The Neuroprotective Role of Acupuncture and Activation of the BDNF Signaling Pathway
Abstract
:1. Introduction
2. Several Kinds of Physical Stimulation Can Activate the BDNF Signaling Pathway
3. The Neuroprotective Roles of BDNF/TrkB Signaling
4. The Neuroprotective Effects of Acupuncture in Brain Function
5. Acupuncture Therapy and BDNF Signaling Pathway in Various Disease States
5.1. Depression
5.2. Cerebral Ischemia Injury
5.3. Memory-Deficits
5.4. Inflammation
5.5. Others
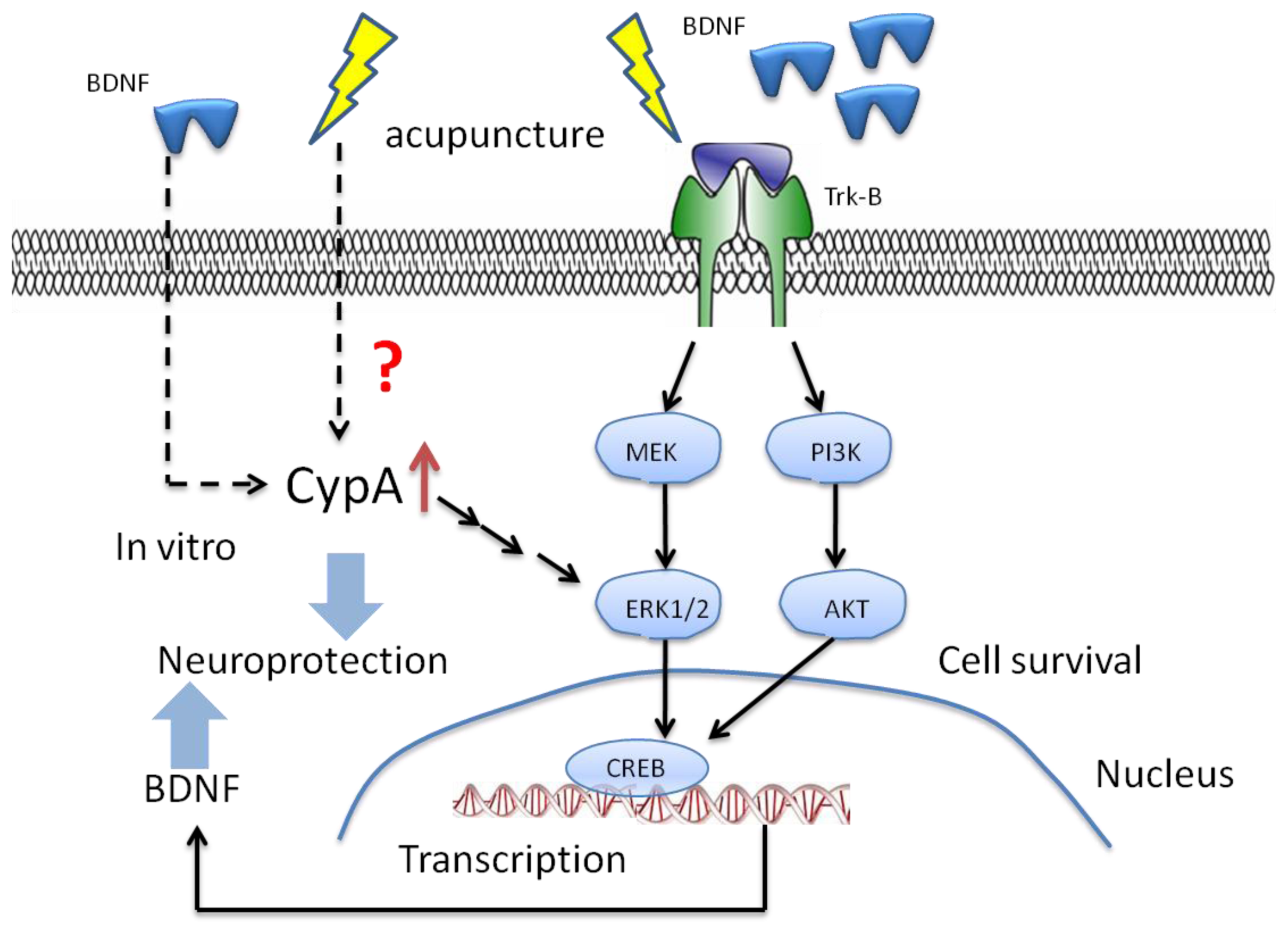
6. The Potential Mechanisms of Acupuncture with Regard to Modulation of BDNF
7. The Potential Targets for the Effect of BDNF with Acupuncture in the Future
8. Conclusions
Acknowledgments
Conflicts of Interest
Reference
- Park, H.J.; Lim, S.; Joo, W.S.; Yin, C.S.; Lee, H.S.; Lee, H.J.; Seo, J.C.; Leem, K.; Son, Y.S.; Kim, Y.J.; et al. Acupuncture prevents 6-hydroxydopamine-induced neuronal death in the nigrostriatal dopaminergic system in the rat parkinson’s disease model. Exp. Neurol 2003, 180, 93–98. [Google Scholar]
- Diehl, D.L.; Kaplan, G.; Coulter, I.; Glik, D.; Hurwitz, E.L. Use of acupuncture by american physicians. J. Altern. Complement. Med 1997, 3, 119–126. [Google Scholar]
- Chuang, C.M.; Hsieh, C.L.; Li, T.C.; Lin, J.G. Acupuncture stimulation at baihui acupoint reduced cerebral infarct and increased dopamine levels in chronic cerebral hypoperfusion and ischemia-reperfusion injured sprague-dawley rats. Am. J. Chin. Med 2007, 35, 779–791. [Google Scholar]
- Donoyama, N.; Ohkoshi, N. Effects of traditional japanese massage therapy on various symptoms in patients with parkinson’s disease: A case-series study. J. Altern. Complement. Med 2012, 18, 294–299. [Google Scholar]
- Choi, Y.G.; Yeo, S.; Hong, Y.M.; Lim, S. Neuroprotective changes of striatal degeneration-related gene expression by acupuncture in an mptp mouse model of parkinsonism: Microarray analysis. Cell. Mol. Neurobiol 2011, 31, 377–391. [Google Scholar]
- Choi, D.C.; Lee, J.Y.; Moon, Y.J.; Kim, S.W.; Oh, T.H.; Yune, T.Y. Acupuncture-mediated inhibition of inflammation facilitates significant functional recovery after spinal cord injury. Neurobiol. Dis 2010, 39, 272–282. [Google Scholar]
- Choi, S.; Lee, G.J.; Chae, S.J.; Kang, S.W.; Yin, C.S.; Lee, S.H.; Choi, S.K.; Park, H.K. Potential neuroprotective effects of acupuncture stimulation on diabetes mellitus in a global ischemic rat model. Physiol. Meas 2010, 31, 633–647. [Google Scholar]
- Doo, A.R.; Kim, S.T.; Kim, S.N.; Moon, W.; Yin, C.S.; Chae, Y.; Park, H.K.; Lee, H.; Park, H.J. Neuroprotective effects of bee venom pharmaceutical acupuncture in acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of parkinson’s disease. Neurol. Res 2010, 32, 88–91. [Google Scholar]
- Hong, M.S.; Park, H.K.; Yang, J.S.; Park, H.J.; Kim, S.T.; Kim, S.N.; Park, J.Y.; Song, J.Y.; Jo, D.J.; Park, S.W.; et al. Gene expression profile of acupuncture treatment in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinson’s disease model. Neurol. Res 2010, 32, 74–78. [Google Scholar]
- Kim, W.S.; Kim, I.S.; Kim, S.J.; Wei, P.; Hyung Choi, D.; Han, T.R. Effect of electroacupuncture on motor recovery in a rat stroke model during the early recovery stage. Brain Res 2009, 1248, 176–183. [Google Scholar]
- Hwang, I.K.; Chung, J.Y.; Yoo, D.Y.; Yi, S.S.; Youn, H.Y.; Seong, J.K.; Yoon, Y.S. Effects of electroacupuncture at zusanli and baihui on brain-derived neurotrophic factor and cyclic amp response element-binding protein in the hippocampal dentate gyrus. J. Vet. Med. Sci 2010, 72, 1431–1436. [Google Scholar]
- Abbracchio, M.P.; Burnstock, G.; Verkhratsky, A.; Zimmermann, H. Purinergic signalling in the nervous system: An overview. Trends Neurosci 2009, 32, 19–29. [Google Scholar]
- Yang, C.P.; Chang, M.H.; Liu, P.E.; Li, T.C.; Hsieh, C.L.; Hwang, K.L.; Chang, H.H. Acupuncture versus topiramate in chronic migraine prophylaxis: A randomized clinical trial. Cephalalgia 2011, 31, 1510–1521. [Google Scholar]
- Fischer, M.; Wille, G.; Klien, S.; Shanib, H.; Holle, D.; Gaul, C.; Broessner, G. Brain-derived neurotrophic factor in primary headaches. J. Headache Pain 2012, 13, 469–475. [Google Scholar]
- Castrén, E.; Zafra, F.; Thoenen, H.; Lindholm, D. Light regulates expression of brain-derived neurotrophic factor mrna in rat visual cortex. Proc. Natl. Acad. Sci. USA 1992, 89, 9444–9448. [Google Scholar]
- Mattson, M.P.; Duan, W.; Wan, R.; Guo, Z. Prophylactic activation of neuroprotective stress response pathways by dietary and behavioral manipulations. NeuroRx 2004, 1, 111–116. [Google Scholar]
- Lee, J.Y.; Kim, S.H.; Ko, A.R.; Lee, J.S.; Yu, J.H.; Seo, J.H.; Cho, B.P.; Cho, S.R. Therapeutic effects of repetitive transcranial magnetic stimulation in an animal model of parkinson’s disease. Brain Res 2013, 1537, 290–302. [Google Scholar]
- Yue, L.; Xiao-Lin, H.; Tao, S. The effects of chronic repetitive transcranial magnetic stimulation on glutamate and gamma-aminobutyric acid in rat brain. Brain Res 2009, 1260, 94–99. [Google Scholar]
- Yukimasa, T.; Yoshimura, R.; Tamagawa, A.; Uozumi, T.; Shinkai, K.; Ueda, N.; Tsuji, S.; Nakamura, J. High-frequency repetitive transcranial magnetic stimulation improves refractory depression by influencing catecholamine and brain-derived neurotrophic factors. Pharmacopsychiatry 2006, 39, 52–59. [Google Scholar]
- Matsuda, F.; Sakakima, H.; Yoshida, Y. The effects of early exercise on brain damage and recovery after focal cerebral infarction in rats. Acta Physiol 2011, 201, 275–287. [Google Scholar]
- Ke, Z.; Yip, S.P.; Li, L.; Zheng, X.X.; Tam, W.K.; Tong, K.Y. The effects of voluntary, involuntary, and forced exercises on motor recovery in a stroke rat model. Conf. Proc. IEEE Eng. Med. Biol. Soc 2011, 2011, 8223–8226. [Google Scholar]
- Ploughman, M.; Windle, V.; MacLellan, C.L.; White, N.; Doré, J.J.; Corbett, D. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke 2009, 40, 1490–1495. [Google Scholar]
- Sakakima, H.; Khan, M.; Dhammu, T.S.; Shunmugavel, A.; Yoshida, Y.; Singh, I.; Singh, A.K. Stimulation of functional recovery via the mechanisms of neurorepair by s-nitrosoglutathione and motor exercise in a rat model of transient cerebral ischemia and reperfusion. Restor. Neurol. Neurosci 2012, 30, 383–396. [Google Scholar]
- Chen, M.J.; Russo-Neustadt, A.A. Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Res. Mol. Brain Res 2005, 135, 181–193. [Google Scholar]
- Ni, Y.Q.; Gan, D.K.; Xu, H.D.; Xu, G.Z.; Da, C.D. Neuroprotective effect of transcorneal electrical stimulation on light-induced photoreceptor degeneration. Exp. Neurol 2009, 219, 439–452. [Google Scholar]
- Gomes, J.R.; Costa, J.T.; Melo, C.V.; Felizzi, F.; Monteiro, P.; Pinto, M.J.; Inácio, A.R.; Wieloch, T.; Almeida, R.D.; Grãos, M.; et al. Excitotoxicity downregulates trkb.Fl signaling and upregulates the neuroprotective truncated trkb receptors in cultured hippocampal and striatal neurons. J. Neurosci 2012, 32, 4610–4622. [Google Scholar]
- Sato, T.; Fujikado, T.; Morimoto, T.; Matsushita, K.; Harada, T.; Tano, Y. Effect of electrical stimulation on igf-1 transcription by l-type calcium channels in cultured retinal müller cells. Jpn. J. Ophthalmol 2008, 52, 217–223. [Google Scholar]
- Zhou, W.T.; Ni, Y.Q.; Jin, Z.B.; Zhang, M.; Wu, J.H.; Zhu, Y.; Xu, G.Z.; Gan, D.K. Electrical stimulation ameliorates light-induced photoreceptor degeneration in vitro via suppressing the proinflammatory effect of microglia and enhancing the neurotrophic potential of müller cells. Exp. Neurol 2012, 238, 192–208. [Google Scholar]
- Mowla, S.J.; Farhadi, H.F.; Pareek, S.; Atwal, J.K.; Morris, S.J.; Seidah, N.G.; Murphy, R.A. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J. Biol. Chem 2001, 276, 12660–12666. [Google Scholar]
- Lin, E.; Hong, C.J.; Hwang, J.P.; Liou, Y.J.; Yang, C.H.; Cheng, D.; Tsai, S.J. Gene-gene interactions of the brain-derived neurotrophic-factor and neurotrophic tyrosine kinase receptor 2 genes in geriatric depression. Rejuvenation Res 2009, 12, 387–393. [Google Scholar]
- Tongiorgi, E. Activity-dependent expression of brain-derived neurotrophic factor in dendrites: Facts and open questions. Neurosci. Res 2008, 61, 335–346. [Google Scholar]
- Murer, M.G.; Yan, Q.; Raisman-Vozari, R. Brain-derived neurotrophic factor in the control human brain, and in alzheimer’s disease and parkinson’s disease. Prog. Neurobiol 2001, 63, 71–124. [Google Scholar]
- Schäbitz, W.R.; Sommer, C.; Zoder, W.; Kiessling, M.; Schwaninger, M.; Schwab, S. Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates bax and bcl-2 expression after temporary focal cerebral ischemia. Stroke 2000, 31, 2212–2217. [Google Scholar]
- Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2000, 103, 211–225. [Google Scholar]
- Hetman, M.; Kanning, K.; Cavanaugh, J.E.; Xia, Z. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J. Biol. Chem 1999, 274, 22569–22580. [Google Scholar]
- Xia, Y.; Wang, C.Z.; Liu, J.; Anastasio, N.C.; Johnson, K.M. Brain-derived neurotrophic factor prevents phencyclidine-induced apoptosis in developing brain by parallel activation of both the erk and pi-3k/akt pathways. Neuropharmacology 2010, 58, 330–336. [Google Scholar]
- Andersson, S.; Lundeberg, T. Acupuncture--from empiricism to science: Functional background to acupuncture effects in pain and disease. Med. Hypotheses 1995, 45, 271–281. [Google Scholar]
- Zhao, Z.Q. Neural mechanism underlying acupuncture analgesia. Prog. Neurobiol 2008, 85, 355–375. [Google Scholar]
- Kaptchuk, T.J. Acupuncture: Theory, efficacy, and practice. Ann. Intern. Med 2002, 136, 374–383. [Google Scholar]
- Guan, X.M.; Wang, C.Y.; Liu, X.C.; Zhang, Y.W.; Zheng, X.Y.; Liang, X.C.; Li, L.L. The influence of ach on the metabolism of 5-ht in the brain during acupuncture analgesia. Zhen Ci Yan Jiu 1988, 13, 314–318. [Google Scholar]
- Manni, L.; Albanesi, M.; Guaragna, M.; Barbaro Paparo, S.; Aloe, L. Neurotrophins and acupuncture. Auton. Neurosci 2010, 157, 9–17. [Google Scholar]
- Yu, Y.P.; Ju, W.P.; Li, Z.G.; Wang, D.Z.; Wang, Y.C.; Xie, A.M. Acupuncture inhibits oxidative stress and rotational behavior in 6-hydroxydopamine lesioned rat. Brain Res 2010, 1336, 58–65. [Google Scholar]
- Han, J.S.; Terenius, L. Neurochemical basis of acupuncture analgesia. Annu. Rev. Pharmacol. Toxicol 1982, 22, 193–220. [Google Scholar]
- Guo, J.; Liu, J.; Fu, W.; Ma, W.; Xu, Z.; Yuan, M.; Zhou, X.; Hu, J. Effect of electroacupuncture stimulation of hindlimb on seizure incidence and supragranular mossy fiber sprouting in a rat model of epilepsy. J. Physiol. Sci 2008, 58, 309–315. [Google Scholar]
- Joh, T.H.; Park, H.J.; Kim, S.N.; Lee, H. Recent development of acupuncture on parkinson’s disease. Neurol. Res 2010, 32, 5–9. [Google Scholar]
- Cakmak, Y.O. Epilepsy, electroacupuncture and the nucleus of the solitary tract. Acupunct. Med 2006, 24, 164–168. [Google Scholar]
- Chen, Y.; Zhou, J.; Li, J.; Yang, S.B.; Mo, L.Q.; Hu, J.H.; Yuan, W.L. Electroacupuncture pretreatment prevents cognitive impairment induced by limb ischemia-reperfusion via inhibition of microglial activation and attenuation of oxidative stress in rats. Brain Res 2012, 1432, 36–45. [Google Scholar]
- Yun, S.J.; Park, H.J.; Yeom, M.J.; Hahm, D.H.; Lee, H.J.; Lee, E.H. Effect of electroacupuncture on the stress-induced changes in brain-derived neurotrophic factor expression in rat hippocampus. Neurosci. Lett 2002, 318, 85–88. [Google Scholar]
- Hu, L.; Liang, J.; Jin, S.Y.; Han, Y.J.; Lu, J.; Tu, Y. Progress of researches on mechanisms of acupuncture underlying improvement of depression in the past five years. Zhen Ci Yan Jiu 2013, 38, 253–258. [Google Scholar]
- Liang, J.; Lu, J.; Cui, S.F.; Wang, J.R.; Tu, Y. Effect of acupuncture on expression of brain-derived neurotrophic factor gene and protein in frontal cortex and hippocampus of depression rats. Zhen Ci Yan Jiu 2012, 37, 20–24. [Google Scholar]
- Zhao, J.X.; Tian, Y.X.; Xiao, H.L.; Hu, M.X.; Chen, W.R. Effects of electroacupuncture on hippocampal and cortical apoptosis in a mouse model of cerebral ischemia-reperfusion injury. J. Tradit. Chin. Med 2011, 31, 349–355. [Google Scholar]
- Zhao, J.; Xu, H.; Tian, Y.; Hu, M.; Xiao, H. Effect of electroacupuncture on brain-derived neurotrophic factor mrna expression in mouse hippocampus following cerebral ischemia-reperfusion injury. J. Tradit. Chin. Med 2013, 33, 253–257. [Google Scholar]
- Tao, J.; Chen, B.; Gao, Y.; Yang, S.; Huang, J.; Jiang, X.; Wu, Y.; Peng, J.; Hong, Z.; Chen, L. Electroacupuncture enhances hippocampal nscs proliferation in cerebral ischemia-reperfusion injured rats via activation of notch signaling pathway. Int. J. Neurosci 2014, 124, 204–212. [Google Scholar]
- Kim, M.W.; Chung, Y.C.; Jung, H.C.; Park, M.S.; Han, Y.M.; Chung, Y.A.; Maeng, L.S.; Park, S.I.; Lim, J.; Im, W.S.; et al. Electroacupuncture enhances motor recovery performance with brain-derived neurotrophic factor expression in rats with cerebral infarction. Acupunct. Med 2012, 30, 222–226. [Google Scholar]
- Chen, A.; Lin, Z.; Lan, L.; Xie, G.; Huang, J.; Lin, J.; Peng, J.; Tao, J.; Chen, L. Electroacupuncture at the quchi and zusanli acupoints exerts neuroprotective role in cerebral ischemia-reperfusion injured rats via activation of the pi3k/akt pathway. Int. J. Mol. Med 2012, 30, 791–796. [Google Scholar]
- Kim, J.H.; Choi, K.H.; Jang, Y.J.; Kim, H.N.; Bae, S.S.; Choi, B.T.; Shin, H.K. Electroacupuncture preconditioning reduces cerebral ischemic injury via bdnf and sdf-1α in mice. BMC Complement. Altern. Med 2013, 13, 22. [Google Scholar] [CrossRef]
- Xia, Y.; Cao, X.; Wu, G.-C.; Cheng, J. Acupuncture Therapy for Neurological Diseases: A Neurobiological View; Springer: Beijing, China, 2010. [Google Scholar]
- Zeng, B.-Y.; Zhao, K.; Liang, F. Neurobiology of Acupuncture, 1st ed.; Academic Press: Waltham, MA, USA, 2013. [Google Scholar]
- Kim, H.; Park, H.J.; Han, S.M.; Hahm, D.H.; Lee, H.J.; Kim, K.S.; Shim, I. The effects of acupuncture stimulation at pc6 (neiguan) on chronic mild stress-induced biochemical and behavioral responses. Neurosci. Lett 2009, 460, 56–60. [Google Scholar]
- Lee, B.; Shim, I.; Lee, H.J.; Yang, Y.; Hahm, D.H. Effects of acupuncture on chronic corticosterone-induced depression-like behavior and expression of neuropeptide y in the rats. Neurosci. Lett 2009, 453, 151–156. [Google Scholar]
- Lee, B.; Sur, B.J.; Kwon, S.; Jung, E.; Shim, I.; Lee, H.; Hahm, D.H. Acupuncture stimulation alleviates corticosterone-induced impairments of spatial memory and cholinergic neurons in rats. Evid. Based Complement. Altern. Med 2012, 2012, 670536. [Google Scholar] [CrossRef]
- Wang, T.; Liu, C.Z.; Yu, J.C.; Jiang, W.; Han, J.X. Acupuncture protected cerebral multi-infarction rats from memory impairment by regulating the expression of apoptosis related genes bcl-2 and bax in hippocampus. Physiol. Behav 2009, 96, 155–161. [Google Scholar]
- Tyler, W.J.; Alonso, M.; Bramham, C.R.; Pozzo-Miller, L.D. From acquisition to consolidation: On the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn. Mem 2002, 9, 224–237. [Google Scholar]
- Liang, C.; Jiang, T. Acupoint autohemotherapy for allergic rhinitis and its effect on serum il-12 and ifn-gamma. Zhongguo Zhen Jiu 2012, 32, 1077–1080. [Google Scholar]
- Xiao, L.; Li, B.; Du, Y.H.; Xiong, J.; Gao, X. Systematic evaluation of the randomized controlled trials about acupuncture and moxibustion treatment of allergic rhinitis. Zhongguo Zhen Jiu 2009, 29, 512–516. [Google Scholar]
- Rom, E. Sensory stimulation for lowering intraocular pressure, improving blood flow to the optic nerve and neuroprotection in primary open-angle glaucoma. Acupunct. Med 2013, 31, 416–421. [Google Scholar]
- Park, H.; Yoo, D.; Kwon, S.; Yoo, T.W.; Park, H.J.; Hahm, D.H.; Lee, H.; Kim, S.T. Acupuncture stimulation at ht7 alleviates depression-induced behavioral changes via regulation of the serotonin system in the prefrontal cortex of maternally-separated rat pups. J. Physiol. Sci 2012, 62, 351–357. [Google Scholar]
- Wang, S.J.; Tan, L.H.; Liu, J.L. Effect of electroacupuncture at different acupoints on expression of cervico-spinal gdnf and bdnf and their receptor genes in neck-incision pain rats. Zhen Ci Yan Jiu 2012, 37, 351–356. [Google Scholar]
- Zhang, X.F.; Zou, Y.; Zhao, Y.; Wang, T.H.; Zhang, W. Effects of electroacupuncture of “Governor vessel” Acupoints on changes of bdnf in the cortical motor area of mice with spinal cord transection. Sichuan Da Xue Xue Bao Yi Xue Ban 2012, 43, 250–253. [Google Scholar]
- Sun, H.; Zhang, H.; Lin, B.S. Effect of acupuncture on the expression of bcl-xl and bdnf of retina in rabbits with chronic intraocular hypertension. Zhongguo Zhen Jiu 2010, 30, 661–664. [Google Scholar]
- Hua, C.; Liu, Q.S. Effects of acupuncture on the expression of brain-derived neurotrophic factor in the ovariectomized rat fracture model. Zhongguo Zhen Jiu 2009, 29, 303–308. [Google Scholar]
- Manni, L.; Aloe, L.; Fiore, M. Changes in cognition induced by social isolation in the mouse are restored by electro-acupuncture. Physiol. Behav 2009, 98, 537–542. [Google Scholar]
- Wang, J.H.; Chen, B.G.; Yin, J.; Wang, G.; Zou, W.G.; Luo, X.J. Effect of electroacupuncture of different acupoints on the excitability of detrusor muscle and the expression of bdnf and trkb in the spinal cord of rats with urinary retention due to spinal cord injury. Zhen Ci Yan Jiu 2009, 34, 387–392. [Google Scholar]
- Jeon, S.; Kim, Y.J.; Kim, S.T.; Moon, W.; Chae, Y.; Kang, M.; Chung, M.Y.; Lee, H.; Hong, M.S.; Chung, J.H.; et al. Proteomic analysis of the neuroprotective mechanisms of acupuncture treatment in a parkinson’s disease mouse model. Proteomics 2008, 8, 4822–4832. [Google Scholar]
- Chen, J.; Qi, J.G.; Zhang, W.; Zhou, X.; Meng, Q.S.; Zhang, W.M.; Wang, X.Y.; Wang, T.H. Electro-acupuncture induced ngf, bdnf and nt-3 expression in spared l6 dorsal root ganglion in cats subjected to removal of adjacent ganglia. Neurosci. Res 2007, 59, 399–405. [Google Scholar]
- Liang, X.B.; Liu, X.Y.; Li, F.Q.; Luo, Y.; Lu, J.; Zhang, W.M.; Wang, X.M.; Han, J.S. Long-term high-frequency electro-acupuncture stimulation prevents neuronal degeneration and up-regulates bdnf mrna in the substantia nigra and ventral tegmental area following medial forebrain bundle axotomy. Brain Res. Mol. Brain Res 2002, 108, 51–59. [Google Scholar]
- Xia, Y.; Wang, H.D.; Ding, Y.; Kang, B.; Liu, W.G. Parkinson’s disease combined with depression treated with electroacupuncture and medication and its effect on serum bdnf. Zhongguo Zhen Jiu 2012, 32, 1071–1074. [Google Scholar]
- Moldenhauer, S.; Burgauner, M.; Hellweg, R.; Lun, A.; Hohenböken, M.; Dietz, E.; Kiesewetter, H.; Salama, A.; Moldenhauer, A. Mobilization of cd133(+)cd34(−) cells in healthy individuals following whole-body acupuncture for spinal cord injuries. J. Neurosci.Res 2010, 88, 1645–1650. [Google Scholar]
- Mattson, M.P.; Cheng, B.; Smith-Swintosky, V.L. Mechanisms of neurotrophic factor protection against calcium- and free radical-mediated excitotoxic injury: Implications for treating neurodegenerative disorders. Exp. Neurol 1993, 124, 89–95. [Google Scholar]
- Ryffel, B.; Woerly, G.; Greiner, B.; Haendler, B.; Mihatsch, M.J.; Foxwell, B.M. Distribution of the cyclosporine binding protein cyclophilin in human tissues. Immunology 1991, 72, 399–404. [Google Scholar]
- Göldner, F.M.; Patrick, J.W. Neuronal localization of the cyclophilin a protein in the adult rat brain. J. Comp. Neurol 1996, 372, 283–293. [Google Scholar]
- Chiu, R.; Rey, O.; Zheng, J.Q.; Twiss, J.L.; Song, J.; Pang, S.; Yokoyama, K.K. Effects of altered expression and localization of cyclophilin a on differentiation of p19 embryonic carcinoma cells. Cell. Mol. Neurobiol 2003, 23, 929–943. [Google Scholar]
- Song, J.; Lu, Y.C.; Yokoyama, K.; Rossi, J.; Chiu, R. Cyclophilin a is required for retinoic acid-induced neuronal differentiation in p19 cells. J. Biol. Chem 2004, 279, 24414–24419. [Google Scholar]
- Boulos, S.; Meloni, B.P.; Arthur, P.G.; Majda, B.; Bojarski, C.; Knuckey, N.W. Evidence that intracellular cyclophilin a and cyclophilin a/cd147 receptor-mediated erk1/2 signalling can protect neurons against in vitro oxidative and ischemic injury. Neurobiol. Dis 2007, 25, 54–64. [Google Scholar]
- Fujimura, H.; Altar, C.A.; Chen, R.; Nakamura, T.; Nakahashi, T.; Kambayashi, J.; Sun, B.; Tandon, N.N. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb. Haemost 2002, 87, 728–734. [Google Scholar]
- Goldman, N.; Chen, M.; Fujita, T.; Xu, Q.; Peng, W.; Liu, W.; Jensen, T.K.; Pei, Y.; Wang, F.; Han, X.; et al. Adenosine a1 receptors mediate local anti-nociceptive effects of acupuncture. Nat. Neurosci 2010, 13, 883–888. [Google Scholar]
- Fang, K.M.; Wang, Y.L.; Huang, M.C.; Sun, S.H.; Cheng, H.; Tzeng, S.F. Expression of macrophage inflammatory protein-1α and monocyte chemoattractant protein-1 in glioma-infiltrating microglia: Involvement of atp and p2×7 receptor. J. Neurosci. Res 2011, 89, 199–211. [Google Scholar]

| Animal research | |||||||
|---|---|---|---|---|---|---|---|
| Reference | Experimental | Tissue | Stimulation | Time of treatment | Acupoint | Signal pathway | Result |
| (Kim, et al., 2013) [56] | cerebral ischemia in mice | cerebral cortex | Electroacupuncture 2 HZ/1 mA/20 min | EA preconditioning for 3 days | GV20/GV14 | Expression of BDNF increased (after 12 h) | |
| (Zhao, et al., 2013) [52] | cerebral ischemia-reperfusion injury(mouse) | hippocampus | Electroacupuncture | once daily for 7 days | BL17/GV20/BL23 | BDNF mRNA expressions Up-regulated | |
| (Chen, et al., 2012) [55] | cerebral ischemia/reperfusion (I/R) injury(rat) | Blood | Electroacupuncture/disperse wave of 1 and 20 Hz | 30 min treated/2 or 24 h after ischemia/reperfusion | LI11/ST36 | PI3K/Akt signaling pathway | increase BDNF and GDNF secretion levels in serum |
| (Kim, et al., 2012) [54] | cerebral ischaemia(rat) | ischaemic hemisphere | Electroacupuncture | once daily for 2 weeks | GV20 | BDNF/trkB | increased expression of BDNF/trkB protein |
| (Lee, et al., 2012) [61] | spatial cognitive impairment induced by repeated corticosterone (CORT)(rat) | hippocampus | Manual acupuncture | once daily for 21 days | TE5/HT7 | Up-regulate BDNF mRNA expressions levels | |
| (Liang, et al., 2012) [50] | chronic stress-induced depression (rat) | prefrontal cortex and hippocampus | Electroacupuncture | once every other day for 28 days | GV 20/EX-HN 3/PC 6 | Up-regulate BDNF mRNA and protein expression levels | |
| (Park, et al., 2012) [67] | depression-like behavioral changes (rat) | prefrontal cortex (PFC) | Manual acupuncture | 7 consecutive days | HT7/ST36 | increased expression of BDNF protein | |
| (Wang, et al., 2012) [68] | neck-incision pain rats | in the cervico-spinal cord (C1–C4) | Electroacupuncture (1–2 mA, 2 Hz/100 Hz) | 30 min | LI 18/PC 6-LI 4/ST 36-GB 34 | BDNF/trkB/trkA | Down-regulated for the BDNF mRNA, TrkA mRNA and TrkB mRNA |
| (Zhang, et al., 2012) [69] | spinal cord transaction between T9 and T10 (mouse) | cortex area | Electroacupuncture | once daily for 14 days | “Governor Vessel” acupoints | increased expression of BDNF protein | |
| (Hwang, et al., 2010) [11] | Normal Wistar rats (13-week-old) | in the dentate gyrus of hippocampus | Electroacupuncture | once daily for 3 weeks | ST36/GV20 | BDNF/CREB | increased expression of BDNF protein |
| (Sun, et al., 2010) [70] | glaucoma model in rabbits | retina | Manual acupuncture | twice a day for 4 weeks | EX-HN 7/GB 20/LR 2 | BDNF/Bcl-xl | increased expression of BDNF protein |
| (Hua, et al., 2009) [71] | ovariectomized rat fracture model | fractural callus and blood samples | Manual acupuncture | once daily for 4 weeks | GB 30/ST 36/GB 34/BL 40 | BDNF/trkB | increased expression of BDNF/trkB protein |
| (Kim, et al., 2009) [10] | middle cerebral artery occlusion (MCAO) rats | Cerebral ischemia area | Electroacupuncture (30 min, 2/15 Hz) | once daily for 16 days | GV20/GV14/LI11/ST36 | BDNF/trkB | no significant change in BDNF |
| (Manni, et al., 2009) [72] | cognition induced by social isolation in the mouse | hypothalamus, striatum and hippocampus | Electroacupuncture (30 min, 1–4 Hz) | once daily for 4 days | ST36 | Decreased expression of BDNF protein | |
| (Wang, et al., 2009)[73] | spinal cord injury (rat) | spinal cord | Electroacupuncture (20 min, 1 mA, 2 Hz/15 Hz) | once daily for 10 days | CV 4/ST 28 | increased expression of BDNF/trkB protein | |
| (Jeon, et al., 2008) [74] | MPTP induced Parkinson’s disease mouse model | substantia nigra | manual acupuncture | once daily for 7 days | B34/SI3/BL62/ST36 | BDNF/CypA | increased expression of CypA following BDNF |
| (Chen, et al., 2007) [75] | cats subjected to removal of adjacent ganglia | L6 dorsal root ganglion (DRG) | Electroacupuncture (30 min, 98 Hz) | once daily for 7 days | ST36/GB39/ST32/SP6 | Up-regulate BDNF mRNA and protein expression levels | |
| (Liang, et al., 2002) [76] | Parkinson’s disease rats model induced by transection of the medial forebrain bundle (MFB) | ventral midbrain/ventral tegmental area/substantia nigra | Electroacupuncture (30 min, 1–2 mA, 2/100 Hz) | once daily for 24 days | GV 14/GV 21 | Up-regulate BDNF mRNA expressions levels | |
| (Yun, et al., 2002) [48] | stress-induced hippocampal degeneration rats | hippocampus | Electroacupuncture (30 min, 2 Hz) | 30 min (one time) | ST36 | Up-regulate BDNF mRNA expressions levels | |
| For clinical research | |||||||
| (Xia, et al., 2012) [77] | Parkinson’s disease combined with depression patients | serum | electroacupuncture | 3 months | GV 20/EX-HN 3/EX-HN 1/LR 3/SP 6 | increased expression of BDNF(compared with that before treatment) | |
| (Moldenhauer, et al., 2010) [78] | Spinal Cord Injuries | serum | Manual acupuncture | <1 h | whole-body acupuncture | Decreased expression of BDNF in 48 h after acupuncture | |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, D.; De La Pena, I.; Lin, L.; Zhou, S.-F.; Borlongan, C.V.; Cao, C. The Neuroprotective Role of Acupuncture and Activation of the BDNF Signaling Pathway. Int. J. Mol. Sci. 2014, 15, 3234-3252. https://doi.org/10.3390/ijms15023234
Lin D, De La Pena I, Lin L, Zhou S-F, Borlongan CV, Cao C. The Neuroprotective Role of Acupuncture and Activation of the BDNF Signaling Pathway. International Journal of Molecular Sciences. 2014; 15(2):3234-3252. https://doi.org/10.3390/ijms15023234
Chicago/Turabian StyleLin, Dong, Ike De La Pena, Lili Lin, Shu-Feng Zhou, Cesar V. Borlongan, and Chuanhai Cao. 2014. "The Neuroprotective Role of Acupuncture and Activation of the BDNF Signaling Pathway" International Journal of Molecular Sciences 15, no. 2: 3234-3252. https://doi.org/10.3390/ijms15023234




