Novel Microwave-Assisted Synthesis of the Immunomodulator Organotellurium Compound Ammonium Trichloro(dioxoethylene-O,O')tellurate (AS101)
Abstract
:1. Introduction
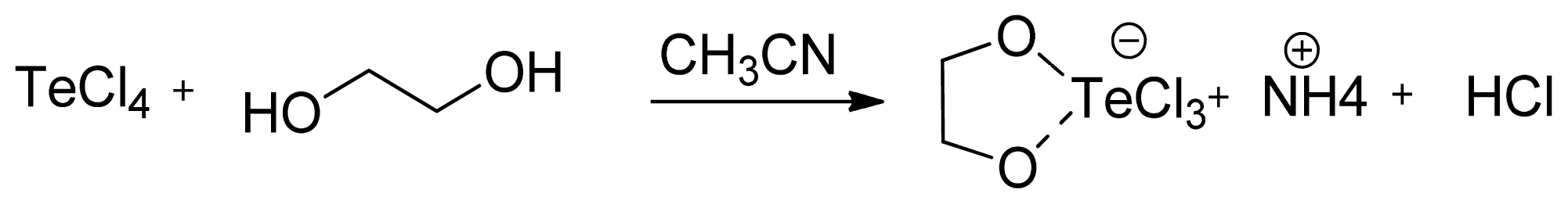
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedure
3.1.1. AS101 Obtention by Method A
- Found (%): C, 7.70; H, 2.58; N, 4.49. C2H8Cl3NO2Te.
- Calculated (%): C, 7.85; H, 2.37; N, 4.42.
- IR (Golden-Gate): v = 3183 (NH): 1390 (NH4+): 1019: 894 cm−1.
- NMR-1H (DMSO-d6, δ, ppm): 4.36 (s, 4H, CH2): 7.16 (t, 4H, J = 50 Hz, NH4+).
- NMR-13C (DMSO-d6, δ, ppm): 68.13.
- EM (m/z): 292 (M+-NH4,1), 290 (M+–NH4+,1), 260 (M+–NH4+–Cl [Te130, 2Cl35],7), 258 (M+–NH4+–Cl [Te128, 2Cl35],13), 256 (M+–NH4+–Cl [Te126, 2Cl35],13), 254 (9), 224 (42), 223 (35), 221 (19), 200 (36), 198 (27), 196 (14), 190 (69), 188 (63), 186 (37), 165 (21), 163 (18), 161 (11), 146 (5), 130 (25), 128 (23), 126 (13).
3.1.2. AS101 Obtention by Method B
- Found (%): C, 7.81; H, 2.33; N, 4.45. C2H8Cl3NO2Te.
- Calculated (%): C, 7.70; H, 2.58; N, 4.49.
- IR (Golden-Gate): 3198 (NH): 1399 (NH4+): 1017: 889 cm−1.
- NMR-1H (DMSO-d6, δ, ppm): 4.36 (s, 4H, CH2): 7.16 (s, 4H, NH4+).
- NMR-13C (DMSO-d6, δ, ppm): 68.10.
- EM (m/z): 292 (M+–NH4+,1), 290 (M+–NH4+,1), 260 (M+–NH4+–Cl [Te130,2Cl35], 11), 258 (M+–NH4+–Cl [Te128, 2Cl35],20), 256 (M+–NH4+–Cl [Te126, 2Cl35],21), 254 (14), 224 (52), 223 (43), 221 ( 23), 200 (46), 198 (36), 196 (18), 190 (100), 188 (92), 186 (54), 165 (27), 163 (23), 161 (14), 146 (7), 130 (32), 128 (30), 126 (18).
3.2. 1H-NMR and 13C-NMR Analysis
3.3. MS Analysis
3.4. Infrared Spectroscopy (FTIR)
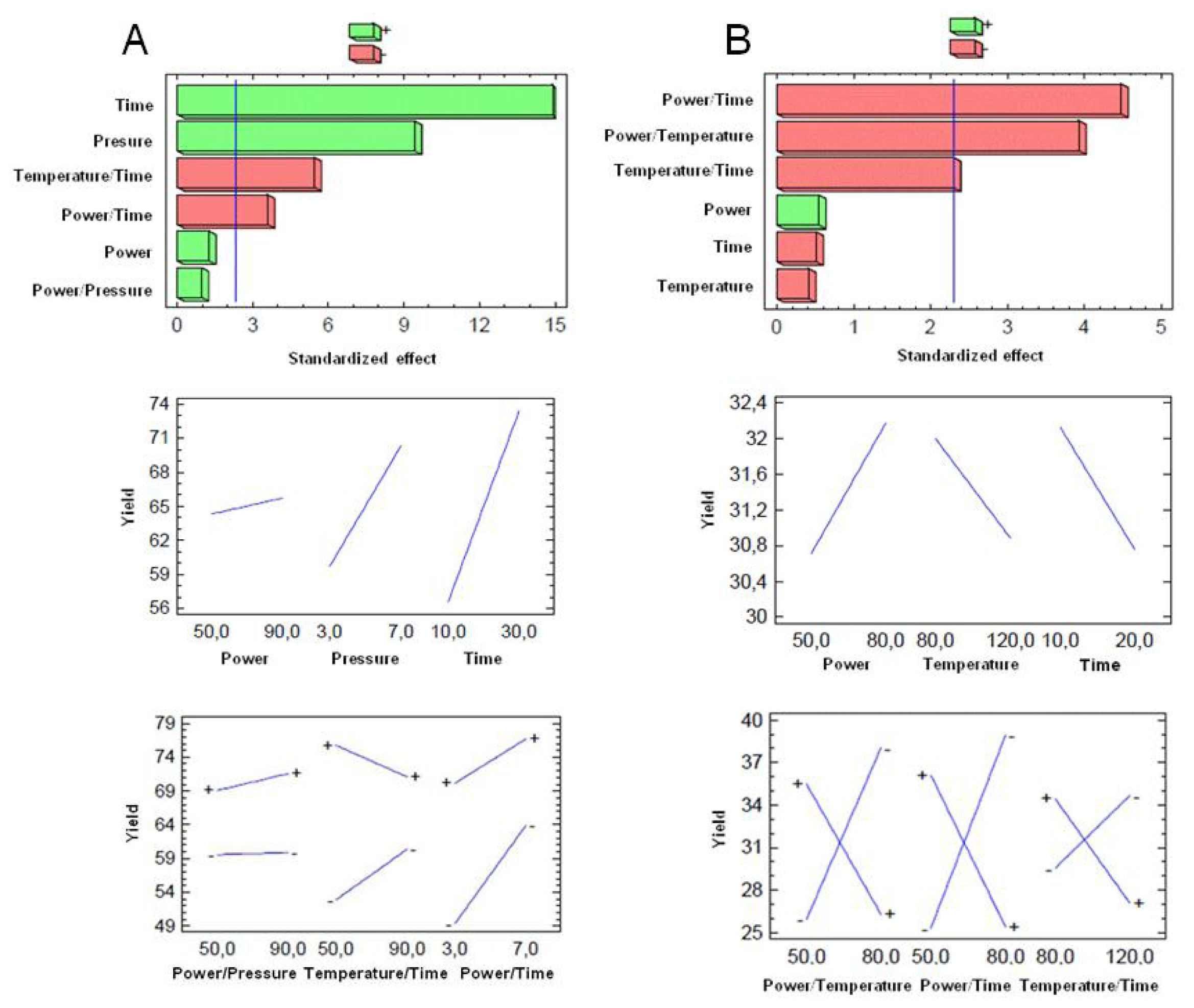
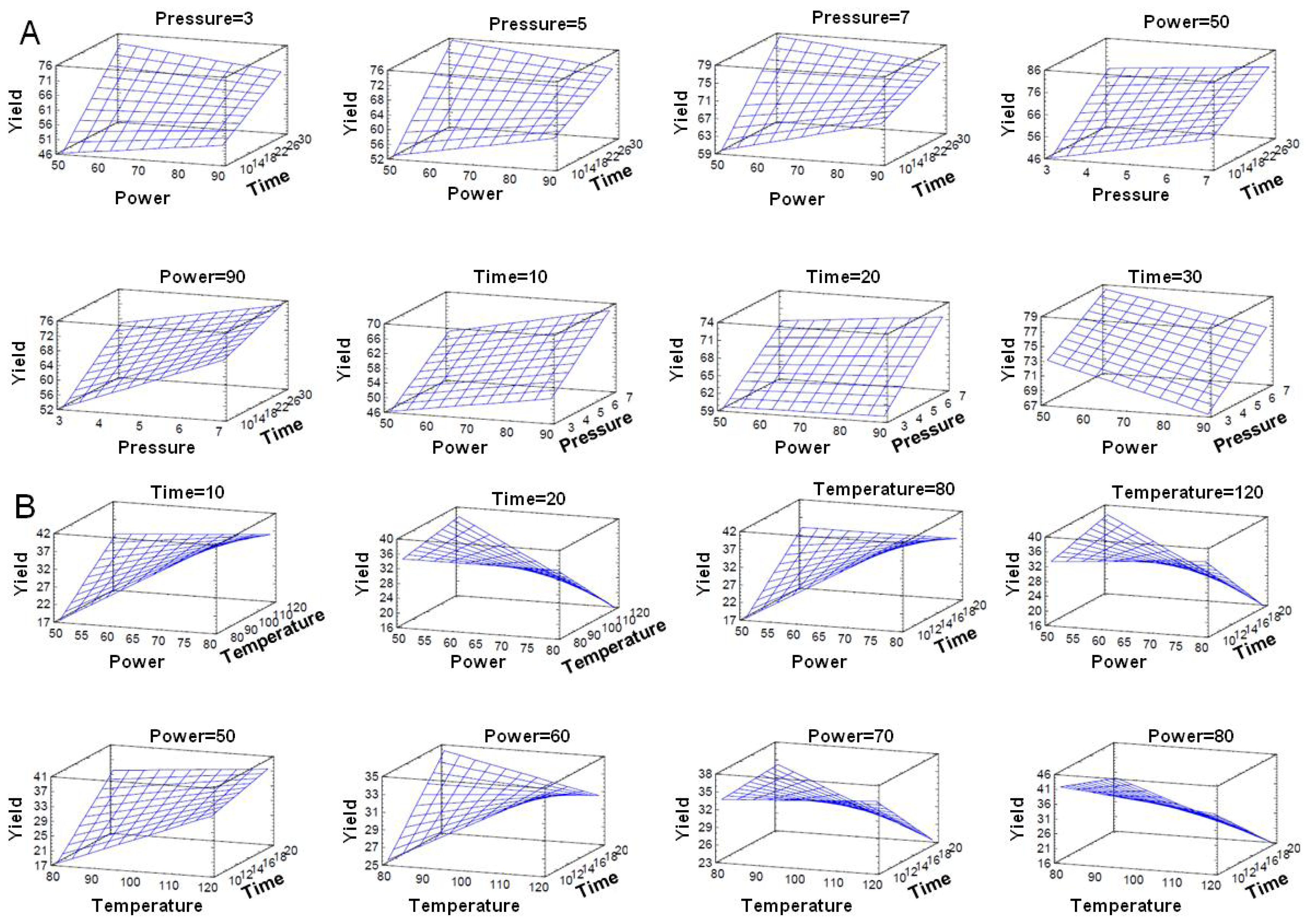
3.5. Optimization of MAOS AS101 Procedure
3.6. Statistical Analyses
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Cohen, B.L. Anomalous behavior of tellurium abundances. Geochim. Cosmochim. Acta 1984, 48, 203–205. [Google Scholar]
- Larner, A.J. Biological effects of tellurium: A review. Trace Elem. Electrolytes 1995, 12, 26–31. [Google Scholar]
- Sredni, B. Immunomodulating tellurium compounds as anti-cancer agents. Semin. Cancer Biol 2012, 22, 60–691. [Google Scholar]
- Tiekink, E.R.T. Therapeutic potential of selenium and tellurium compounds: Opportunities yet unrealised. Dalton Trans 2012, 41, 6390–6395. [Google Scholar]
- Chasteen, T.G.; Bentley, R. Biomethylation of selenium and tellurium: Microorganisms and plants. Chem. Rev 2003, 103, 1–25. [Google Scholar]
- Dittmer, D.C. Tellurium. Chem. Eng. News 2003, 81, 128. [Google Scholar]
- Nicolaou, K.C.; Baran, P.S.; Zhong, Y.-L.; Barluenga, S.; Hunt, K.W.; Kranich, R.; Vega, J.A. Iodine(V) reagents in organic synthesis. Part 3. New routes to heterocyclic compounds via o-iodoxybenzoic acid-mediated cyclizations: Generality, scope, and mechanism. J. Am. Chem. Soc 2002, 124, 2233–2244. [Google Scholar]
- Musalova, M.V.; Potapov, V.A.; Amosova, S.V. Synthesis of novel E-2-chlorovinyltellurium compounds based on the stereospecific anti-addition of tellurium tetrachloride to acetylene. Molecules 2012, 17, 5770–5779. [Google Scholar]
- Sredni, B.; Caspi, R.R.; Klein, A.; Kalechman, Y.; Danziger, Y.; Ya’akov, M.B.; Tamari, T.; Shalit, F.; Albeck, M. A new immunomodulating compound (AS101) with potential therapeutic application. Nature 1987, 330, 173–176. [Google Scholar]
- Daniel-Hoffmann, M.; Benjamin Sredni, B.; Nitzan, Y. Bactericidal activity of the organo-tellurium compound AS101 against Enterobacter cloacae. J. Antimicrob. Chemother 2012, 67, 2165–2172. [Google Scholar]
- Daniel-Hoffmann, M.; Albeck, M.; Sredni, B.; Nitzan, Y. A potential antimicrobial treatment against ESBL-producing Klebsiella pneumoniae using the tellurium compound AS101. Arch. Microbiol 2009, 191, 631–638. [Google Scholar]
- Wieslander, E.; Engman, L.; Svensjö, E.; Erlansson, M.; Johansson, U.; Linden, M.; Andersson, C.M.; Brattsand, R. Antioxidative properties of organotellurium compounds in cell systems. Biochem. Pharmacol 1998, 55, 573–584. [Google Scholar]
- Brodsky, M.; Halpert, G.; Albeck, M.; Sredni, B. The anti-inflammatory effects of the tellurium redox modulating compound, AS101, are associated with regulation of NFκB signaling pathway and nitric oxide induction in macrophages. J. Inflamm. (Lond.) 2010, 7, 3. [Google Scholar]
- Naor, Y.; Hayun, M.; Sredni, B.; Don, J. Multiple signal transduction pathways are involved in G2/M growth arrest and apoptosis induced by the immunomodulator AS101 in multiple myeloma. Leuk. Lymphoma 2013, 54, 160–166. [Google Scholar]
- Sredni, B.; Xu, R.H.; Albeck, M.; Gafter, U.; Gal, R.; Shani, A.; Tichler, T.; Shapira, J.; Bruderman, I.; Catane, R.; et al. The protective role of the immunomodulator AS101 against chemotherapy-induced alopecia: Studies on human and animal models. Int. J. Cancer 1996, 65, 97–103. [Google Scholar]
- Sredni, B.; Geffen-Aricha, R.; Duan, W.; Albeck, M.; Shalit, F.; Lander, H.M.; Kinor, N.; Sagi, O.; Albeck, A.; Yosef, S.; et al. Multifunctional tellurium molecule protects and restores dopaminergic neurons in Parkinson’s disease models. FASEB J 2007, 21, 1870–1873. [Google Scholar]
- Halperin-Sheinfeld, M.; Gertler, A.; Okun, E.; Sredni, B.; Cohen, H.Y. The Tellurium compound, AS101, increases SIRT1 level and activity and prevents type 2 diabetes. Aging (Albany N. Y.) 2012, 4, 436–447. [Google Scholar]
- Lee, J.H.; Halperin-Sheinfeld, M.; Baatar, D.; Mughal, M.R.; Tae, H.J.; Kim, J.W.; Carter, A.; Lustig, A.; Snir, O.; Lavie, G.; et al. Tellurium compound AS101 ameliorates experimental autoimmune encephalomyelitis by VLA-4 inhibition and suppression of monocyte and T cell infiltration into the CNS. NeuroMol. Med 2013. [Google Scholar] [CrossRef]
- Kalich-Philosoph, L.; Roness, H.; Carmely, A.; Fishel-Bartal, M.; Ligumsky, H.; Paglin, S.; Wolf, I.; Kanety, H.; Sredni, B.; Meirow, D. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci. Transl. Med 2013, 5, 185ra62. [Google Scholar]
- Sredni-Kenigsbuch, D.; Shohat, M.; Shohat, B.; Ben-Amitai, D.; Chan, C.C.; David, M. The novel tellurium immunomodulator AS101 inhibits interleukin-10 production and p38 MAPK expression in atopic dermatitis. J. Dermatol. Sci 2008, 50, 232–235. [Google Scholar]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and organotellurium compounds: Toxicology and pharmacology. Chem. Rev 2004, 104, 6255–6286. [Google Scholar]
- Indenbaum, V.; Bin, H.; Makarovsky, D.; Weil, M.; Shulman, L.M.; Albeck, M.; Sredni, B.; Mendelson, E. In vitro and in vivo activity of AS101 against West Nile virus (WNV). Virus Res 2012, 166, 68–76. [Google Scholar]
- Nascimento-Júnior, N.M.; Kümmerle, A.E.; Barreiro, E.J.; Fraga, C.A.M. MAOS and medicinal chemistry: Some important examples from the last years. Molecules 2011, 16, 9274–9297. [Google Scholar]
- Kappe, C.O.; Dallinger, D. The impact of microwave synthesis on drug discovery. Nat. Rev. Drug Discov 2006, 5, 51–64. [Google Scholar]
- Larhed, M.; Hallberg, A. Microwave-assisted high-speed chemistry: A new technique in drug discovery. Drug Discov. Today 2001, 6, 406–416. [Google Scholar]
- Shipe, W.D.; Wolkenberg, S.E.; Lindsley, C.W. Accelerating lead development by microwaveenhanced medicinal chemistry. Drug Discov. Today Technol 2005, 2, 155–161. [Google Scholar]
- Lidstrom, P.; Tierney, J.; Wathey, B. Microwave assisted organic synthesis: A review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar]
- Kappe, C.O.; Dallinger, D.; Murphree, S.S. Practical Microwave Synthesis for Organic Chemistry. Strategies, Instruments, and Protocols; Wiley-VCH: West Sussex, UK, 2009. [Google Scholar]
- Santagada, V.; Frecentese, F.; Perissutti, E.; Fiorino, F.; Severino, B.; Caliendo, G. Microwave assisted synthesis: A new technology in drug discovery. Mini Rev. Med. Chem 2009, 9, 340–358. [Google Scholar]
- Nuchter, M.; Ondruschka, B.; Bonrath, W.; Gum, A. Microwave assisted synthesis—A critical technology overview. Green Chem 2004, 6, 128–141. [Google Scholar]
- Microwaves in Organic Synthesis; Loupy, A. (Ed.) Wiley-VCH: West Sussex, UK, 2006.
- Alcazar, J.; Oehlrich, D. Recent applications of microwave irradiation to medicinal chemistry. Future Med. Chem 2010, 2, 169–178. [Google Scholar]
- Roberts, B.A.; Strauss, C.R. Toward rapid “green” predictable microwave-assisted synthesis. Acc. Chem. Res 2005, 38, 653–661. [Google Scholar]
- Seijas, J.A.; Vázquez-Tato, M.P.; Martínez, M.M.; Rodríguez-Parga, J. Microwave enhanced synthesis of acridines. A new aspect in the Bernthsen reaction. Green Chem 2002, 4, 390–391. [Google Scholar]
- Braña, M.F.; Sánchez-Migallón, A. Anticancer: Drug discovery and pharmaceutical chemistry: A history. Clin. Transl. Oncol 2006, 8, 717–728. [Google Scholar]
- Lombardino, J.G.; Lowe, J.A., 3rd. The role of the medicinal chemist in drug discovery—Then and now. Nat. Rev. Drug Discov 2004, 3, 853–862. [Google Scholar]
- Mavandadi, F.; Pilotti, A. The impact of microwave-assisted organic synthesis in drug discovery. Drug Discov. Today 2006, 11, 165–174. [Google Scholar]
- Wathey, B.; Tierney, J.; Lidström, P.; Westman, J. The impact of microwave assisted organic chemistry on drug discovery. Drug Discov. Today 2002, 7, 373–380. [Google Scholar]
- Vázquez-Tato, M.P. Microwave mediated synthesis of amides. Synlett 1993, 7, 506. [Google Scholar]
- Seijas, J.A.; Vázquez-Tato, M.P.; Martínez, M.M.; Núñez-Corredoira, G. Direct synthesis of imides from dicarboxylic acids using microwaves. J. Chem. Res. (S) 1999, 420–421. [Google Scholar] [CrossRef]
- Seijas, J.A.; Vázquez-Tato, M.P.; Martínez, M.M. Microwave enhanced synthesis of 4-aminoquinazolines. Tetrahedron Lett 2000, 41, 2215–2217. [Google Scholar]
- Seijas, J.A.; Vázquez-Tato, M.P.; González-Bande, C.; Pacios-López, B. Procedure for Preparing Thalidomide Using Microwave Oven Radiation. Spanish Pat. Appl. P200002113, 22 August 2000. [Google Scholar]
- Seijas, J.A.; Vázquez-Tato, M.P.; González-Bande, C.; Pacios-López, B. Microwave promoted synthesis of a rehabilitated drug: Thalidomide. Synthesis 2001, 7, 999–1000. [Google Scholar]
- Albeck, M.; Tamari, T.; Sredni, B. Synthesis and properties of ammonium trichloro(dioxyethylene-O,O′)tellurate (AS101). A new immunomodulating compound. Synthesis 1989, 8, 635–636. [Google Scholar]
- Rajender, S.V.; Rajender, D. Sodium borohydride on wet clay: Solvent-free reductive amination of carbonyl compounds using microwaves. Tetrahedron 1998, 54, 6293–6298. [Google Scholar]
- Roberts, B.A.; Strauss, C.R. Development of predictive tools for optimizing organic reactions. Molecules 2004, 9, 459–465. [Google Scholar]
| Key | High level (+) | Low level (−) | Unit | |
|---|---|---|---|---|
| Method A | ||||
| Power | X1a | 90 | 50 | watt |
| Pressure | X2a | 7 | 3 | bar |
| Time | X3a | 30 | 10 | minute |
| Method B | ||||
| Power | X1b | 80 | 50 | watt |
| Temperature | X2b | 120 | 80 | °C |
| Time | X3b | 20 | 10 | minute |
| X1a | X2a | X3a | X1b | X2b | X3b | Yield Method A | Yield Method B | |
|---|---|---|---|---|---|---|---|---|
| 1 | + | + | + | + | − | − | 73.5 ± 2.1 | 20.0 ± 4.2 |
| 2 | − | + | + | − | + | − | 78.5 ± 0.7 | 34.5 ± 2.1 |
| 3 | − | − | + | + | − | + | 67.0 ± 2.8 | 38.0 ± 5.7 |
| 4 | + | − | − | + | + | − | 53.0 ± 1.4 | 45.0 ± 0.3 |
| 5 | − | + | − | + | + | + | 78.5 ± 0.7 | 36.5 ± 0.7 |
| 6 | + | − | + | − | + | + | 75.5 ± 0.7 | 31.0 ± 2.8 |
| 7 | + | + | − | − | − | + | 53.0 ± 1.4 | 33.0 ± 1.4 |
| 8 | − | − | − | − | − | − | 0 | 0 |
| Source | Sum of squares | df | Mean square | F value | p value Prob > F |
|---|---|---|---|---|---|
| X1a | 7.99 | 1 | 7.99 | 1.57 | 0.2452 |
| X2a | 449.122 | 1 | 449.122 | 88.36 | <0.0001 |
| X3a | 1128.12 | 1 | 1128.12 | 221.94 | <0.0001 |
| X1a* X2a | 4.74 | 1 | 4.74 | 0.93 | 0.3624 |
| X1a* X3a | 149.51 | 1 | 149.51 | 29.41 | <0.0001 |
| X2a* X3a | 64.52 | 1 | 64.52 | 12.69 | 0.0074 |
| Total error | 40.66 | 8 | 5.08 | ||
| Cor total | 1846.32 | 15 | |||
| X1b | 8.57 | 1 | 8.57 | 0.29 | 0.6048 |
| X2b | 4.98 | 1 | 4.98 | 0.17 | 0.6921 |
| X3b | 7.54 | 1 | 7.54 | 0.26 | 0.6269 |
| X1b* X2b | 455.07 | 1 | 455.07 | 15.40 | 0.0044 |
| X1b* X3b | 592.31 | 1 | 592.31 | 20.05 | 0.0021 |
| X2b* X3b | 155.68 | 1 | 155.68 | 5.27 | 0.0505 |
| Total error | 236.35 | 8 | 29.54 | ||
| Cor total | 1472.22 | 15 | |||
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vázquez-Tato, M.P.; Mena-Menéndez, A.; Feás, X.; Seijas, J.A. Novel Microwave-Assisted Synthesis of the Immunomodulator Organotellurium Compound Ammonium Trichloro(dioxoethylene-O,O')tellurate (AS101). Int. J. Mol. Sci. 2014, 15, 3287-3298. https://doi.org/10.3390/ijms15023287
Vázquez-Tato MP, Mena-Menéndez A, Feás X, Seijas JA. Novel Microwave-Assisted Synthesis of the Immunomodulator Organotellurium Compound Ammonium Trichloro(dioxoethylene-O,O')tellurate (AS101). International Journal of Molecular Sciences. 2014; 15(2):3287-3298. https://doi.org/10.3390/ijms15023287
Chicago/Turabian StyleVázquez-Tato, M. Pilar, Alberto Mena-Menéndez, Xesús Feás, and Julio A. Seijas. 2014. "Novel Microwave-Assisted Synthesis of the Immunomodulator Organotellurium Compound Ammonium Trichloro(dioxoethylene-O,O')tellurate (AS101)" International Journal of Molecular Sciences 15, no. 2: 3287-3298. https://doi.org/10.3390/ijms15023287







