Pereira, A.A.; Bonatto, D.; Henriques, J.A.P.; Eleutherio, E.C. A. Evaluation of heavy metal toxicity in eukaryotes using a simple functional assay. Metallomics 2011, 3, 1355–1361. [Google Scholar]
- Han, Y.; Wilson, D.B.; Lei, X.G. Expression of an Aspergillus nigerPhytase Gene (phyA) in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1999, 65, 1915–1918. [Google Scholar] [PubMed]
- Jeong, M.-J.; Park, S.-C.; Kwon, H.-B.; Byun, M.-O. Isolation and Haracterization of the gene encoding glyceraldehyde-3-phosphate dehydrogenase. Biochem. Biophys. Res. Commun. 2000, 278, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Posas, F.; Chambers, J.R.; Heyman, J.A.; Hoeffler, J.P.; de Nadal, E.; Ariño, J.N. The transcriptional response of yeast to saline stress. J. Biol. Chem. 2000, 275, 17249–17255. [Google Scholar] [CrossRef] [PubMed]
- Serrano, R.; Rodriguez-Navarro, A. Ion homeostasis during salt stress in plants. Curr. Opin. Cell Biol. 2001, 13, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.-M.; Luo, F.; Li, D.-D.; Zhang, J.; Liu, Z.-H.; Xu, W.-L.; Huang, G.-Q.; Li, X.-B. Cotton BCP genes encoding putative blue copper-binding proteins are functionally expressed in fiber development and involved in response to high-salinity and heavy metal stresses. Physiol. Plant. 2011, 141, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, K.; Lovely, C.; Glenn, E. Growth differences among widely separated geographic accessions of fourwing saltbush (Atriplex canescens) in the great basin desert, New Mexico, USA. Arid Soil Res. Rehabil. 1998, 12, 87–94. [Google Scholar]
- Li, J.; Sun, X.; Yu, G.; Jia, C.; Liu, J.; Pan, H. Generation and analysis of expressed sequence tags (ESTs) from halophyte atriplex canescens to explore salt-responsive related genes. Int. J. Mol. Sci. 2014, 15, 11172–11189. [Google Scholar] [CrossRef] [PubMed]
- Pufahl, R.A.; Singer, C.P.; Peariso, K.L.; Lin, S.-J.; Schmidt, P.J.; Fahrni, C.J.; Culotta, V.C.; Penner-Hahn, J.E.; O’Halloran, T.V. Metal ion chaperone function of the soluble Cu(I) receptor atx1. Science 1997, 278, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Sahlman, L.; Skarfstad, E.G. Mercuric ion binding abilities of MerP variants containing only one cysteine. Biochem. Biophys. Res. Commun. 1993, 196, 583–588. [Google Scholar] [CrossRef] [PubMed]
- BLASTX Program. Available online: http://blast.ncbi.nlm.nih.gov/Blast (accessed on 2 June 2014).
- Pfam. Available online: http://pfam.janelia.org (accessed on 2 June 2014).
- BLASTP Servers. Available online: http://blast.ncbi.nlm.nih.gov (accessed on 2 June 2014).
- Phyre Server. Available online: http://www.sbg.bio.ic.ac.uk/phyre2/html (accessed on 2 June 2014).
- Wang, L.; Xu, C.; Wang, C.; Wang, Y. Characterization of a eukaryotic translation initiation factor 5A homolog from Tamarix androssowii involved in plant abiotic stress tolerance. BMC Plant Biol. 2012, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.R.; Dhankher, O.P. A novel stress-associated protein “AtSAP10” from Arabidopsis thaliana confers tolerance to nickel, manganese, zinc, and high temperature stress. PLoS One 2011, 6, e20921. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jing, R.; Mao, X.; Chang, X.; Li, A. TaABC1, a member of the activity of bc1 complex protein kinase family from common wheat, confers enhanced tolerance to abiotic stresses in Arabidopsis. J. Exp. Bot. 2011, 62, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Ausubel, F.M.; Brent, R.; Kingston, R.E.; Moore, D.D.; Seidman, J.G.; Smith, J.A.; Struhl, K.E. Current Protocols in Molecular Biology; Greene and John Wiley & Sons: New York, NY, USA, 1989. [Google Scholar]
- Ito, H.; Fukuda, Y.; Murata, K.; Kimura, A. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 1983, 153, 163–168. [Google Scholar] [PubMed]
- Giniger, E.; Varnum, S.M.; Ptashne, M. Specific DNA binding of GAL4, a positive regulatory protein of yeast. Cell 1985, 40, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Noël, L.D.; Cagna, G.; Stuttmann, J.; Wirthmüller, L.; Betsuyaku, S.; Witte, C.-P.; Bhat, R.; Pochon, N.; Colby, T.; Parker, J.E. Interaction between SGT1 and cytosolic/nuclear HSC70 chaperones regulates arabidopsis immune responses. Plant Cell Online 2007, 19, 4061–4076. [Google Scholar]
- Kim, D.; Gustin, J.L.; Lahner, B.; Persans, M.W.; Baek, D.; Yun, D.-J.; Salt, D.E. The plant CDF family member TgMTP1 from the Ni/Zn hyperaccumulator Thlaspi goesingense acts to enhance efflux of Zn at the plasma membrane when expressed in Saccharomyces cerevisiae. Plant J. 2004, 39, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Tian, H.; Park, S.; Sreevidya, C.S.; Ward, J.M.; Hirschi, K.D. AtCCX3 is an Arabidopsis endomembrane H+-dependent K+ transporter. Plant Physiol. 2008, 148, 1474–1486. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-J.; Pufahl, R.A.; Dancis, A.; O’Halloran, T.V.; Culotta, V.C. A Role for the Saccharomyces cerevisiae ATX1 gene in copper trafficking and iron transport. J. Biol. Chem. 1997, 272, 9215–9220. [Google Scholar] [CrossRef] [PubMed]
- Himelblau, E.; Mira, H.; Lin, S.-J.; Cizewski Culotta, V.; Peñarrubia, L.; Amasino, R.M. Identification of a functional homolog of the yeast copper homeostasis gene ATX1 from arabidopsis. Plant Physiol. 1998, 117, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Baxter, I.; Tchieu, J.; Sussman, M.R.; Boutry, M.; Palmgren, M.G.; Gribskov, M.; Harper, J.F.; Axelsen, K.B. Genomic comparison of P-type ATPase Ion pumps in arabidopsis and rice. Plant Physiol. 2003, 132, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.D.; Jones, C.E.; Dameron, C.T. Copper chaperones: Function, structure and copper-binding properties. JBIC 1999, 4, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.S.; Stearman, R.; Dancis, A.; Dunn, T.; Beeler, T.; Klausner, R.D. The Menkes/Wilson disease gene homologue in yeast provides copper to a ceruloplasmin-like oxidase required for iron uptake. Proc. Natl. Acad. Sci. USA 1995, 92, 2632–2636. [Google Scholar] [CrossRef] [PubMed]
- Stearman, R.; Yuan, D.S.; Yamaguchi-Iwai, Y.; Klausner, R.D.; Dancis, A. A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science 1996, 271, 1552–1557. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.S.; Gralla, E.B. Delivering copper inside yeast and human cells. Science 1997, 278, 817–818. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Eija, V.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Slekar, K.H.; Kosman, D.J.; Culotta, V.C. The yeast copper/zinc superoxide dismutase and the pentose Phosphate pathway play overlapping roles in oxidative stress protection. J. Biol. Chem. 1996, 271, 28831–28836. [Google Scholar] [CrossRef] [PubMed]
- Shin, L.-J.; Lo, J.-C.; Yeh, K.-C. Copper chaperone antioxidant protein1 is essential for copper homeostasis. Plant Physiol. 2012, 159, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Ravet, K.; Pilon, M. Copper and iron homeostasis in plants: The challenges of oxidative stress. Antioxid. Redox Signal. 2013, 19, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Thapa, G.; Sadhukhan, A.; Panda, S.; Sahoo, L. Molecular mechanistic model of plant heavy metal tolerance. Biometals 2012, 25, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Barth, O.; Vogt, S.; Uhlemann, R.; Zschiesche, W.; Humbeck, K. Stress induced and nuclear localized HIPP26 from Arabidopsis thaliana interacts via its heavy metal associated domain with the drought stress related zinc finger transcription factor ATHB29. Plant Mol. Biol. 2009, 69, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Solioz, M.; Stoyanov, J.V. Copper homeostasis in Enterococcus hirae. FEMS Microbiol. Rev. 2003, 27, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Cobine, P.; Wickramasinghe, W.A.; Harrison, M.D.; Weber, T.; Solioz, M.; Dameron, C.T. The Enterococcus hirae copper chaperone CopZ delivers copper(I) to the CopY repressor. FEBS Lett. 1999, 445, 27–30. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sun, X.-H.; Yu, G.; Li, J.-T.; Jia, P.; Zhang, J.-C.; Jia, C.-G.; Zhang, Y.-H.; Pan, H.-Y. A Heavy Metal-Associated Protein (AcHMA1) from the Halophyte, Atriplex canescens (Pursh) Nutt., Confers Tolerance to Iron and Other Abiotic Stresses When Expressed in Saccharomyces cerevisiae. Int. J. Mol. Sci. 2014, 15, 14891-14906. https://doi.org/10.3390/ijms150814891
Sun X-H, Yu G, Li J-T, Jia P, Zhang J-C, Jia C-G, Zhang Y-H, Pan H-Y. A Heavy Metal-Associated Protein (AcHMA1) from the Halophyte, Atriplex canescens (Pursh) Nutt., Confers Tolerance to Iron and Other Abiotic Stresses When Expressed in Saccharomyces cerevisiae. International Journal of Molecular Sciences. 2014; 15(8):14891-14906. https://doi.org/10.3390/ijms150814891
Chicago/Turabian StyleSun, Xin-Hua, Gang Yu, Jing-Tao Li, Pan Jia, Ji-Chao Zhang, Cheng-Guo Jia, Yan-Hua Zhang, and Hong-Yu Pan. 2014. "A Heavy Metal-Associated Protein (AcHMA1) from the Halophyte, Atriplex canescens (Pursh) Nutt., Confers Tolerance to Iron and Other Abiotic Stresses When Expressed in Saccharomyces cerevisiae" International Journal of Molecular Sciences 15, no. 8: 14891-14906. https://doi.org/10.3390/ijms150814891
APA StyleSun, X.-H., Yu, G., Li, J.-T., Jia, P., Zhang, J.-C., Jia, C.-G., Zhang, Y.-H., & Pan, H.-Y. (2014). A Heavy Metal-Associated Protein (AcHMA1) from the Halophyte, Atriplex canescens (Pursh) Nutt., Confers Tolerance to Iron and Other Abiotic Stresses When Expressed in Saccharomyces cerevisiae. International Journal of Molecular Sciences, 15(8), 14891-14906. https://doi.org/10.3390/ijms150814891



