Two Diterpenoids and a Cyclopenta[c]pyridine Derivative from Roots of Salvia digitaloids
Abstract
:1. Introduction
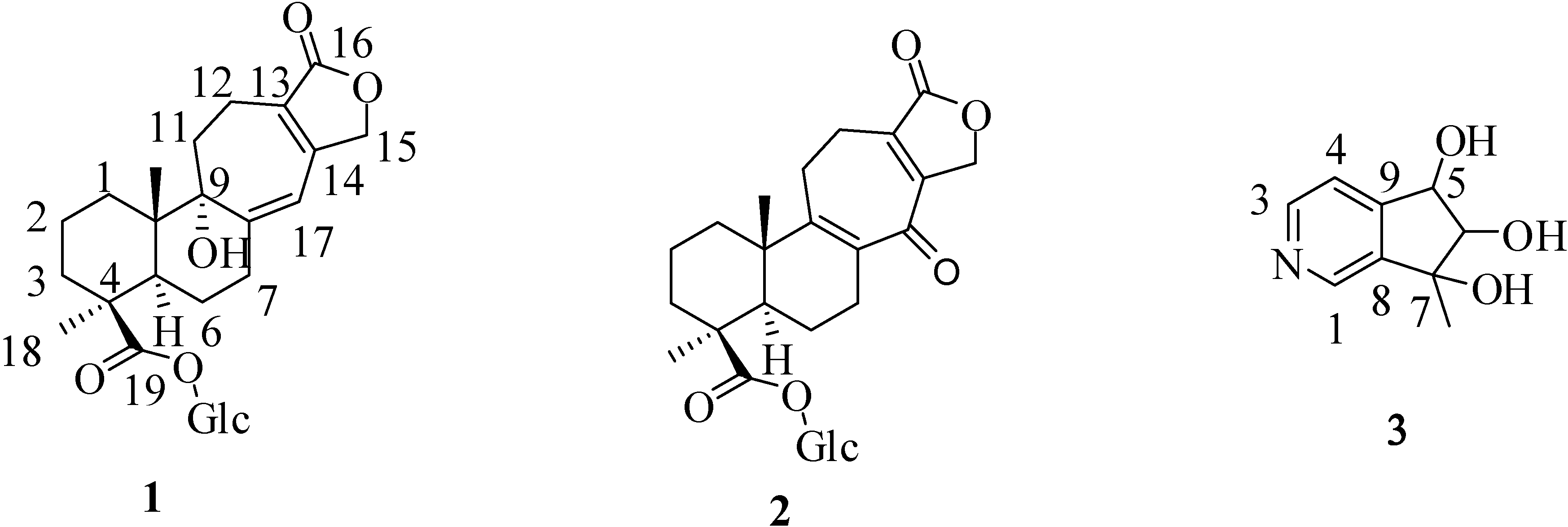
2. Results and Discussion
2.1. Purification and Characterization
2.2. Structural Elucidation of Compounds 1–3
 + 56.3, c 0.19, MeOH). The high resolution electrospray ionization mass spectroscopy (HRESI-MS) of 1 showed an ion peak at m/z 531.2209, which was consistent with the molecular formula of C26H36O10Na. This formula implied nine degrees of unsaturation. The UV spectrum of 1 showed absorption maxima at 220 and 283 nm, indicating the presence of α,β,γ,δ-unsaturated ketone moiety in the molecule. The IR spectrum showed strong absorption peaks for OH (3387 cm−1), carbonyl group (1728 cm−1) and furan ring (763 cm−1). The proton signals for an anomeric proton at δ 5.46 (d, J = 8.0 Hz), oxygenated methylene at δ 3.82 (1H, dd, J = 11.6, 1.4 Hz) and 3.69 (1H, dd, J = 11.6, 4.0 Hz), and oxygenated methines at δ 3.45–3.30 (4H, m) suggested the presence of one sugar moiety. In the 13C NMR spectrum (Table 1) of 1, the carbon resonances at δ 95.5, 74.1, 78.5, 71.1, 78.8, 62.4 further confirmed that 1 was substituted with one glucose [13]. Furthermore, the 13C NMR spectrum showed 20 remaining carbon signals, including a vinyl carbon (δ 117.0), three quaternary olefinic (δ 160.4, 157.4 and 129.9), two carbonyl (δ 177.8 and 176.7), two quaternary (δ 46.2 and 45.8), one oxygenated quaternary (δ 81.4), one methine (δ 49.0), eight methylene (δ 72.4, 38.7, 38.1, 36.3, 33.9, 26.5, 20.9, and 19.4), and two methyl (δ 29.6 and 16.8) carbons. These results were confirmed by the heteronuclear singular quantum correlation (HSQC) spectrum. The 1H NMR spectrum of 1 (Table 1) showed signals for two methyls (δ 1.32 and 0.79) and an oxygen-bearing methylene (H-15, δ 4.77), which are the typical lactone protons. The correlation spectroscopy (COSY) spectrum showed the following proton–proton cross-peaks: H-11 (δ 1.85 and 2.15) to H-12 (δ 2.30 and 2.46), H-6 (δ 2.04) to H-7 (δ 2.30 and 2.91)/H-5, (δ 2.25), and H-2 (δ 1.55, 1.96) to H-1 (δ 1.53, 1.79)/H-3 (δ 1.14, 2.19). The seven-membered C-ring was established by the correlations of H-11 (δ 1.85) with C-8 (δ 160.4), C-12 (δ 19.4) and C-13 (δ 129.9), of H-12 (δ 2.30, 2.46) with C-9 (δ 81.4), C-11 (δ 36.3), C-13 (δ 129.9) and C-14 (δ 157.4), and of H-17 (δ 6.05) with C-8 (δ 160.4), C-9 (δ 81.4) and C-13 (δ 129.9) in the heteronuclear multiple bond correlation (HMBC) spectrum (Table 1, Figure 1). The HMBC spectrum of 1 also showed the conjugated cross-peaks of H-15 (δ 4.77) to C-13 (δ 129.9)/C-14 (δ 157.4)/C-16 (δ 176.7), H-11 (δ 1.85) to C-8 (δ 160.4)/C-13 (δ 129.9) and H-17 (δ 6.05) to C-8 (δ 160.4)/C-9 (δ 81.4)/C-13 (δ 129.9)/C-15 (δ 72.4), indicating that 1 was a γ-lactone ring α,β-fused to a novel 6/6/7 tricyclic-skeleton diterpene containing two double bond conjugated with the carbonyl group. The 3J HMBC correlations of the methyl protons (H-18) at δ 1.32 with C-3 (δ 38.7), C-4 (δ 45.8), C-5 (δ 49.0), and C-19 (δ 177.8) indicated that the quaternary C-4 was substituted with both methyl and carboxylic acid groups. Moreover, the correlation of the anomeric proton at δ 5.46 with C-19 (δ 177.8) in HMBC spectrum suggested that the glucose was connected to the C-19 carboxylic acid group. In addition, the 3J HMBC correlations of the oxygenated quaternary carbon (C-9) at δ 81.4 with H-7 (δ 2.30), H-12 (δ 2.30), H-17 (δ 6.05), and H-20 (δ 0.79) showed that the C-9 was substituted with a hydroxyl group. The stereochemistry was confirmed by a nuclear overhauser enhancement spectroscopy (NOESY) experiment, which showed correlations of H-5/Me-18, H-5/H-6α, Me-20/H-2β, Me-20/H-6β, and Me-20/H-11β (Figure 2). Thus, the methyl substituents at C-4 and C-10 have the α- and β-orientation, respectively. Based on the above-mentioned observations, the structure of salviadigitoside A was assigned as 1.
+ 56.3, c 0.19, MeOH). The high resolution electrospray ionization mass spectroscopy (HRESI-MS) of 1 showed an ion peak at m/z 531.2209, which was consistent with the molecular formula of C26H36O10Na. This formula implied nine degrees of unsaturation. The UV spectrum of 1 showed absorption maxima at 220 and 283 nm, indicating the presence of α,β,γ,δ-unsaturated ketone moiety in the molecule. The IR spectrum showed strong absorption peaks for OH (3387 cm−1), carbonyl group (1728 cm−1) and furan ring (763 cm−1). The proton signals for an anomeric proton at δ 5.46 (d, J = 8.0 Hz), oxygenated methylene at δ 3.82 (1H, dd, J = 11.6, 1.4 Hz) and 3.69 (1H, dd, J = 11.6, 4.0 Hz), and oxygenated methines at δ 3.45–3.30 (4H, m) suggested the presence of one sugar moiety. In the 13C NMR spectrum (Table 1) of 1, the carbon resonances at δ 95.5, 74.1, 78.5, 71.1, 78.8, 62.4 further confirmed that 1 was substituted with one glucose [13]. Furthermore, the 13C NMR spectrum showed 20 remaining carbon signals, including a vinyl carbon (δ 117.0), three quaternary olefinic (δ 160.4, 157.4 and 129.9), two carbonyl (δ 177.8 and 176.7), two quaternary (δ 46.2 and 45.8), one oxygenated quaternary (δ 81.4), one methine (δ 49.0), eight methylene (δ 72.4, 38.7, 38.1, 36.3, 33.9, 26.5, 20.9, and 19.4), and two methyl (δ 29.6 and 16.8) carbons. These results were confirmed by the heteronuclear singular quantum correlation (HSQC) spectrum. The 1H NMR spectrum of 1 (Table 1) showed signals for two methyls (δ 1.32 and 0.79) and an oxygen-bearing methylene (H-15, δ 4.77), which are the typical lactone protons. The correlation spectroscopy (COSY) spectrum showed the following proton–proton cross-peaks: H-11 (δ 1.85 and 2.15) to H-12 (δ 2.30 and 2.46), H-6 (δ 2.04) to H-7 (δ 2.30 and 2.91)/H-5, (δ 2.25), and H-2 (δ 1.55, 1.96) to H-1 (δ 1.53, 1.79)/H-3 (δ 1.14, 2.19). The seven-membered C-ring was established by the correlations of H-11 (δ 1.85) with C-8 (δ 160.4), C-12 (δ 19.4) and C-13 (δ 129.9), of H-12 (δ 2.30, 2.46) with C-9 (δ 81.4), C-11 (δ 36.3), C-13 (δ 129.9) and C-14 (δ 157.4), and of H-17 (δ 6.05) with C-8 (δ 160.4), C-9 (δ 81.4) and C-13 (δ 129.9) in the heteronuclear multiple bond correlation (HMBC) spectrum (Table 1, Figure 1). The HMBC spectrum of 1 also showed the conjugated cross-peaks of H-15 (δ 4.77) to C-13 (δ 129.9)/C-14 (δ 157.4)/C-16 (δ 176.7), H-11 (δ 1.85) to C-8 (δ 160.4)/C-13 (δ 129.9) and H-17 (δ 6.05) to C-8 (δ 160.4)/C-9 (δ 81.4)/C-13 (δ 129.9)/C-15 (δ 72.4), indicating that 1 was a γ-lactone ring α,β-fused to a novel 6/6/7 tricyclic-skeleton diterpene containing two double bond conjugated with the carbonyl group. The 3J HMBC correlations of the methyl protons (H-18) at δ 1.32 with C-3 (δ 38.7), C-4 (δ 45.8), C-5 (δ 49.0), and C-19 (δ 177.8) indicated that the quaternary C-4 was substituted with both methyl and carboxylic acid groups. Moreover, the correlation of the anomeric proton at δ 5.46 with C-19 (δ 177.8) in HMBC spectrum suggested that the glucose was connected to the C-19 carboxylic acid group. In addition, the 3J HMBC correlations of the oxygenated quaternary carbon (C-9) at δ 81.4 with H-7 (δ 2.30), H-12 (δ 2.30), H-17 (δ 6.05), and H-20 (δ 0.79) showed that the C-9 was substituted with a hydroxyl group. The stereochemistry was confirmed by a nuclear overhauser enhancement spectroscopy (NOESY) experiment, which showed correlations of H-5/Me-18, H-5/H-6α, Me-20/H-2β, Me-20/H-6β, and Me-20/H-11β (Figure 2). Thus, the methyl substituents at C-4 and C-10 have the α- and β-orientation, respectively. Based on the above-mentioned observations, the structure of salviadigitoside A was assigned as 1.| Compound | 1 | 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | HMBC | δH (J in Hz) | δC | HMBC | |||
| 1 | β (eq): 1.53 m; | 33.9 | C-2, C-10, | α (ax): 1.26 td (13.9, 3.8) | 37.1 | C-2, C-20 | ||
| 2 | β (ax): 1.55 m; | 20.9 | C-3 | α (eq): 1.61 dq' (13.9, 3.8) | 20.5 | |||
| 3 | α (ax): 1.14 td (13.6, 3.2); | 38.7 | C-4, C-19 | α (ax): 1.12 td (13.9, 3.8) | 38.3 | C-2, C-4, | ||
| 4 | 45.8 | 45.3 | ||||||
| 5 | α (ax): 2.25 d (9.6) | 49.0 | C-4, C-6, | α (ax): 1.44 d (13.2) | 54.4 | C-4, C-6, C-7, | ||
| 6 | 2.04 m (2H) | 26.5 | C-7, C-8, C-10 | β (ax): 1.83 qd (13.2, 6.0) | 21.1 | C-5, C-7, | ||
| 7 | β (eq): 2.30 m; | 38.1 | C-9 | α (ax): 2.02 td (13.2, 6.0) | 29.0 | C-5, C-6, C-8, | ||
| 8 | 160.4 | 137.3 | ||||||
| 9 | 81.4 | 159.3 | ||||||
| 10 | 46.2 | 43.1 | ||||||
| 11 | β (ax): 1.85 t (13.6); | 36.3 | C-8, C-12, | 2.59 m (2H) | 27.4 | C-8, C-10, | ||
| 12 | α (ax): 2.30 m; | 19.4 | C-9, C-11, C-13, C-14, C-16 | α (ax): 2.40 m | 24.6 | C-9, C-13, | ||
| 13 | 129.9 | 139.9 | ||||||
| 14 | 157.4 | 154.1 | ||||||
| 15 | 4.77 m (2H) | 72.4 | C-13, C-14, | 4.84 dd (17.4, 2.4) | 71.4 | C-14, C-16 | ||
| 16 | 176.7 | 176.0 | ||||||
| 17 | 6.05 s | 117.0 | C-8, C-9, | 192.1 | ||||
| 18 | α (eq): 1.32 s | 29.6 | C-3, C-4, | α (eq): 1.31 s | 28.7 | C-3, C-4, | ||
| 19 | 177.8 | 177.6 | ||||||
| 20 | β (ax): 0.79 s | 16.8 | C-1, C-5, | β (ax): 1.04 s | 17.8 | C-1, C-5, | ||
| 1' | 5.46 d (8.0) | 95.5 | C-19 | 5.48 d (7.9) | 95.6 | C-19 | ||
| 2' | 3.30–3.45 m | 74.1 | 3.32–3.42 m | 74.2 | ||||
| 3' | 3.30–3.45 m | 78.5 * | 3.32–3.42 m | 78.4 | ||||
| 4' | 3.30–3.45 m | 71.1 | 3.32–3.42 m | 71.1 | ||||
| 5' | 3.30–3.45 m | 78.8 * | 3.32–3.42 m | 78.7 | ||||
| 6' | 3.69 dd (11.6, 4.0) | 62.4 | 3.68 dd (11.8, 4.0) | 62.4 | ||||
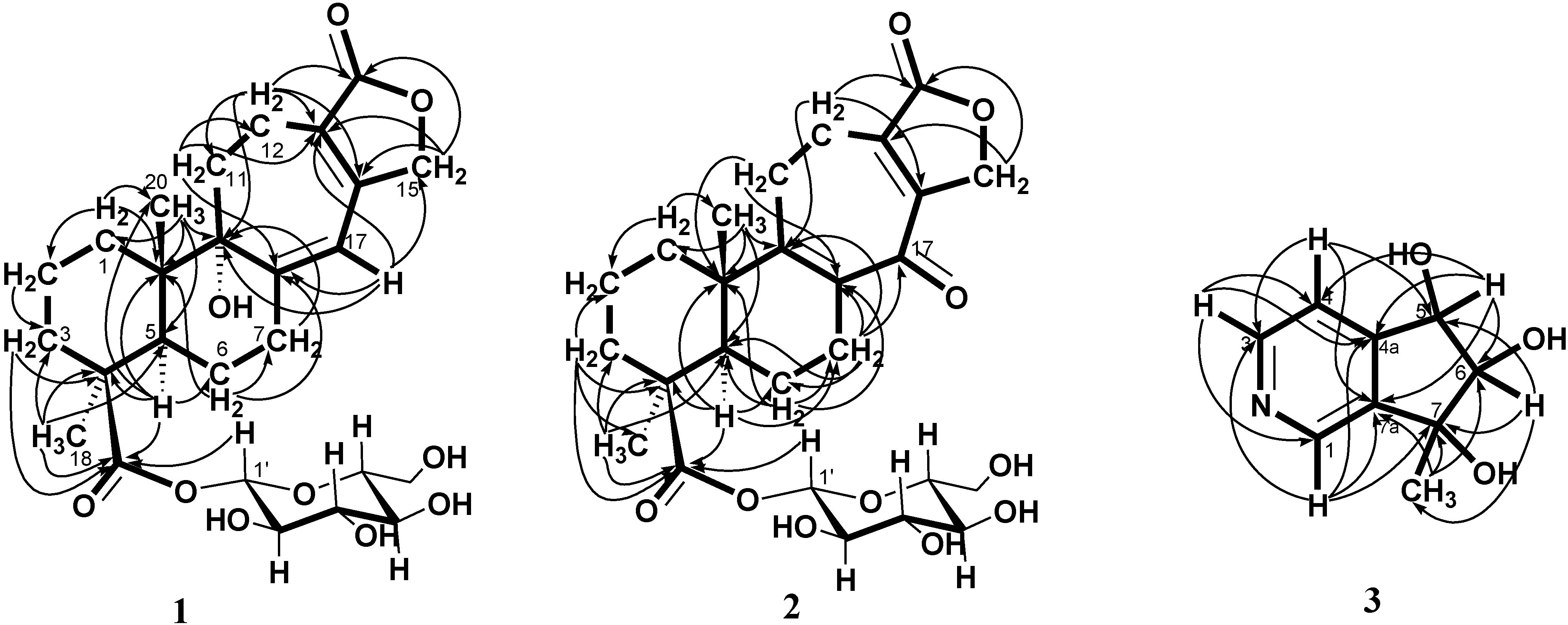
 + 107.8, c 0.47, MeOH). The HRESI-MS of 2 showed a ion peak at m/z 529.2053, consistent with the molecular formula of C26H34O10Na, implied ten degrees of unsaturation. The UV spectrum showed an absorption maximum at 251 and 313 nm, and the IR spectrum showed strong absorptions at 3383 and 1751 cm−1 for hydroxyl and carbonyl group in the molecule, respectively. The structure of 2 was similar to that of 1, according to the 1H NMR and 13C NMR spectral data (Table 1). The differences observed in the 1H NMR and 13C NMR spectra of 2 compared with those of 1 were occurrence of signals for C-8 and C-9 at δ 137.3 and 159.3 instead of δ 160.4 and 81.4, respectively. In addition, the H-17 signal in 2 disappeared and the carbonyl signal at δ 117.0 in 1 shifted to δ 192.1 in 2, suggesting that the seven-membered C-ring of 2 was formed the α,β-unsaturated ketone group. Therefore, the structure of salviatalinA-19-O-β-glucoside was assigned as 2, which was supported by COSY, HSQC, HMBC (Table 1, Figure 1), and NOESY (Figure 3) experiments.
+ 107.8, c 0.47, MeOH). The HRESI-MS of 2 showed a ion peak at m/z 529.2053, consistent with the molecular formula of C26H34O10Na, implied ten degrees of unsaturation. The UV spectrum showed an absorption maximum at 251 and 313 nm, and the IR spectrum showed strong absorptions at 3383 and 1751 cm−1 for hydroxyl and carbonyl group in the molecule, respectively. The structure of 2 was similar to that of 1, according to the 1H NMR and 13C NMR spectral data (Table 1). The differences observed in the 1H NMR and 13C NMR spectra of 2 compared with those of 1 were occurrence of signals for C-8 and C-9 at δ 137.3 and 159.3 instead of δ 160.4 and 81.4, respectively. In addition, the H-17 signal in 2 disappeared and the carbonyl signal at δ 117.0 in 1 shifted to δ 192.1 in 2, suggesting that the seven-membered C-ring of 2 was formed the α,β-unsaturated ketone group. Therefore, the structure of salviatalinA-19-O-β-glucoside was assigned as 2, which was supported by COSY, HSQC, HMBC (Table 1, Figure 1), and NOESY (Figure 3) experiments.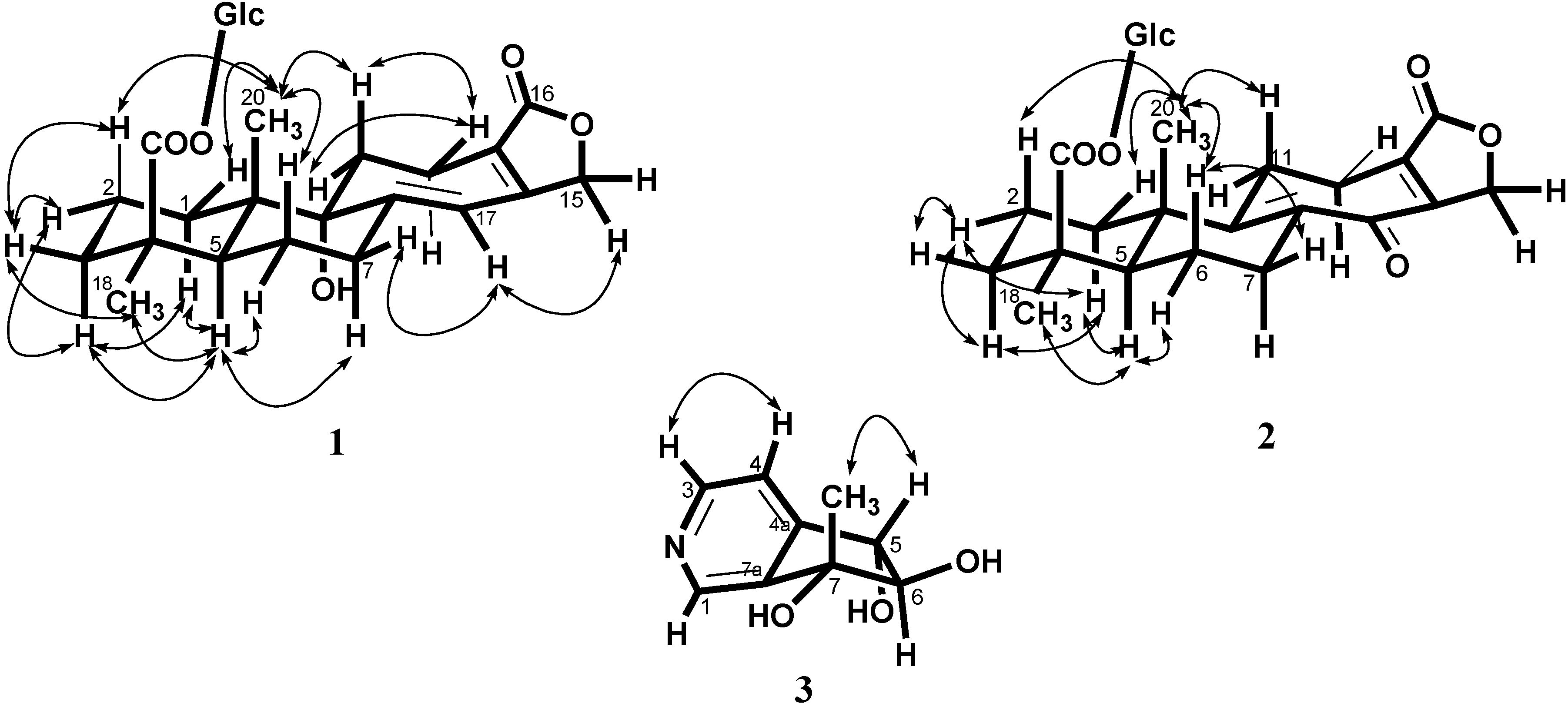
| Compound | 3 | ||
|---|---|---|---|
| δH (J in Hz) | δC | HMBC | |
| 1 | 8.53 s | 145.4 | C-3, C-4a, C-7, C-7a |
| 3 | 8.48 d (5.1) | 149.4 | C-1, C-4, C-4a |
| 4 | 7.43 d (5.1) | 120.4 | C-3, C-5, C-7a |
| 4a | 151.1 | ||
| 5 | 4.68 d (7.7) | 76.8 | C-4, C-4a, C-6, C-7a |
| 6 | 4.04 d (7.7) | 90.3 | C-5, C-7, 7-CH3 |
| 7 | 78.2 | ||
| 7-CH3 | 1.36 s | 23.1 | C-6, C-7, C-7a |
| 7a | 143.2 | ||
 + 15.5 (c 0.09, MeOH). The molecular formula, C9H11NO3, was established by HRESI-MS (182.0816 [M + H]+, calcd. for 182.0817), implying five degrees of unsaturation. The IR spectrum displayed absorption characteristic of hydroxyl and aromatic groups (3321 and 1608 cm−1), and the UV spectrum showed absorbance at λmax 259 nm. The presence of α,β,γ-disubstituted pyridine was revealed by 1H NMR signals at δ 7.43 (d, J = 5.1 Hz, H-4), 8.48 (d, J = 5.1 Hz, H-3) and 8.53 (s, H-1), together with 13C NMR signals at δ 120.4 (d), 143.2 (s), 145.4 (d), 149.4 (d), and 151.1 (s). The signals at δ 76.8 (d) and 90.3 (d) in the 13C NMR spectrum, as well as a resonance at δ 4.04 (1H, d, J = 7.7 Hz) and 4.68 (1H, d, J = 7.7 Hz) in the 1H NMR spectrum, provided evidence for the presence of the fragment –CH(OH)–CH(OH)– in 3. In the HMBC experiment (Table 2, Figure 1) of 3, correlations of H-5 (δ 4.68)/C-4 (δ 120.4), C-4a (δ 151.1), C-6 (δ 90.3), and C-7a (δ 143.2), and of the methyl group (δ 1.36)/C-6 (δ 90.3), C-7 (δ 78.2), and C-7a (δ 143.2) suggested that the planar structure of salviadiginine A was established as 3. The relative stereochemistry of three hydroxyls located toward pseudo equatorial orientation was due to the presence of nuclear overhauser effect (NOE) correlation between H-5 and 7-CH3 and the absence of NOE correlation between H-5/7-CH3 and H-6 in the NOESY spectrum (Figure 3). The absolute configuration of the 5,6,7-triol moiety was determined by application of the circular dichroic (CD) exciton chirality method [14]. The negative Cotton effect at 225 nm (Δε −1.19), the positive Cotton effect at 268 nm (Δε +2.19) and the negative Cotton effect at 291 nm (Δε −0.12) were correlated to the 5S, 6R, 7R configuration. Thus, compound 3 was determined to be (5S,6R,7R)-6,7-dihydro-7-methyl-5H-cyclopenta[c]pyridine-5α,6α,7α-triol.
+ 15.5 (c 0.09, MeOH). The molecular formula, C9H11NO3, was established by HRESI-MS (182.0816 [M + H]+, calcd. for 182.0817), implying five degrees of unsaturation. The IR spectrum displayed absorption characteristic of hydroxyl and aromatic groups (3321 and 1608 cm−1), and the UV spectrum showed absorbance at λmax 259 nm. The presence of α,β,γ-disubstituted pyridine was revealed by 1H NMR signals at δ 7.43 (d, J = 5.1 Hz, H-4), 8.48 (d, J = 5.1 Hz, H-3) and 8.53 (s, H-1), together with 13C NMR signals at δ 120.4 (d), 143.2 (s), 145.4 (d), 149.4 (d), and 151.1 (s). The signals at δ 76.8 (d) and 90.3 (d) in the 13C NMR spectrum, as well as a resonance at δ 4.04 (1H, d, J = 7.7 Hz) and 4.68 (1H, d, J = 7.7 Hz) in the 1H NMR spectrum, provided evidence for the presence of the fragment –CH(OH)–CH(OH)– in 3. In the HMBC experiment (Table 2, Figure 1) of 3, correlations of H-5 (δ 4.68)/C-4 (δ 120.4), C-4a (δ 151.1), C-6 (δ 90.3), and C-7a (δ 143.2), and of the methyl group (δ 1.36)/C-6 (δ 90.3), C-7 (δ 78.2), and C-7a (δ 143.2) suggested that the planar structure of salviadiginine A was established as 3. The relative stereochemistry of three hydroxyls located toward pseudo equatorial orientation was due to the presence of nuclear overhauser effect (NOE) correlation between H-5 and 7-CH3 and the absence of NOE correlation between H-5/7-CH3 and H-6 in the NOESY spectrum (Figure 3). The absolute configuration of the 5,6,7-triol moiety was determined by application of the circular dichroic (CD) exciton chirality method [14]. The negative Cotton effect at 225 nm (Δε −1.19), the positive Cotton effect at 268 nm (Δε +2.19) and the negative Cotton effect at 291 nm (Δε −0.12) were correlated to the 5S, 6R, 7R configuration. Thus, compound 3 was determined to be (5S,6R,7R)-6,7-dihydro-7-methyl-5H-cyclopenta[c]pyridine-5α,6α,7α-triol.2.3. Anti-Inflammatory Activity
| Compound | Superoxide Anion | Elastase Release |
|---|---|---|
| Inh % | Inh % | |
| 1 | 11.14 ± 3.27 * | 11.83 ± 6.06 |
| 2 | 9.35 ± 4.85 | 11.12 ± 2.76 * |
| 3 | 7.88 ± 5.53 | 2.83 ± 4.19 |
| LY294002 a | 1.0± 0.3 | 1.5± 0.4 |
3. Experimental Section
3.1. General
3.2. Plant Materials
3.3. Extraction and Isolation
3.1.1. Salviadigitoside A (1)
 + 56.3 (c 0.19, MeOH); UV (MeOH), λmax (log ε) 220 (3.60), 283 (3.90) nm; IR (KBr) νmax cm−1: 3387, 1728, 763 cm−1; 1H and 13C NMR see Table 1; electrospray ionization mass spectroscopy (ESI-MS) m/z (rel. int.): 531 [M + Na]+; HRESI-MS m/z: 531.2209 [M + Na]+ (calcd. for C26H36O10Na, 531.2206).
+ 56.3 (c 0.19, MeOH); UV (MeOH), λmax (log ε) 220 (3.60), 283 (3.90) nm; IR (KBr) νmax cm−1: 3387, 1728, 763 cm−1; 1H and 13C NMR see Table 1; electrospray ionization mass spectroscopy (ESI-MS) m/z (rel. int.): 531 [M + Na]+; HRESI-MS m/z: 531.2209 [M + Na]+ (calcd. for C26H36O10Na, 531.2206).3.1.2. SalviatalinA-19-O-β-glucoside (2)
 + 107.8 (c 0.47, MeOH ); UV (MeOH), λmax (log ε) 251 (3.92), 313 (3.51) nm; IR (KBr) νmax cm−1: 3383, 1751, 759 cm−1 ; 1H and 13C NMR see Table 1; ESI-MS m/z (rel. int.): 529 [M + Na]+; HRESI-MS m/z: 529.2053 [M + Na]+ (calcd. for C26H34O10Na, 529.2050).
+ 107.8 (c 0.47, MeOH ); UV (MeOH), λmax (log ε) 251 (3.92), 313 (3.51) nm; IR (KBr) νmax cm−1: 3383, 1751, 759 cm−1 ; 1H and 13C NMR see Table 1; ESI-MS m/z (rel. int.): 529 [M + Na]+; HRESI-MS m/z: 529.2053 [M + Na]+ (calcd. for C26H34O10Na, 529.2050).3.1.3. Salviadiginine A (3)
 + 15.5 (c 0.09, MeOH); UV (MeOH) λmax (log ε) nm: 259 (3.46), 265 (3.38) nm; IR (KBr) νmax cm−1: 3321 cm−1; 1H and 13C NMR see Table 2; ESI-MS m/z (rel. int.): 182 [M + H]+; HRESI-MS m/z: 182.0816 [M + H]+ (calcd. for C9H12NO3, 182.0817); CD (MeOH, 2.76 × 10−4 M) (Δε) 291 (−0.12), 268 (2.19), 225 (−1.19) nm.
+ 15.5 (c 0.09, MeOH); UV (MeOH) λmax (log ε) nm: 259 (3.46), 265 (3.38) nm; IR (KBr) νmax cm−1: 3321 cm−1; 1H and 13C NMR see Table 2; ESI-MS m/z (rel. int.): 182 [M + H]+; HRESI-MS m/z: 182.0816 [M + H]+ (calcd. for C9H12NO3, 182.0817); CD (MeOH, 2.76 × 10−4 M) (Δε) 291 (−0.12), 268 (2.19), 225 (−1.19) nm.3.2. Biological Assay
3.2.1. Preparation of Human Neutrophils
3.2.2. Measurement of Superoxide Anion Generation
3.2.3. Elastase Release Assays.
4. Conclusions

Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, C.Y.; Li, H.W. On the evolution and distribution in Labeatae. Acta Bot. Yunnan 1982, 4, 97–118. [Google Scholar]
- Ryu, S.Y.; Lee, C.O.; Choi, S.U. In vitro cytotoxicity of tanshinones from Salvia miltiorrhiza. Planta Medica 1997, 63, 339–342. [Google Scholar]
- Ryu, S.Y.; Oak, M.H.; Kim, K.M. Inhibition of mast cell degranulation by tanshinones from the roots of Salvia miltiorrhiza. Planta Medica 1999, 65, 654–655. [Google Scholar]
- Cao, E.H.; Liu, X.Q.; Wang, J.-J.; Xu, N.-F. Effect of natural antioxidant tanshinone II-A on DNA damage by lipid peroxidation in liver cells. Free Radic. Biol. Med. 1996, 20, 801–806. [Google Scholar]
- Honda, G.; Koezuka, Y.; Tabata, M. Isolation of an antidermatophytic substance from the root of Salvia miltiorrhiza. Chem. Pharm. Bull. 1988, 36, 408–411. [Google Scholar]
- Lin, H.C.; Ding, H.Y.; Chang, W.L. Two new fatty diterpenoids from Salvia miltiorrhiza. J. Nat. Prod. 2001, 64, 648–650. [Google Scholar]
- Meng, L.H.; Zhang, J.S.; Ding, J. Salvicine, a novel DNA topoisomerase II inhibitor, exerting its effects by trapping enzyme-DNA cleavage complexes. Biochem. Pharmacol. 2001, 62, 733–741. [Google Scholar]
- Munro, T.A.; Rizzacasa, M.A.; Roth, B.L.; Toth, B.A.; Yan, F. Studies toward the pharmacophore of Salvinorin A, a potent κ opioid receptor agonist. J. Med. Chem. 2005, 48, 345–348. [Google Scholar]
- Wang, X.H.; Bastow, K.F.; Sun, C.M.; Lin, Y.L.; Yu, H.J.; Don, M.J.; Wu, T.S.; Nakamura, S.; Lee, K.H. Antitumor agents 239. Isolation, structure elucidation, total synthesis, and anti-breast cancer activity of neo-tanshinlactone from Salvia miltiorrhiza. J. Med. Chem. 2004, 47, 5816–5819. [Google Scholar]
- Lin, F.W.; Damu, A.G.; Wu, T.S. Abietane diterpene alkaloids from Salvia yunnanensis. J. Nat. Prod. 2006, 69, 93–96. [Google Scholar]
- Wu, S.J.; Chan, H.H.; Hwang, T.L.; Qian, K.; Morris-Natschke, S.; Lee, K.H.; Wu, T.S. Salviatalin A and salvitrijudin A, two diterpenes with novel skeletons from roots of Salvia digitaloides and anti-inflammatory evaluation. Tetrahedron Lett. 2010, 51, 4287–4290. [Google Scholar]
- Xu, G.; Yang, J.; Wang, Y.Y.; Peng, L.Y.; Yang, X.W.; Pan, Z.H.; Liu, E.D.; Li, Y.; Zhao, Q.S. Diterpenoid constituents of the roots of Salvia digitaloides. J. Agric. Food. Chem. 2010, 58, 12157–12161. [Google Scholar]
- Matsuda, H.; Nishida, N.; Yoshikawa, M. Antidiabetic principles of natural medicines. V. (1) Aldose reductase inhibitors from Myrcia multiflora DC. (2): Structures of myrciacitrins III, IV, and V. Chem. Pharm. Bull. 2002, 50, 429–431. [Google Scholar]
- Ohba, M.; Izuta, R.; Shimizu, E. A new access to cyclopenta[c]pyridine ring system: Syntheses of (−)-plectrodorine and (+)-oxerine. Tetrahedron Lett. 2000, 41, 10251–10255. [Google Scholar]
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Investig. 2000, 80, 617–653. [Google Scholar]
- Borregaard, N. The human neutrophil. Function and dysfunction. Eur. J. Haematol. 1998, 41, 401–413. [Google Scholar]
- Roos, D.; van Bruggen, R.; Meischl, C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003, 5, 1307–1315. [Google Scholar]
- Faurschou, M.; Borregaard, N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003, 5, 1317–1327. [Google Scholar]
- Yang, S.C.; Chung, P.J.; Ho, C.M.; Kuo, C.Y.; Hung, M.F.; Huang, Y.T.; Chang, W.Y.; Chang, Y.W.; Chan, K.H.; Hwang, T.L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973, 52, 741–744. [Google Scholar]
- Hwang, T.L.; Li, G.L.; Lan, Y.H.; Chia, Y.C.; Shieh, P.W.; Wu, Y.H.; Wu, Y.C. Potent inhibition of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii. Free Radic. Biol. Med. 2009, 46, 520–528. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, S.-J.; Huang, C.-H.; Chan, Y.-Y.; Liao, Y.-R.; Hwang, T.-L.; Wu, T.-S. Two Diterpenoids and a Cyclopenta[c]pyridine Derivative from Roots of Salvia digitaloids. Int. J. Mol. Sci. 2014, 15, 11566-11577. https://doi.org/10.3390/ijms150711566
Wu S-J, Huang C-H, Chan Y-Y, Liao Y-R, Hwang T-L, Wu T-S. Two Diterpenoids and a Cyclopenta[c]pyridine Derivative from Roots of Salvia digitaloids. International Journal of Molecular Sciences. 2014; 15(7):11566-11577. https://doi.org/10.3390/ijms150711566
Chicago/Turabian StyleWu, Shwu-Jen, Chieh-Hung Huang, Yu-Yi Chan, Yu-Ren Liao, Tsong-Long Hwang, and Tian-Shung Wu. 2014. "Two Diterpenoids and a Cyclopenta[c]pyridine Derivative from Roots of Salvia digitaloids" International Journal of Molecular Sciences 15, no. 7: 11566-11577. https://doi.org/10.3390/ijms150711566
APA StyleWu, S.-J., Huang, C.-H., Chan, Y.-Y., Liao, Y.-R., Hwang, T.-L., & Wu, T.-S. (2014). Two Diterpenoids and a Cyclopenta[c]pyridine Derivative from Roots of Salvia digitaloids. International Journal of Molecular Sciences, 15(7), 11566-11577. https://doi.org/10.3390/ijms150711566





