Synthesis and Antioxidant Activity Evaluation of New Compounds from Hydrazinecarbothioamide and 1,2,4-Triazole Class Containing Diarylsulfone and 2,4-Difluorophenyl Moieties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
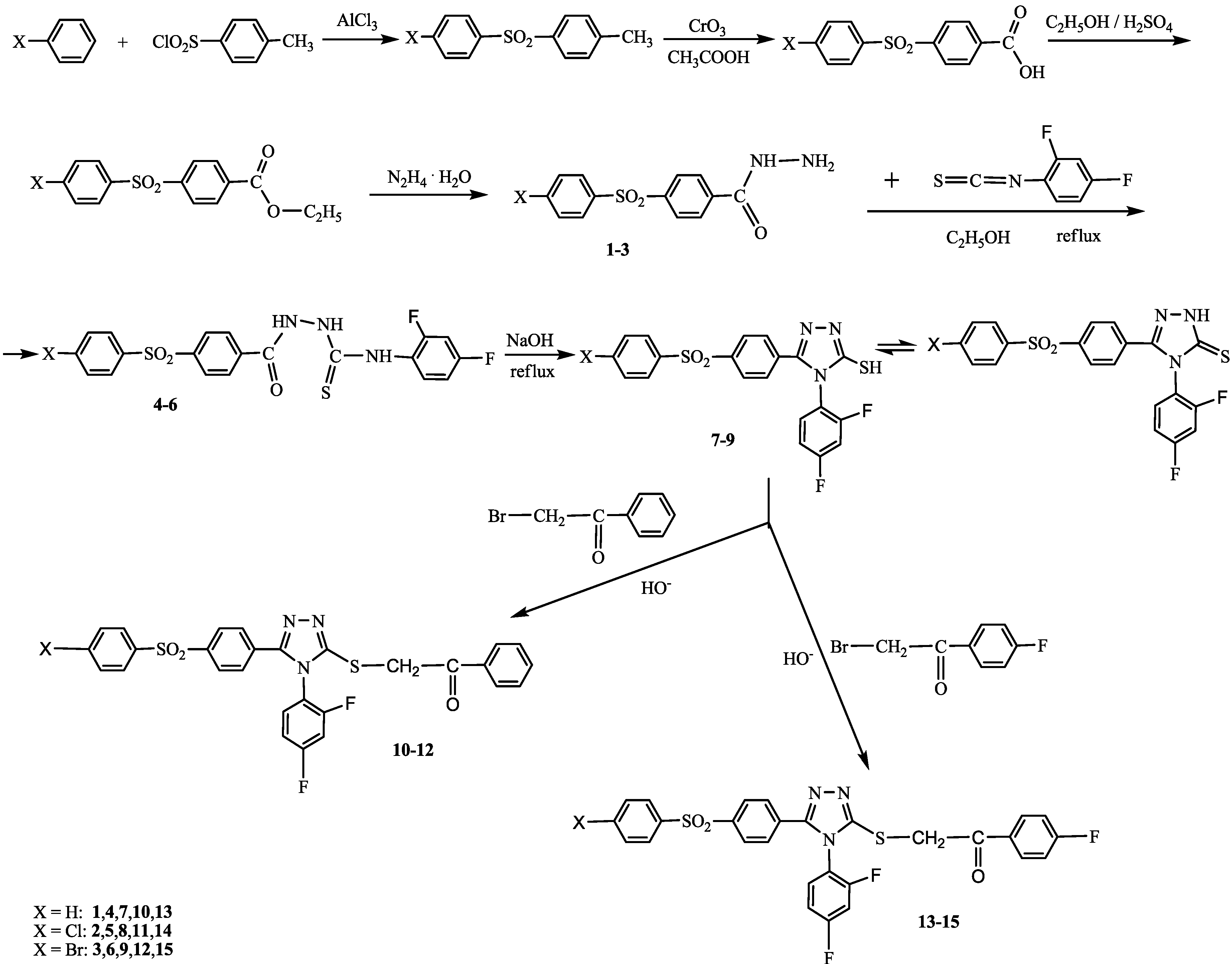
2.2. Antioxidant Activity
| Compd. | Scavenging Effect (%) | IC50 (μM) | |||||
|---|---|---|---|---|---|---|---|
| - | 25 μM | 50 μM | 75 μM | 100 μM | 125 μM | 250 μM | - |
| 4 | 30.54 ± 1.32 | 64.37 ± 1.35 | 74.86 ± 1.40 | 85.39 ± 1.45 | 95.99 ± 1.50 | 97.18 ± 1.42 | 39.39 |
| 5 | 30.39 ± 1.18 | 63.58 ± 1.62 | 74.12 ± 1.34 | 84.69 ± 1.83 | 95.36 ± 1.87 | 96.90 ± 1.39 | 39.79 |
| 6 | 29.14 ± 1.53 | 59.28 ± 1.23 | 71.23 ± 1.32 | 83.23 ± 1.42 | 95.35 ± 1.18 | 97.11 ± 1.12 | 42.32 |
| 7 | 15.88 ± 1.03 | 24.74 ± 1.32 | 33.30 ± 1.67 | 37.93 ± 1.49 | 46.14 ± 1.45 | 67.70 ± 1.68 | 147.79 |
| 8 | 15.56 ± 0.95 | 24.36 ± 1.19 | 32.18 ± 1.48 | 40.58 ± 1.41 | 48.38 ± 1.54 | 72.45 ± 1.42 | 133.80 |
| 9 | 13.96 ± 0.97 | 22.99 ± 1.05 | 31.74 ± 1.56 | 38.63 ± 1.59 | 43.03 ± 1.63 | 58.52 ± 1.55 | 182.60 |
| AA | 0.70 ± 1.00 | 1.08 ± 0.84 | 17.48 ± 1.03 | 34.91 ± 0.69 | 84.12 ± 0.48 | 91.26 ± 0.49 | 107.67 |
| BHA | 23.27 ± 1.39 | 48.99 ± 1.42 | 64.77 ± 1.32 | 73.89 ± 1.59 | 81.74 ± 1.45 | 89.30 ± 1.37 | 51.62 |
| BHT | - | - | - | - | - | 23.05 ± 1.32 | 423.37 |
| Compd. | Concentration (μM) | Scavenging Effect (%) |
|---|---|---|
| 10 | 250 | 12.67 ± 0.82 |
| 11 | 250 | 8.24 ± 1.20 |
| 12 | 250 | 7.73 ± 0.96 |
| 13 | 250 | 13.23 ± 0.48 |
| 14 | 250 | 15.04 ± 0.43 |
| 15 | 250 | 12.73 ± 0.50 |
| AA | 250 | 91.26 ± 0.49 |
| BHA | 250 | 89.30 ± 1.37 |
| BHT | 250 | 23.05 ± 1.32 |
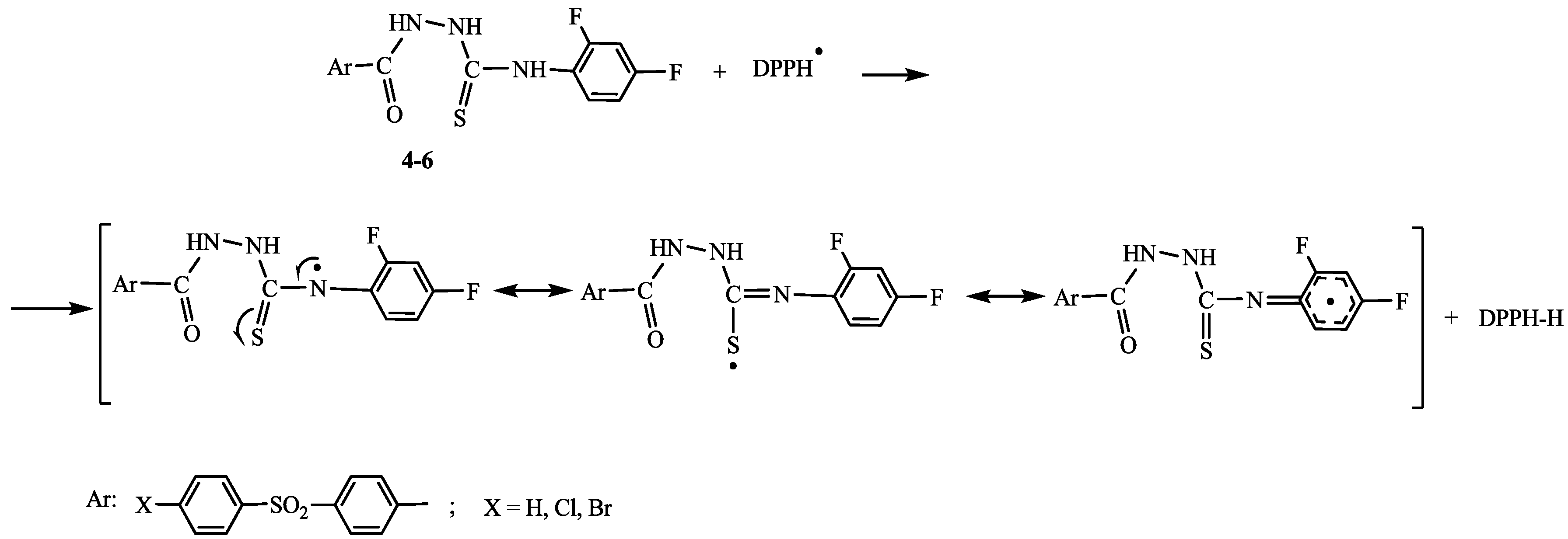
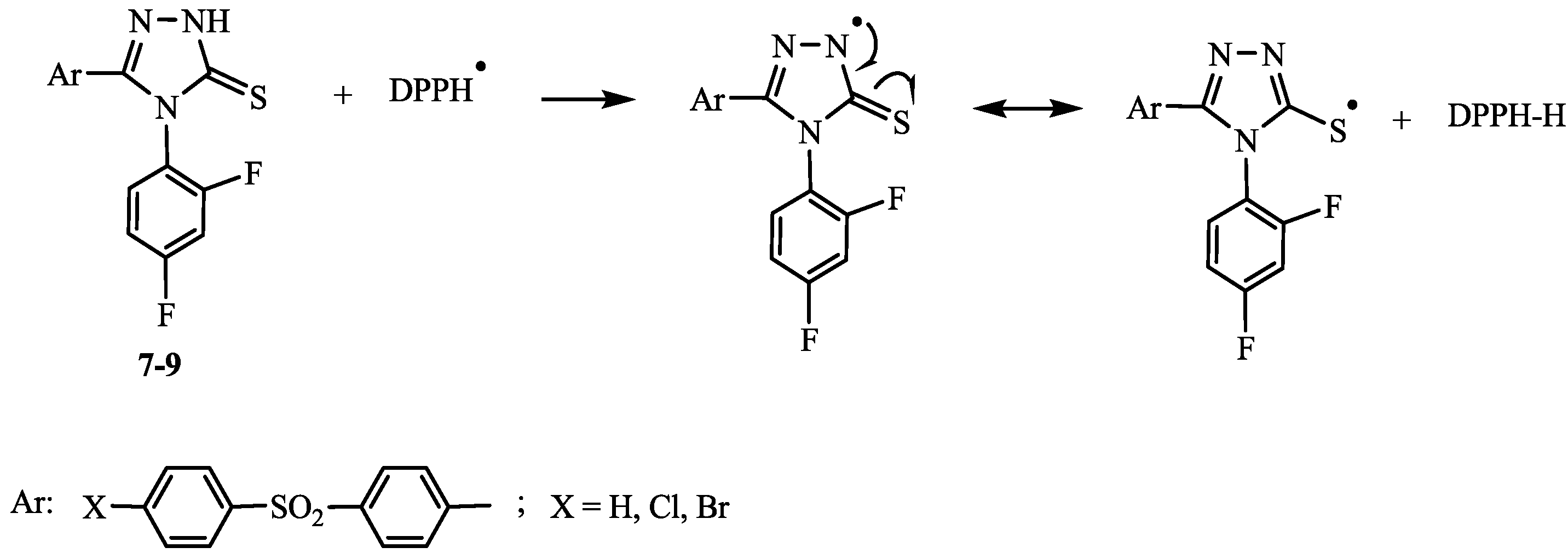
3. Experimental
3.1. Chemistry
3.1.1. General Procedure for the Preparation of 2-(4-(4-X-Phenylsulfonyl)benzoyl)-N-(2,4-difluorophenyl)hydrazinecarbothioamides 4–6
3.1.2. General Procedure for the Preparation of 5-(4-(4-X-Phenylsulfonyl)phenyl)-4-(2,4-difluorophenyl)-2H-1,2,4-triazole-3(4H)-thiones 7–9
3.1.3. General Procedure for the Preparation of 2-(5-(4-(4-X-Phenylsulfonyl)phenyl)-4-(2,4-difluorophenyl)-4H-1,2,4-triazol-3-ylthio)-1-(phenyl/4-fluorophenyl)ethanones 10–15
3.2. Antioxidant Activity
4. Conclusions
Supplementary Files
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Ślusarczyk, S.; Hajnos, M.; Skalicka-Woźniak, K.; Matkowski, A. Antioxidant activity of polyphenols from Lycopus lucidus Turcz. Food Chem. 2009, 113, 134–138. [Google Scholar]
- Dakubo, G.D. Mitochondrial Genetics and Cance; Springer-Verlag Berlin Heidelberg: Berlin, Germany, 2010. [Google Scholar] [CrossRef]
- Torreggiani, A.; Tamba, M. Free radical scavenging and metal chelating activity of some therapeutic heterocyclic agents. Trends Heterocycl. Chem. 2005, 10, 115–137. [Google Scholar]
- Karalı, N.; Güzel, Ӧ.; Ӧzsoy, N.; Ӧzbey, S.; Salman, A. Synthesis of new spiroindolinones incorporating a benzothiazole moiety as antioxidant agents. Eur. J. Med. Chem. 2010, 45, 1068–1077. [Google Scholar]
- Patil, V.P.; Markad, V.L.; Kodam, K.M.; Waghmode, S.B. Facile preparation of tetrahydro-5H-pyrido[1,2,3-de]-1,4-benzoxazines via reductive cyclization of 2-(8-quinolinyloxy)ethanones and their antioxidant activity. Bioorg. Med. Chem. Lett. 2013, 23, 6259–6263. [Google Scholar]
- Azam, F. Therapeutic Potential of Free Radical Scavengers in Neurological Disorders in Handbook of Free Radicals: Formation, Types and Effects; Chapter 2; Kozyrev, D., Slutsky, V., Eds.; Nova Science Pub. Inc.: Hauppauge, NY, USA, 2010; pp. 57–97. [Google Scholar]
- Groll, A.H.; Kolve, H. Antifungal agents: In vitro susceptibility testing, pharmacodynamics, and prospects for combination therapy. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 256–270. [Google Scholar]
- Kathiravan, M.K.; Salake, A.B.; Chothe, A.S.; Dudhe, P.B.; Watode, R.P.; Mukta, M.S.; Gadhwe, S. The biology and chemistry of antifungal agents: A review. Bioorg. Med. Chem. 2012, 20, 5678–5698. [Google Scholar]
- Thompson, G.R., III; Cadena, J.; Patterson, T.F. Overview of antifungal agents. Clin. Chest Med. 2009, 30, 203–215. [Google Scholar]
- Balfour, H.H., Jr. Antiviral drugs. N. Engl. J. Med. 1999, 340, 1255–1268. [Google Scholar]
- Murthy, N.; Rao, A.R.; Sastry, G.N. Aromatase inhibitors: A new paradigm in breast cancer treatment. Curr. Med. Chem. Anticancer Agents 2004, 4, 523–534. [Google Scholar]
- Koparir, M.; Orek, C.; Parlak, A.E.; Söylemez, A.; Koparir, P.; Karatepe, M.; Dastan, S.D. Synthesis and biological activities of some novel aminomethyl derivatives of 4-substituted-5-(2-thienyl)-2,4-dihydro-3H-1,2,4-triazole-3-thiones. Eur. J. Med. Chem. 2013, 63, 340–346. [Google Scholar]
- Yehye, W.A.; Rahman, N.A.; Alhadi, A.A.; Khaledi, H.; Ng, S.W.; Ariffin, A. Butylated hydroxytoluene analogs: Synthesis and evaluation of their multipotent antioxidant activities. Molecules 2012, 17, 7645–7665. [Google Scholar]
- Kuş, C.; Ayhan-Kılcıgil, G.; Özbey, S.; Kaynak, F.B.; Kaya, M.; Çoban, T.; Can-Eke, B. Synthesis and antioxidant properties of novel N-methyl-1,3,4-thiadiazol-2-amine and 4-methyl-2H-1,2,4-triazole-3(4H)-thione derivatives of benzimidazole class. Bioorg. Med. Chem. 2008, 16, 4294–4303. [Google Scholar]
- Zoumpoulakis, P.; Camoutsis, C.; Pairas, G.; Soković, M.; Glamočlija, J.; Potamitis, C.; Pitsas, A. Synthesis of novel sulfonamide-1,2,4-triazoles, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles, as potential antibacterial and antifungal agent. Biological evaluation and conformational analysis studies. Bioorg. Med. Chem. 2012, 20, 1569–1583. [Google Scholar]
- Eswaran, S.; Adhikari, A.V.; Shetty, N.S. Synthesis and antimicrobial activities of novel quinoline derivatives carrying 1,2,4-triazole moiety. Eur. J. Med. Chem. 2009, 44, 4637–4647. [Google Scholar]
- Hassan, G.S.; El-Messery, S.M.; Al-Omary, F.A.M.; Al-Rashood, S.T.; Shabayek, M.I.; Abulfadl, Y.S.; Habib, E.-S.E.; El-Hallouty, S.M.; Fayad, W.; Mohamed, K.M.; et al. Nonclassical antifolates, part 4. 5-(2-Aminothiazol-4-yl)-4-phenyl-4H-1,2,4-triazole-3-thiols as a new class of DHFR inhibitors: Synthesis, biological evaluation and molecular modeling study. Eur. J. Med. Chem. 2013, 66, 135–145. [Google Scholar]
- Turan-Zitouni, G.; Kaplancikli, Z.A.; Yildiz, M.T.; Chevallet, P.; Kaya, D. Synthesis and antimicrobial activity of 4-phenyl/cyclohexyl-5-(1-phenoxyethyl)-3-[N-(2-thiazolyl)acetamido]-thio-4H-1,2,4-triazole derivatives. Eur. J. Med. Chem. 2005, 40, 607–613. [Google Scholar]
- Duran, A.; Dogan, H.N.; Rollas, S. Synthesis and preliminary anticancer activity of new 1,4-dihydro-3-(3-hydroxy-2-naphthyl)-4-substituted-5H-1,2,4-triazoline-5-thiones. Farmaco 2002, 57, 559–564. [Google Scholar]
- Idrees, G.A.; Aly, O.M.; Abuo-Rahma, G.E.D.A.A.; Radwan, M.F. Design, synthesis and hypolipidemic activity of novel 2-(naphthalen-2-yloxy)propionic acid derivatives as desmethyl fibrate analogs. Eur. J. Med. Chem. 2009, 44, 3973–3980. [Google Scholar]
- Özadalı, K.; Özkanlı, F.; Jain, S.; Rao, P.P.N.; Velázquez-Martínez, C.A. Synthesis and biological evaluation of isoxazolo[4,5-d]pyridazin-4-(5H)-one analogues as potent anti-inflammatory agents. Bioorg. Med. Chem. 2012, 20, 2912–2922. [Google Scholar]
- Orek, C.; Koparir, P.; Koparir, M. N-cyclohexyl-2-[5-(4-pyridyl)-4-(p-tolyl)-4H-1,2,4-triazol-3-ylsulfanyl]-acetamide dihydrate: Synthesis, experimental, theoretical characterization and biological activities. Spectrochim. Acta A 2012, 97, 923–934. [Google Scholar]
- Navidpour, L.; Shafaroodi, H.; Abdi, K.; Amini, M.; Ghahremani, M.H.; Dehpour, A.R.; Shafiee, A. Design, synthesis, and biological evaluation of substituted 3-alkylthio-4,5-diaryl-4H-1,2,4-triazoles as selective COX-2 inhibitors. Bioorg. Med. Chem. 2006, 14, 2507–2517. [Google Scholar]
- Stefanska, J.; Szulczyk, D.; Koziol, A.E.; Miroslaw, B.; Kedzierska, E.; Fidecka, S.; Busonera, B.; Sanna, G.; Giliberti, G.; La Colla, P.; et al. Disubstituted thiourea derivatives and their activity on CNS: Synthesis and biological evaluation. Eur. J. Med. Chem. 2012, 55, 205–213. [Google Scholar]
- Šarkanj, B.; Molnar, M.; Čačić, M.; Gille, L. 4-Methyl-7-hydroxycoumarin antifungal and antioxidant activity enhancement by substitution with thiosemicarbazide and thiazolidinone moieties. Food Chem. 2013, 139, 488–495. [Google Scholar]
- Kuş, C.; Ayhan-KIlcIgil, G.; Eke, B.C.; Işcan, M. Synthesis and antioxidant properties of some novel benzimidazole derivatives on lipid peroxidation in the rat liver. Arch. Pharm. Res. 2004, 27, 156–163. [Google Scholar]
- Shelke, S.; Mhaske, G.; Gadakh, S.; Gill, C. Green synthesis and biological evaluation of some novel azoles as antimicrobial agents. Bioorg. Med. Chem. Lett. 2010, 20, 7200–7204. [Google Scholar]
- Sriram, D.; Yogeeswari, P.; Priya, D.Y. Antimycobacterial activity of novel N-(substituted)-2-isonicotinoylhydrazinocarbothioamide endowed with high activity towards isoniazid resistant tuberculosis. Biomed. Pharmacother. 2009, 63, 36–39. [Google Scholar]
- Elslager, E.F.; Gavrilis, Z.B.; Phillips, A.A.; Worth, D.F. Repository drugs. IV., 4',4'''-Sulfonylbisacetanilide (acedapsone, DADDS) and related sulfanilylanilides with prolonged antimalarial and antileprotic action. J. Med. Chem. 1969, 12, 357–363. [Google Scholar]
- McMahon, J.B.; Gulakowski, R.J.; Weislow, O.S.; Schultz, R.J.; Narayanan, V.L.; Clanton, D.J.; Pedemonte, R.; Wassmundt, F.W.; Buckheit, R.W., Jr.; Decker, W.D.; et al. Diarylsulfones, a new chemical class of nonnucleoside antiviral anhibitors of human immunodeficiency virus Type 1 Reverse Transcriptase. Antimicrob. Agents Chemother. 1993, 37, 754–760. [Google Scholar]
- Saeed, A.; Shaheen, U.; Hameed, A.; Kazmi, F. Synthesis and antimicrobial activity of some novel 2-(substituted fluorobenzoylimino)-3-(substituted fluorophenyl)-4-methyl-1,3-thiazolines. J. Fluorine Chem. 2010, 131, 333–339. [Google Scholar]
- Barbuceanu, S.-F.; Saramet, G.; Almajan, G.L.; Draghici, C.; Barbuceanu, F.; Bancescu, G. New heterocyclic compounds from 1,2,4-triazole and 1,3,4-thiadiazole class bearing diphenylsulfone moieties. Synthesis, characterization and antimicrobial activity evaluation. Eur. J. Med. Chem. 2012, 49, 417–423. [Google Scholar]
- Barbuceanu, S.-F.; Bancescu, G.; Saramet, G.; Barbuceanu, F.; Draghici, C.; Radulescu, F.S.; Ionescu, A.; Negres, S. Synthesis and biological evaluation of some new N1-[4-(4-Chlorophenylsulfonyl)benzoyl]-N4-(aryl)-thiosemicarbazides and products of their cyclization. Heteroat. Chem. 2013, 24, 309–321. [Google Scholar]
- Almajan, G.L.; Innocenti, A.; Puccetti, L.; Manole, G.; Barbuceanu, S.; Saramet, I.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of the cytosolic and tumor-associated carbonic anhydrase isozymes I, II, and IX with a series of 1,3,4-thiadiazole- and 1,2,4-triazole-thiols. Bioorg. Med. Chem. Lett. 2005, 15, 2347–2352. [Google Scholar]
- Socea, L.-I.; Apostol, T.V.; Şaramet, G.; Bărbuceanu, Ş.-F.; Drăghici, C.; Dinu, M. Synthesis and root growth activity of some new acetylhydrazinecarbothioamides and 1,2,4-triazoles substituted with 5H-dibenzo[a,d]annulene moiety. J. Serb. Chem. Soc. 2012, 77, 1541–1549. [Google Scholar]
- Şaramet, I.; Almăjan, G.-L.; Barbuceanu, Ş.; Drăghici, C.; Banciu, M.D. Synthesis of some substituted aroyl thiosemicarbazides, -mercaptotriazoles and -aminothiadiazoles. Rev. Roum. Chim. 2005, 50, 19–27. [Google Scholar]
- Mavrodin, A.; Zotta, V.; Stoenescu, V.M.; Oteleanu, D. Sulfones. IV. New sulfone-hydrazide derivatives. Pharm. Zentr. Deutsch. 1956, 95, 353–361. [Google Scholar]
- Khan, I.; Ali, S.; Hameed, S.; Rama, N.H.; Hussain, M.T.; Wadood, A.; Uddin, R.; Ul-Haq, Z.; Khan, A.; Ali, S.; et al. Synthesis, antioxidant activities and urease inhibition of some new 1,2,4-triazole and 1,3,4-thiadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5200–5207. [Google Scholar]
- Kumar, H.; Javed, S.A.; Khan, S.A.; Amir, M. 1,3,4-Oxadiazole/thiadiazole and 1,2,4-triazole derivatives of biphenyl-4-yloxy acetic acid: Synthesis and preliminary evaluation of biological properties. Eur. J. Med. Chem. 2008, 43, 2688–2698. [Google Scholar]
- Liesen, A.P.; de Aquino, T.M.; Carvalho, C.S.; Lima, V.T.; de Araújo, J.M.; de Lima, J.G.; de Faria, A.R.; de Melo, E.J.T.; Alves, A.J.; Alves, E.W.; et al. Synthesis and evaluation ofanti-Toxoplasma gondii and antimicrobial activities of thiosemicarbazides, 4-thiazolidinones and 1,3,4-thiadiazoles. Eur. J. Med. Chem. 2010, 45, 3685–3691. [Google Scholar]
- Akhtar, T.; Hameed, S.; Al-Masoudi, N.A.; Khan, K.M. Synthesis and anti-HIV activity of new chiral 1,2,4-triazoles and 1,3,4-thiadiazoles. Heteroat. Chem. 2007, 18, 316–322. [Google Scholar]
- Salgın-Gökșen, U.; Gökhan-Kelekçi, N.; Göktaș, Ö.; Köysal, Y.; Kılıç, E.; Ișık, Ș.; Aktay, G.; Özalp, M. 1-Acylthiosemicarbazides, 1,2,4-triazole-5(4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: Synthesis, analgesic-anti-inflammatory and antimicrobial activ. Bioorg. Med. Chem. 2007, 15, 5738–5751. [Google Scholar]
- Al-Deeb, O.A.; Al-Omar, M.A.; El-Brollosy, N.R.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and antiinflammatory activities of novel 2-[3-(1-adamantyl)-4-substituted-5-tioxo-1,2,4-triazolin-1-yl]acetic acids, 2-[3-(1-adamantyl)-4-substituted-5-tioxo-1,2,4-triazolin-1-yl]-propionic acids and related derivatives. Arzneim.-Forsch./Drug Res. 2006, 56, 40–47. [Google Scholar]
- Saadeh, H.A.; Mosleh, I.M.; Al-Bakri, A.G.; Mubarak, M.S. Synthesis and antimicrobial activity of new 1,2,4-triazole-3-thiol metronidazole derivatives. Monatsh. Chem. 2010, 141, 471–478. [Google Scholar]
- Kumar, A.; Sharma, P.; Kumari, P.; Kalal, B.L. Exploration of antimicrobial and antioxidant potential of newly synthesized 2,3-disubstituted quinazoline-4(3H)-ones. Bioorg. Med. Chem. Lett. 2011, 21, 4353–4357. [Google Scholar]
- Zhou, B.; Li, B.; Yi, W.; Bu, X.; Ma, L. Synthesis, antioxidant, and antimicrobial evaluation of some 2-arylbenzimidazole derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 3759–3763. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 2001, 46, 3–26. [Google Scholar]
- Duan, X.-J.; Zhang, W.-W.; Li, X.-M.; Wang, B.-G. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem. 2006, 95, 37–43. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barbuceanu, S.-F.; Ilies, D.C.; Saramet, G.; Uivarosi, V.; Draghici, C.; Radulescu, V. Synthesis and Antioxidant Activity Evaluation of New Compounds from Hydrazinecarbothioamide and 1,2,4-Triazole Class Containing Diarylsulfone and 2,4-Difluorophenyl Moieties. Int. J. Mol. Sci. 2014, 15, 10908-10925. https://doi.org/10.3390/ijms150610908
Barbuceanu S-F, Ilies DC, Saramet G, Uivarosi V, Draghici C, Radulescu V. Synthesis and Antioxidant Activity Evaluation of New Compounds from Hydrazinecarbothioamide and 1,2,4-Triazole Class Containing Diarylsulfone and 2,4-Difluorophenyl Moieties. International Journal of Molecular Sciences. 2014; 15(6):10908-10925. https://doi.org/10.3390/ijms150610908
Chicago/Turabian StyleBarbuceanu, Stefania-Felicia, Diana Carolina Ilies, Gabriel Saramet, Valentina Uivarosi, Constantin Draghici, and Valeria Radulescu. 2014. "Synthesis and Antioxidant Activity Evaluation of New Compounds from Hydrazinecarbothioamide and 1,2,4-Triazole Class Containing Diarylsulfone and 2,4-Difluorophenyl Moieties" International Journal of Molecular Sciences 15, no. 6: 10908-10925. https://doi.org/10.3390/ijms150610908






