2.1. The Sequence and Expression of the Zebrafish Ortholog of Zipper-Interacting Protein Kinase (ZIPK)
Reversible phosphorylation of the regulatory light chain of type II myosin is a critical regulatory mechanism for controlling the actin cytoskeleton [
22]. MLC2 phosphorylation is required for numerous cellular processes including cellular morphogenesis, cell movement, smooth muscle contraction, cytokinesis, and tumor cell invasion [
22,
23]. MLC2 is phosphorylated, primarily at serine 19, but also threonine 18, by a number of protein kinases, including Myosin Light Chain Kinase (MLCK), Rho-Associated Protein Kinase (ROCK), MRCKs, Integrin-linked kinase (ILK), and Zipper-Interacting Protein Kinase (ZIPK) [
24]. In smooth muscle MLC2 is most often regulated by calcium-dependent pathways that leads to MLCK activation or by the RhoA-ROCK pathway [
25]. ZIPK is a major regulator of calcium-independent smooth muscle contraction and has been shown to be an effector of ROCK activity in both smooth muscle and non-muscle cells [
14]. The dephosphorylation of both sites on MLC2 is mediated by a highly conserved Myosin Phosphatase (MP) complex consisting of a targeting subunit Mypt1 and the catalytic subunit Protein Phosphatase 1 Beta (PP1β) [
26]. MLC2 kinases and phosphatases are in turn precisely regulated by reversible phosphorylation in response to a variety of signaling pathways [
24]. Importantly, not only do ROCK and ZIPK phosphorylate MLC2, but they also phosphorylate and inhibit the myosin phosphatase [
26].
While many aspects of ZIPK function are highly conserved, ZIPK from murine species have diverged significantly in both its
C-terminus and in its mechanism of regulation [
18]. Rat and mouse ZIPK are localized primarily in the nucleus and require a binding partner PAR-4 to exit the nucleus. Previous work has indicated that the zebrafish ortholog may actually be more functionally similar to the human ortholog than are the murine orthologs [
18]. In this work, we undertake a comparative biochemical approach to better understand the conservation of function of ZIPK, with the primary focus on the zebrafish ortholog. The amino acid sequence alignment of zebrafish ZIPK with mouse and human reveals a high level of sequence homology between the orthologs (
Figure 1). Importantly, critical regulatory phosphorylation sites are conserved in the zebrafish ortholog (
Figure 1). Mammalian ZIPK requires phosphorylation of threonines 180, 225, and 265 for maximal kinase activation, each of these sites is conserved in zebrafish ZIPK (indicated by a * in
Figure 1). In the human ZIPK phosphorylation of threonines 299 and 300 mediates nuclear export and is required for regulation of the cytoskeleton. Threonine 299 and 300 are conserved in zebrafish ZIPK, but are replaced by alanine in both mouse and rat ZIPK (indicated by a # in
Figure 1).
Figure 1.
Conservation of regulatory domains of zipper-interacting protein kinase (ZIPK) homologs. A protein sequence alignment of zebrafish ZIPK with human and rat ZIPK; The amino acids marked with a * are phosphorylation sites required for kinase activation in the human ortholog; Amino acids marked with a # are phosphorylation sites that modulate nucleo-cytoplasmic shuttling in the human ortholog; Numbered regions indicate the four conserved nuclear localization sequences; LZ indicates the leucine zipper; amino acids N-terminal to the V are the kinase domain; accession numbers are available in the experimental section.
Figure 1.
Conservation of regulatory domains of zipper-interacting protein kinase (ZIPK) homologs. A protein sequence alignment of zebrafish ZIPK with human and rat ZIPK; The amino acids marked with a * are phosphorylation sites required for kinase activation in the human ortholog; Amino acids marked with a # are phosphorylation sites that modulate nucleo-cytoplasmic shuttling in the human ortholog; Numbered regions indicate the four conserved nuclear localization sequences; LZ indicates the leucine zipper; amino acids N-terminal to the V are the kinase domain; accession numbers are available in the experimental section.
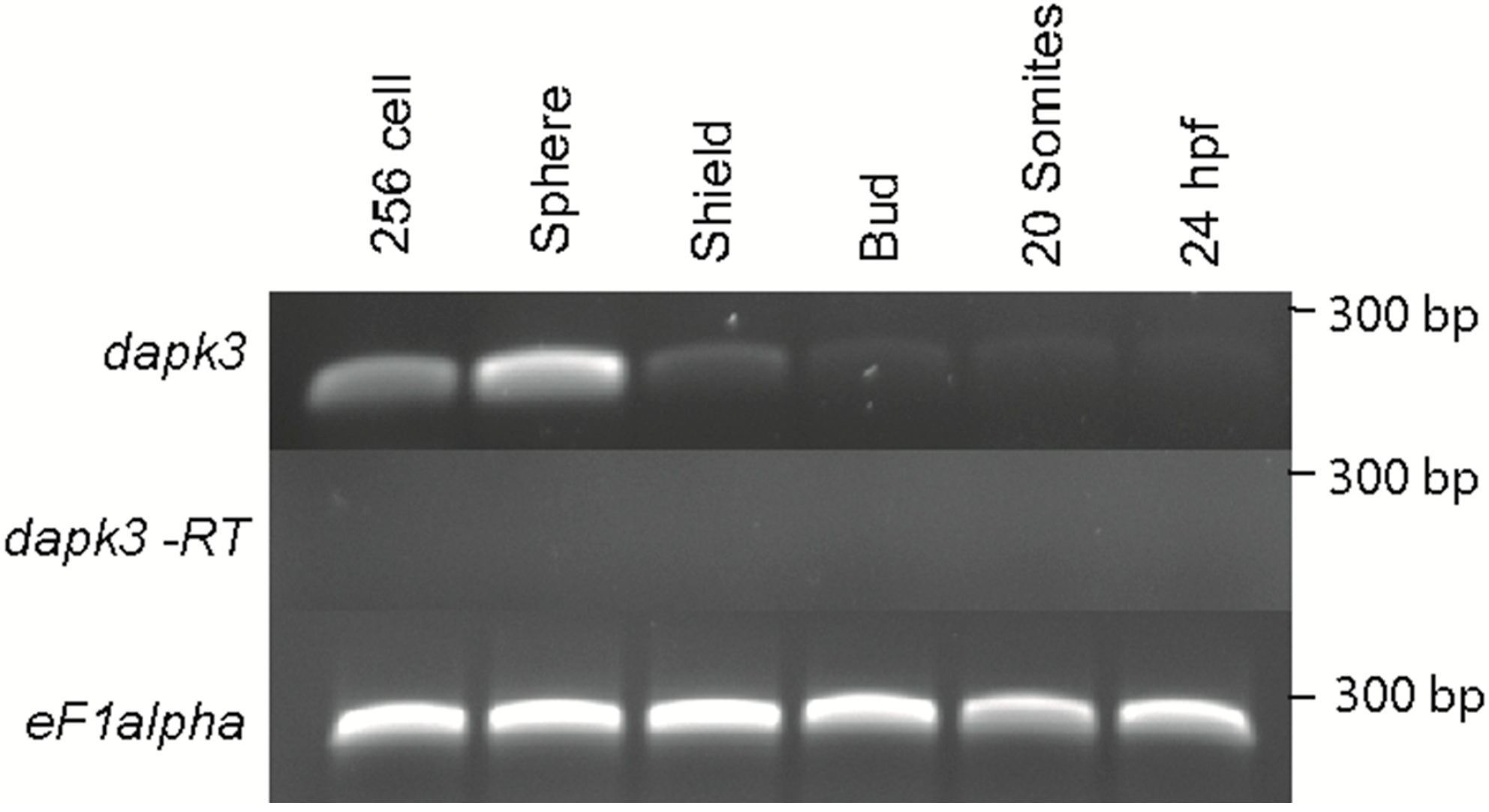
Given the critical role of regulators of MLC2 in early development in zebrafish we set out to determine if zebrafish
zipk is expressed during developmental stages where MLC2 and its regulators are expressed. In order to determine the spatiotemporal expression of zebrafish
zipk, the cDNA for
zipk was used to produce probes for
in situ hybridization and the level of expression was confirmed with semi-quantitative RT-PCR. The
zipk mRNA was expressed maternally and zygotically (
Figure 2A) and was ubiquitously during the 256 cell stage and sphere stages (
Figure 2A and
Figure 3). After the midblastula transition
zipk expression appeared to be lower, with reduced expression at shield stage (
Figure 2A), bud stage (
Figure 2A), the 20 somite stage and 24 hpf (
Figure 2A). The expression pattern of
zipk mRNA was confirmed using two alternate primer pairs (data not shown). Quantification of
zipk levels using real-time PCR showed that maternal expression and zygotic expression levels were statistically different and that levels dropped an average of 15-fold from sphere to shield stage (
Figure S1). No statistically significant difference was observed between each maternal stage expression levels or between each zygotic stage levels (
Figure S1). Until mid-somite stages
zipk appeared to be ubiquitously expressed and showed no visible spatial localization (
Figure 3A–F). During late somitogenesis
zipk became enriched anteriorly (
Figure 3G). After 24 hpf
zipk expression was sufficiently low that it was difficult to distinguish from background in
in situ analysis (data not shown). A sense control did not show background staining at any of the tested stages (256 cell stage in
Figure 3B, others not shown). Other key regulators of actomyosin contractility, such as the rho-dependent kinase
rock2 [
27], the myosin phosphatase targeting subunit
mypt1 [
28], and catalytic subunits
ppp1ca and
ppp1cb [
29] are expressed ubiquitously, but are also abundant in both zygotically and maternally.
Figure 2.
Temporal expression of zipk (dapk3) during early zebrafish development. Gene specific primers were used to detect zipk (286 bp) in various stages of development by RT-PCR. The zipk mRNA was expressed maternally and zygotically throughout early development. Amplification of eF1 alpha and total RNA without addition of reverse transcriptase were used as controls.
Figure 2.
Temporal expression of zipk (dapk3) during early zebrafish development. Gene specific primers were used to detect zipk (286 bp) in various stages of development by RT-PCR. The zipk mRNA was expressed maternally and zygotically throughout early development. Amplification of eF1 alpha and total RNA without addition of reverse transcriptase were used as controls.
Figure 3.
Spatial expression of zipk (dapk3) during early zebrafish development. Detection of zipk mRNA (dapk3) was carried out by whole-mount in situ hybridization using gene-specific probes on staged embryos at the 256 cells stage (A); sphere stage (C); shield stage (D); bud stage ((E) lateral view and (F) dorsal view); 20 somite stage (G); Negative control sense probes for zipk did not show staining at the 256 cell stage (B) or other stages (data not shown). Because of the difference in expression maternal stages (A–C) were developed for a shorter time than zygotic stages (D–G).
Figure 3.
Spatial expression of zipk (dapk3) during early zebrafish development. Detection of zipk mRNA (dapk3) was carried out by whole-mount in situ hybridization using gene-specific probes on staged embryos at the 256 cells stage (A); sphere stage (C); shield stage (D); bud stage ((E) lateral view and (F) dorsal view); 20 somite stage (G); Negative control sense probes for zipk did not show staining at the 256 cell stage (B) or other stages (data not shown). Because of the difference in expression maternal stages (A–C) were developed for a shorter time than zygotic stages (D–G).
2.2. Zebrafish ZIPK Regulates the Actin Cytoskeleton by Increasing Type-II Myosin (MLC2) Phosphorylation
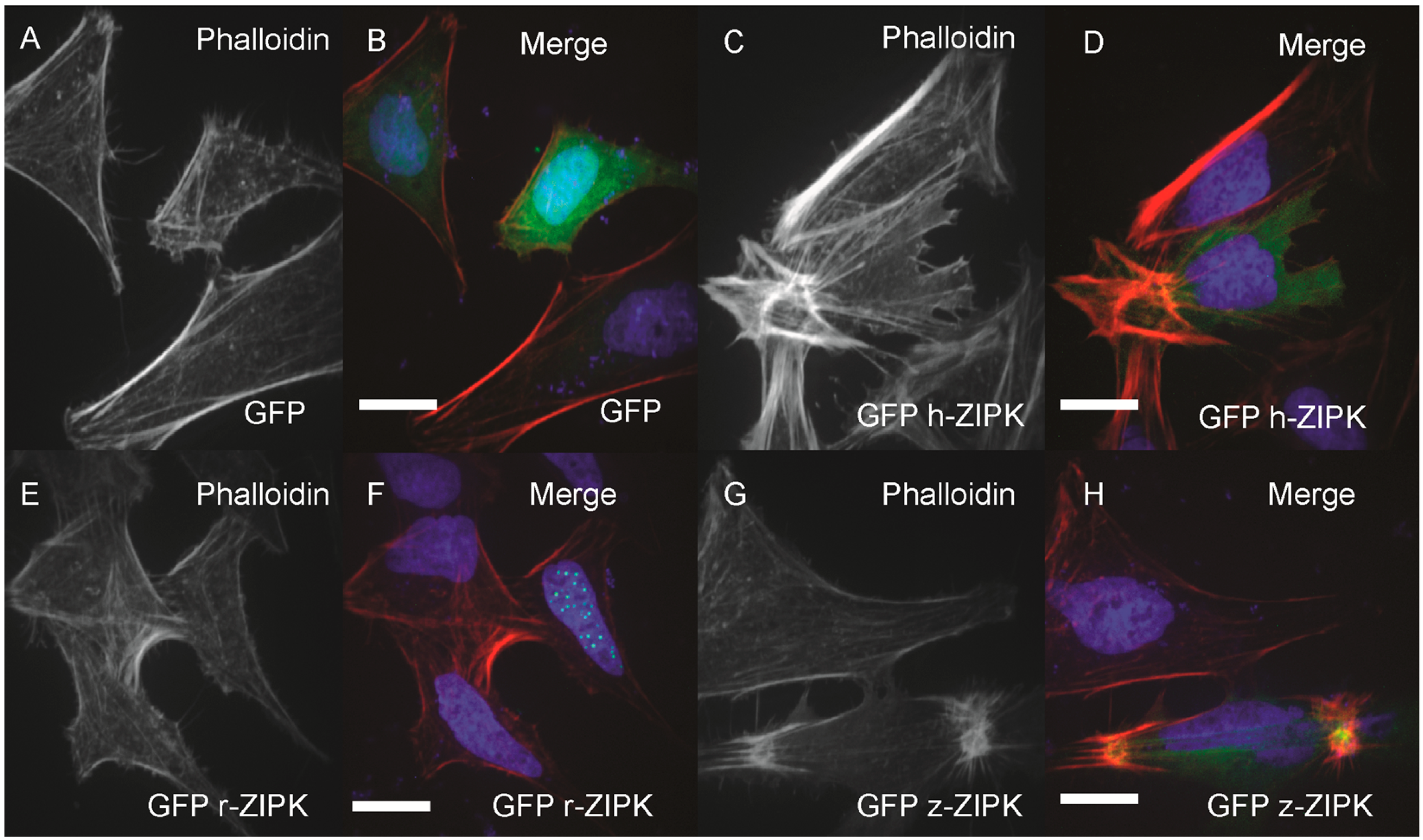
In order to examine the evolutionary conservation of ZIPK regulatory mechanisms we turned to heterologous expression in HeLa cells. Using the HeLa cells we could simultaneously analyze the effect on the actin cytoskeleton and take note of the subcellular localization of ZIPK. HeLa cells were transfected with either a vector-only GFP control, or GFP-tagged versions of the human, rat, or zebrafish orthologs of ZIPK. The cells were then fixed and stained with phalloidin to mark the actin cytoskeleton and DAPI to stain the nucleus. Heterologously expressed GFP was localized throughout the cell with noticeable enrichment in the nucleus, but resulted in no detectible change in the actin cytoskeleton relative to untransfected cells (
Figure 4A,B; quantification in
Figure S2). Expression of the human ortholog of ZIPK resulted in the accumulation of excessive as well as highly focused and disorganized stress fiber bundles (
Figure 4C,D; quantification in
Figure S2). In contrast, expression of the rat ZIPK resulted in accumulation of ZIPK in a punctate pattern in the nucleus and no detectible change in the actin cytoskeleton (
Figure 4E,F; quantification in
Figure S2). Zebrafish ZIPK was present in both the cytoplasm and nucleus and also resulted in an accumulation of excessive and highly focused stress fibers (
Figure 4G,H; quantification in
Figure S2). Similar results have been noted when a number of MLC2 kinases are overexpressed [
14,
30,
31].
Figure 4.
Differential regulation of the actin cytoskeleton by ZIPK homologs. HeLa cells were transfected with either GFP alone (A, B); human GFP-ZIPK (C, D); rat GFP-ZIPK (E, F); zebrafish GFP-ZIPK (G, H). All cells were fixed and stained with DAPI and Alexa 568-phalloidin and imaged with confocal microscopy. Black and white images show phalloidin staining, while color images are a merge of DAPI (blue), GFP (green) and phalloidin (red). Each experiment was replicated a minimum of five times with at least 25 cells assayed per experiment. White bar indicates 20 μm.
Figure 4.
Differential regulation of the actin cytoskeleton by ZIPK homologs. HeLa cells were transfected with either GFP alone (A, B); human GFP-ZIPK (C, D); rat GFP-ZIPK (E, F); zebrafish GFP-ZIPK (G, H). All cells were fixed and stained with DAPI and Alexa 568-phalloidin and imaged with confocal microscopy. Black and white images show phalloidin staining, while color images are a merge of DAPI (blue), GFP (green) and phalloidin (red). Each experiment was replicated a minimum of five times with at least 25 cells assayed per experiment. White bar indicates 20 μm.
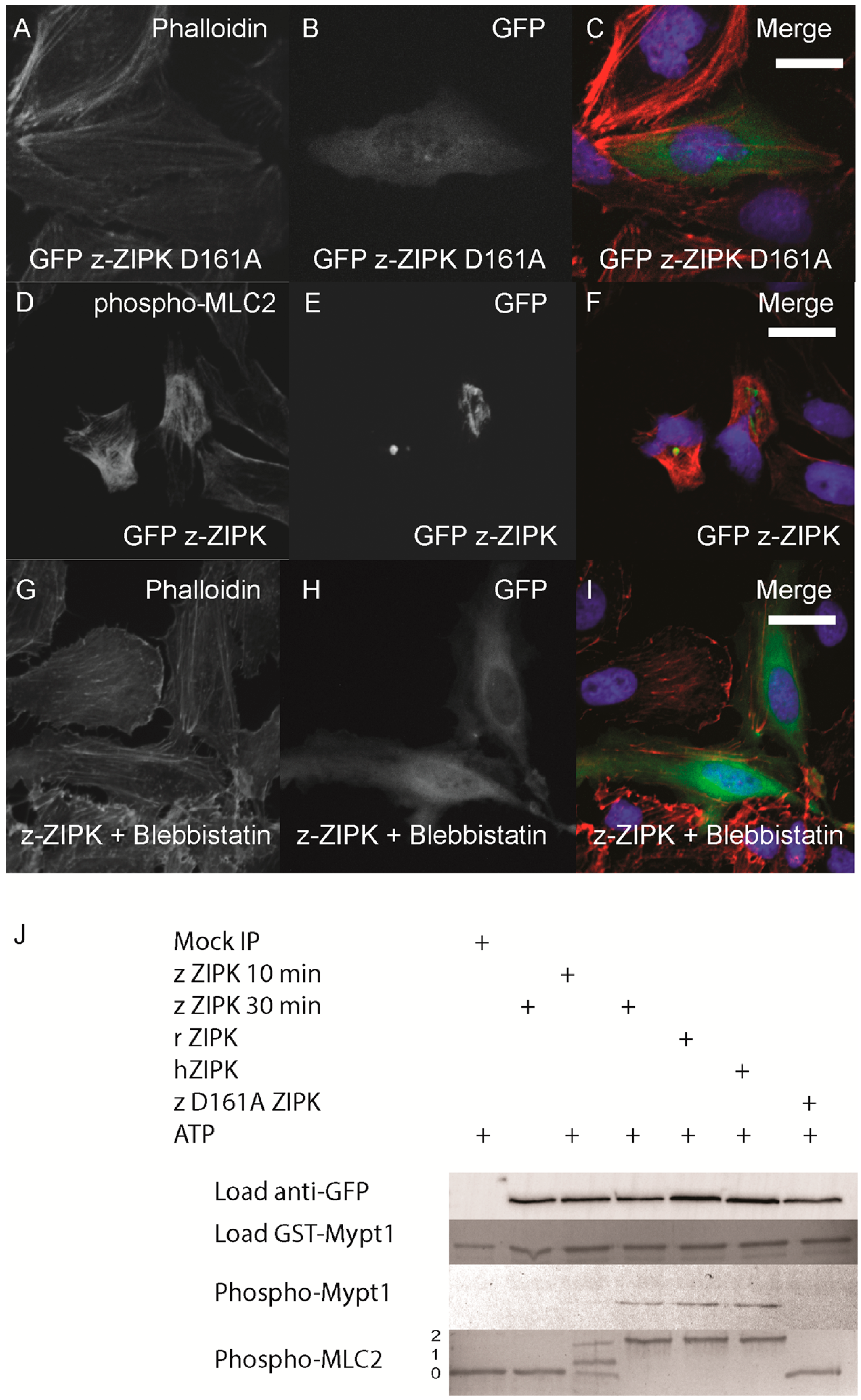
The cytoskeletal phenotype of ZIPK in mammals is mediated by increasing phosphorylation of MLC2 either by direct phosphorylation or indirectly by inhibition of the myosin phosphatase or other MLC2 regulators. We next sought to determine if the zebrafish ortholog functions on the cytoskeleton in a conserved manner. In the human ortholog, mutation of D161 to alanine results in a kinase dead version of ZIPK [
16]. We generated the same mutation in the zebrafish ortholog. Expression of the D161A mutant version of zebrafish ZIPK in HeLa cells resulted in no observable change in the actin cytoskeleton, indicating that kinase activity is required for the observed cellular phenotype (
Figure 5A–C; quantified in
Figure S2). In addition, immunostaining cells transfected with zebrafish ZIPK using an anti-phospho-MLC2 antibody showed a noticeable increase in the intensity of MLC2 phosphorylation (
Figure 5D–F) Similarly, expression of the human ortholog increased MLC2 staining, but not the rat ortholog or the kinase-dead zebrafish ZIPK (data not shown). Treatment of transfected cells with 50 μM blebbistatin (a type II myosin inhibitor) for two hours resulted in a dissolution of the excess stress fibers in zebrafish ZIPK transfected cells (
Figure 5G–I). Blebbistatin blocks myosin activity even if MLC2 is phosphorylated, indicating that the ZIPK stress fiber phenotype is indeed MLC2 dependent. Finally, we carried out
in vitro phosphorylation assays with ZIPK orthologs immunoprecipitated from HEK293T cell lysates. The three orthologs, but not the kinase dead zebrafish ZIPK mutant, were able to phosphorylate two of the primary ZIPK substrates: the
C-terminus of Mypt1 and MLC2 (
Figure 5J) and showed no statistically significant difference in activity against MLC2 (
Figure S3). Together these results provide direct evidence that the increased and disorganized stress fibers induced by ZIPK overexpression is caused by excess MLC2 phosphorylation. One of the unique regulatory mechanisms of the murine ZIPKs is the requirement to bind PAR-4 in order to exit the nucleus and regulate the cytoskeleton. We cloned the zebrafish ortholog of
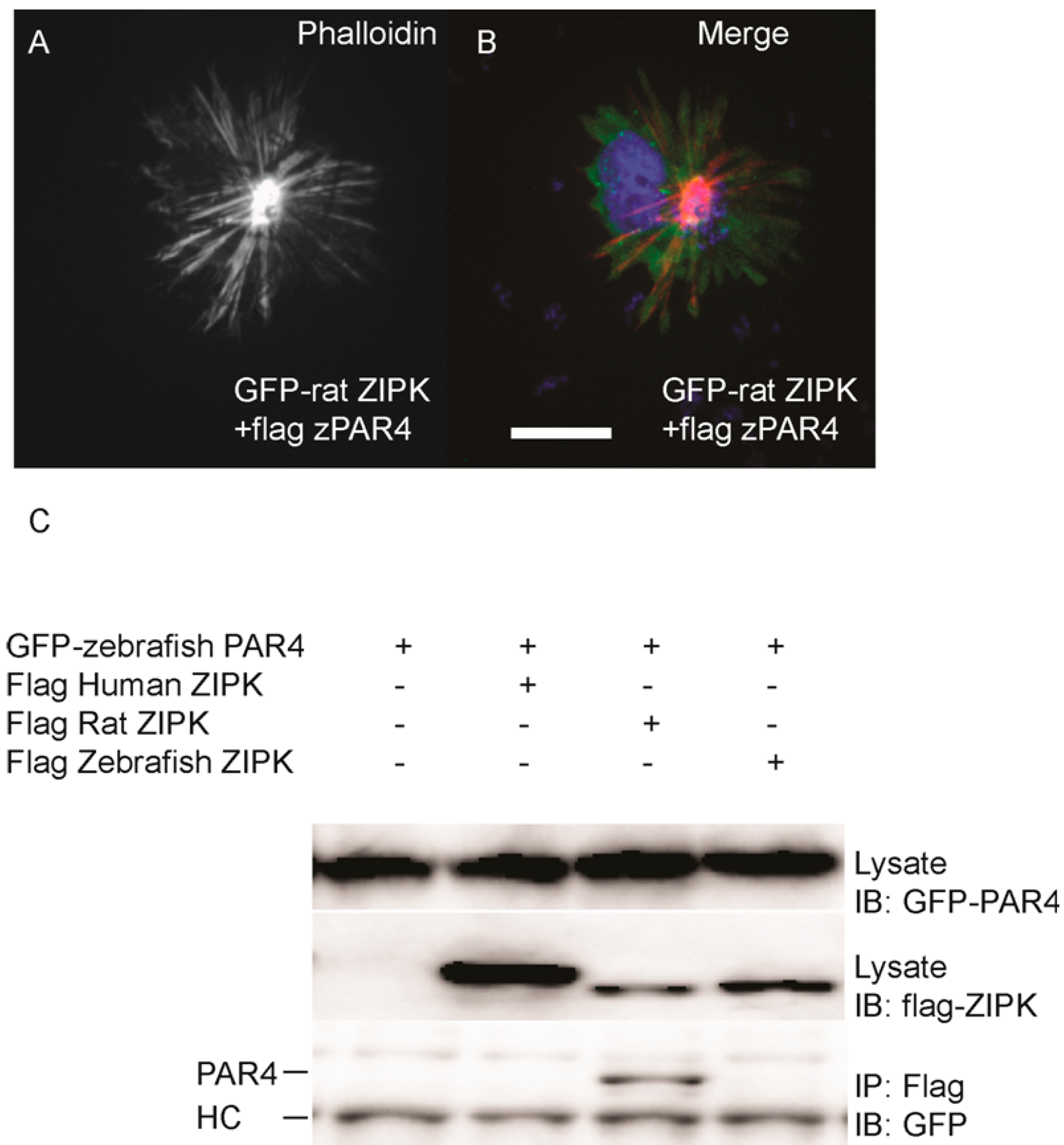
par-4 (
pawl) and sought to determine if it had any ability to regulate the zebrafish ZIPK. To confirm that zebrafish PAR-4’s ability to regulate ZIPK was conserved we co-expressed flag-tagged PAR-4 with rat GFP-ZIPK. In contrast to when rat ZIPK was expressed alone (
Figure 4), when co-expressed with PAR-4 the rat ZIPK exited the nucleus and resulted in a large scale rearrangement of the actin cytoskeleton, causing brightly staining and highly focused stress fibers (
Figure 6A and
Figure 6B; quantified in
Figure S2). Protein complexes were immunoprecipitated from HEK 293T cells co-expressing GFP-tagged PAR-4 and flag-tagged versions of ZIPK using an anti-flag antibody. The rat ortholog of ZIPK bound zebrafish PAR-4, but, neither the human, nor the zebrafish ZIPK showed any interaction with PAR-4 (
Figure 6C), consistent with studies of the human PAR-4 [
18]. Similar results were observed when cells were immunoprecipitated with GFP antibodies and blotted with FLAG (data not shown). Thus, our findings are consistent with other observations [
18,
19] that PAR-4 binding is unique to murine orthologs of ZIPK.
Figure 5.
Zebrafish ZIPK controls the actin cytoskeleton by regulating type-II myosin (MLC2) phosphorylation. HeLa cells were transfected with a kinase dead D161A zebrafish GFP-ZIPK (A, B, C) and fixed and stained with DAPI and Alexa 568-phalloidin; HeLa cells were transfected with a zebrafish GFP-ZIPK (D, E, F) and immunostained using an anti-phospho myosin light chain 2 antibody, co-stained with DAPI; HeLa cells were treated with media containing either 0.1% DMSO (not shown) or 50 μM blebbistatin (G, H, I) for 4 h; Black and white images show phalloidin staining, while color images are a merge of DAPI (blue), GFP (green), and phalloidin (red). (J) Zebrafish, Rat and Human ZIPK immunoprecipitated from HEK 293T cells were used to phosphorylate purified GST-Mypt1 and GST-MLC2. Unphosphoryled (0), mono (1) and di-phosphorylation (2) MLC2 was detected by band shift using a phos-tag SDS-PAGE gel and stained with Coomassie. Phosphorylation of Mypt1 was detected using a phospho-specific antibody (T696). Each experiment was replicated a minimum of three times. White bar indicates a 20 μm scale bar.
Figure 5.
Zebrafish ZIPK controls the actin cytoskeleton by regulating type-II myosin (MLC2) phosphorylation. HeLa cells were transfected with a kinase dead D161A zebrafish GFP-ZIPK (A, B, C) and fixed and stained with DAPI and Alexa 568-phalloidin; HeLa cells were transfected with a zebrafish GFP-ZIPK (D, E, F) and immunostained using an anti-phospho myosin light chain 2 antibody, co-stained with DAPI; HeLa cells were treated with media containing either 0.1% DMSO (not shown) or 50 μM blebbistatin (G, H, I) for 4 h; Black and white images show phalloidin staining, while color images are a merge of DAPI (blue), GFP (green), and phalloidin (red). (J) Zebrafish, Rat and Human ZIPK immunoprecipitated from HEK 293T cells were used to phosphorylate purified GST-Mypt1 and GST-MLC2. Unphosphoryled (0), mono (1) and di-phosphorylation (2) MLC2 was detected by band shift using a phos-tag SDS-PAGE gel and stained with Coomassie. Phosphorylation of Mypt1 was detected using a phospho-specific antibody (T696). Each experiment was replicated a minimum of three times. White bar indicates a 20 μm scale bar.
![Ijms 15 11597 g005]()
Figure 6.
Differential interaction of ZIPK homologs with zebrafish PAR-4. HeLa cells were transfected with rat GFP-ZIPK and flag zebrafish PAR-4 (A, B). The cells were fixed and stained with DAPI and Alexa 568-phalloidin. Black and white images show phalloidin staining, while color images are a merge of DAPI (blue), GFP (green), and phalloidin (red).White bar indicates a 20 μm scale; (C) HEK293T cells were co-transfected with GFP-tagged zebrafish PAR-4 along with flag-tagged ZIPK from human, rat and zebrafish. Protein complexes were immunoprecipitated with anti-FLAG antibodies. The IPs were immunoblotted with anti-GFP antibodies and anti-FLAG antibodies. HC indicates the IgG heavy chain which is the same size as zebrafish and mouse ZIPK, while human is slightly heavier. Each experiment was repeated a minimum of three times.
Figure 6.
Differential interaction of ZIPK homologs with zebrafish PAR-4. HeLa cells were transfected with rat GFP-ZIPK and flag zebrafish PAR-4 (A, B). The cells were fixed and stained with DAPI and Alexa 568-phalloidin. Black and white images show phalloidin staining, while color images are a merge of DAPI (blue), GFP (green), and phalloidin (red).White bar indicates a 20 μm scale; (C) HEK293T cells were co-transfected with GFP-tagged zebrafish PAR-4 along with flag-tagged ZIPK from human, rat and zebrafish. Protein complexes were immunoprecipitated with anti-FLAG antibodies. The IPs were immunoblotted with anti-GFP antibodies and anti-FLAG antibodies. HC indicates the IgG heavy chain which is the same size as zebrafish and mouse ZIPK, while human is slightly heavier. Each experiment was repeated a minimum of three times.
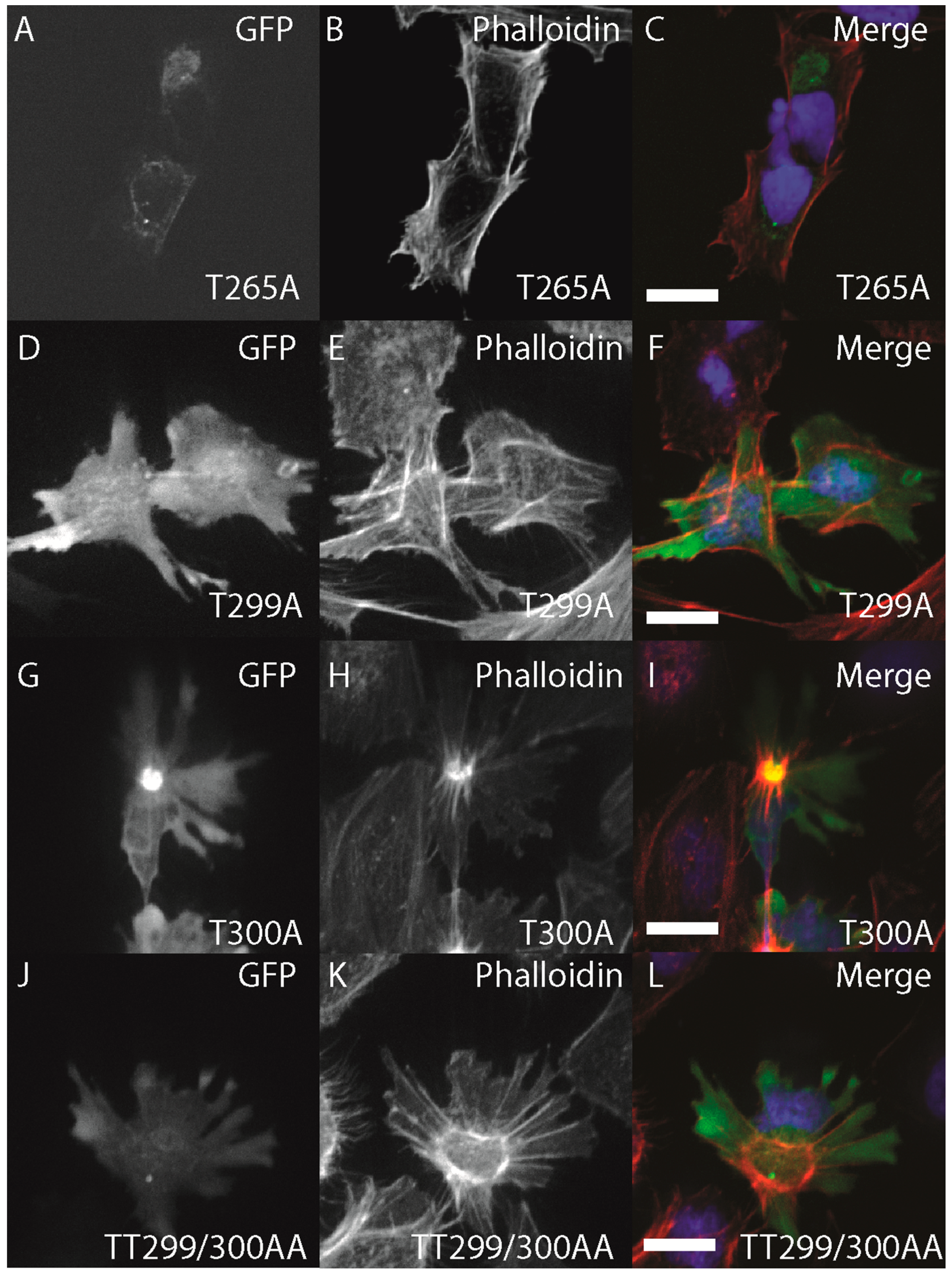
2.3. Conservation of the Structure-Function of Zebrafish ZIPK Activity and Nucleo-Cytoplasmic Shuttling
To characterize the conservation of ZIPK regulation by multi-site phosphorylation we used site-directed mutagenesis to generate a series of mutant zebrafish GFP-ZIPK lacking critical conserved phosphorylation sites. Mutation of T265 to alanine did not alter subcellular localization, but eliminated the ability of ZIPK to regulate the cytoskeleton (
Figure 7A–C; quantified in
Figure S2), indicating a conserved role of phosphorylation in the activation of zebrafish ZIPK. Mutation of T299A, T300A alone or in combination resulted in no observable change in localization or cytoskeletonal regulation (
Figure 7D–L; quantified in
Figure S2). Interestingly, expression of all three mutant ZIPK constructs resulted in accumulation of excess, focused stress fibers like the WT version (
Figure 7D–L; quantified in
Figure S2). The double mutant T299A/T300A ZIPK also increased immunocytochemical staining for phospho MLC2 (data not shown). This is in stark contrast to the human ZIPK, where mutation of T299 and T300 to alanines resulted in nuclear accumulation [
19].
Figure 7.
Structure-function of zebrafish ZIPK. HeLa cells were transfected with either GFP-ZIPK constructs generated by site-directed mutagenesis mutating a proposed activating phosphorylation site T265 or phosphorylation sites involved in controlling subcellular localization in the human paralog T299 and T300. All cells were fixed and stained with DAPI and Alexa 568-phalloidin. (A, D, G, J) show phalloidin staining; (B, E, H, K) show GFP; while color images (C, F, I, L) are a merge of DAPI (blue), GFP (green) and phalloidin (red). Each experiment was performed at least five times with a minimum of 25 cells assayed per experiment. White bar indicates 20 μm.
Figure 7.
Structure-function of zebrafish ZIPK. HeLa cells were transfected with either GFP-ZIPK constructs generated by site-directed mutagenesis mutating a proposed activating phosphorylation site T265 or phosphorylation sites involved in controlling subcellular localization in the human paralog T299 and T300. All cells were fixed and stained with DAPI and Alexa 568-phalloidin. (A, D, G, J) show phalloidin staining; (B, E, H, K) show GFP; while color images (C, F, I, L) are a merge of DAPI (blue), GFP (green) and phalloidin (red). Each experiment was performed at least five times with a minimum of 25 cells assayed per experiment. White bar indicates 20 μm.
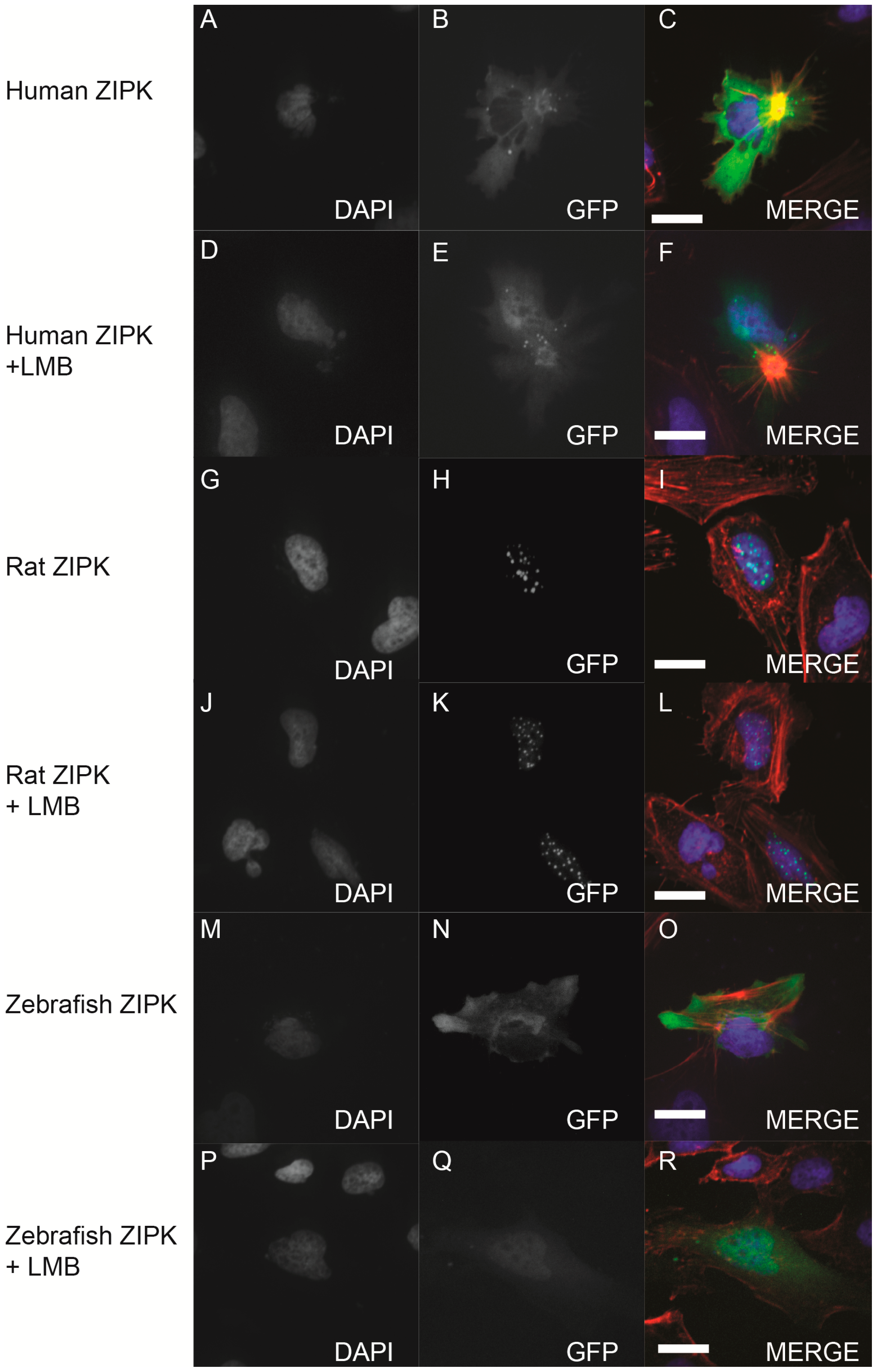
Given that the mutation of T299/T300 in zebrafish ZIPK had no apparent effect on activity or localization we set out to determine if the zebrafish ortholog like the mammalian orthologs undergoes nucleocytoplasmic shuttling. To determine this we expressed human, rat and zebrafish orthologs in HeLa cells and either treated with the nuclear export inhibitor leptomycin B or a DMSO control (
Figure 8). Rat ZIPK remained in the nucleus under either condition (
Figure 8G–L). In contrast, both the human (
Figure 8A–F) and the zebrafish ZIPK (
Figure 8M–R) accumulated in the nucleus after leptomycin B treatment, confirming that both shuttle in and out of the nucleus. In addition, the kinase dead D161A and the double mutant T299A/T300A behaved similarly to the WT zebrafish ortholog (data not shown). This raises the possibility that the subcellular localization of zebrafish ZIPK may be unregulated or regulated by a unique mechanism.
Figure 8.
Nucleo-cytoplasmic shuttling of zebrafish ZIPK. HeLa cells were transfected with either human GFP-ZIPK (A–F); rat GFP-ZIPK (G–L); or zebrafish GFP-ZIPK (M–R). All cells were fixed and stained with DAPI and Alexa 568-phalloidin. The cells in (A–C, G–I, M–O) were treated with 0.1% DMSO; while the cells in (D–F, J–L, P–R) were treated with 50 nM leptomycin B for four hours. (A, D, G, J, M, P) are stained with DAPI; (B, E, H, K, N, Q) show subcellular localization of the GFP-fusion protein, while color images are a merge of DAPI (blue), GFP (green), and phalloidin (red). Each experiment was repeated three times with a minimum of 25 cells assayed per experiment. White bar indicates 20 μm.
Figure 8.
Nucleo-cytoplasmic shuttling of zebrafish ZIPK. HeLa cells were transfected with either human GFP-ZIPK (A–F); rat GFP-ZIPK (G–L); or zebrafish GFP-ZIPK (M–R). All cells were fixed and stained with DAPI and Alexa 568-phalloidin. The cells in (A–C, G–I, M–O) were treated with 0.1% DMSO; while the cells in (D–F, J–L, P–R) were treated with 50 nM leptomycin B for four hours. (A, D, G, J, M, P) are stained with DAPI; (B, E, H, K, N, Q) show subcellular localization of the GFP-fusion protein, while color images are a merge of DAPI (blue), GFP (green), and phalloidin (red). Each experiment was repeated three times with a minimum of 25 cells assayed per experiment. White bar indicates 20 μm.
MLC2 phosphorylation has been found to regulate a variety of developmental processes in zebrafish. Knockdown of ROCK results reduced MLC2 phosphorylation, abnormal cell shape, disrupted cytokinesis, motility defects during gastrulation, reduced epithelial contraction, primordial cell migration and left right asymmetry defects [
27,
32,
33,
34]. Loss of myosin phosphatase activity in zebrafish results in hyper-contractile cells and causes defects in cell movements during gastrulation, failure of liver development, disorganized somites, and over-contractility of the neural epithelium leading to neural fold defects [
28,
29,
35,
36,
37]. Given the critical role of MLC2 phosphorylation in many crucial developmental processes and the broad expression pattern of
zipk it will be interesting to analyze loss-of-function experiments for
zipk during early developmental stages to determine if ZIPK regulates MLC2 dependent functions.