Biochemical Properties and Possible Roles of Ectophosphatase Activities in Fungi
Abstract
:1. Introduction
2. Occurrence of Ectophosphatase Activities in Fungi Cells
2.1. Ectophosphatases
2.2. Secreted Phosphatases
3. Biochemical Properties of Fungal Ectophosphatases
3.1. Substrate Specificity of Ectophosphatase Activities
3.2. pH Influence on Ectophosphatase Activities
3.3. Modulation of Ectophosphatase Activities by Divalent Metals
3.4. Sensibility of Ectophosphatase Activities to Classical Phosphatase Inhibitors
3.5. Modulation of Ectophosphatase Activities by Exogenous Pi Content
4. Possible Roles of Fungal Ectophosphatase Activities
4.1. Differential Expression of Fungal Ectophosphatase Activities
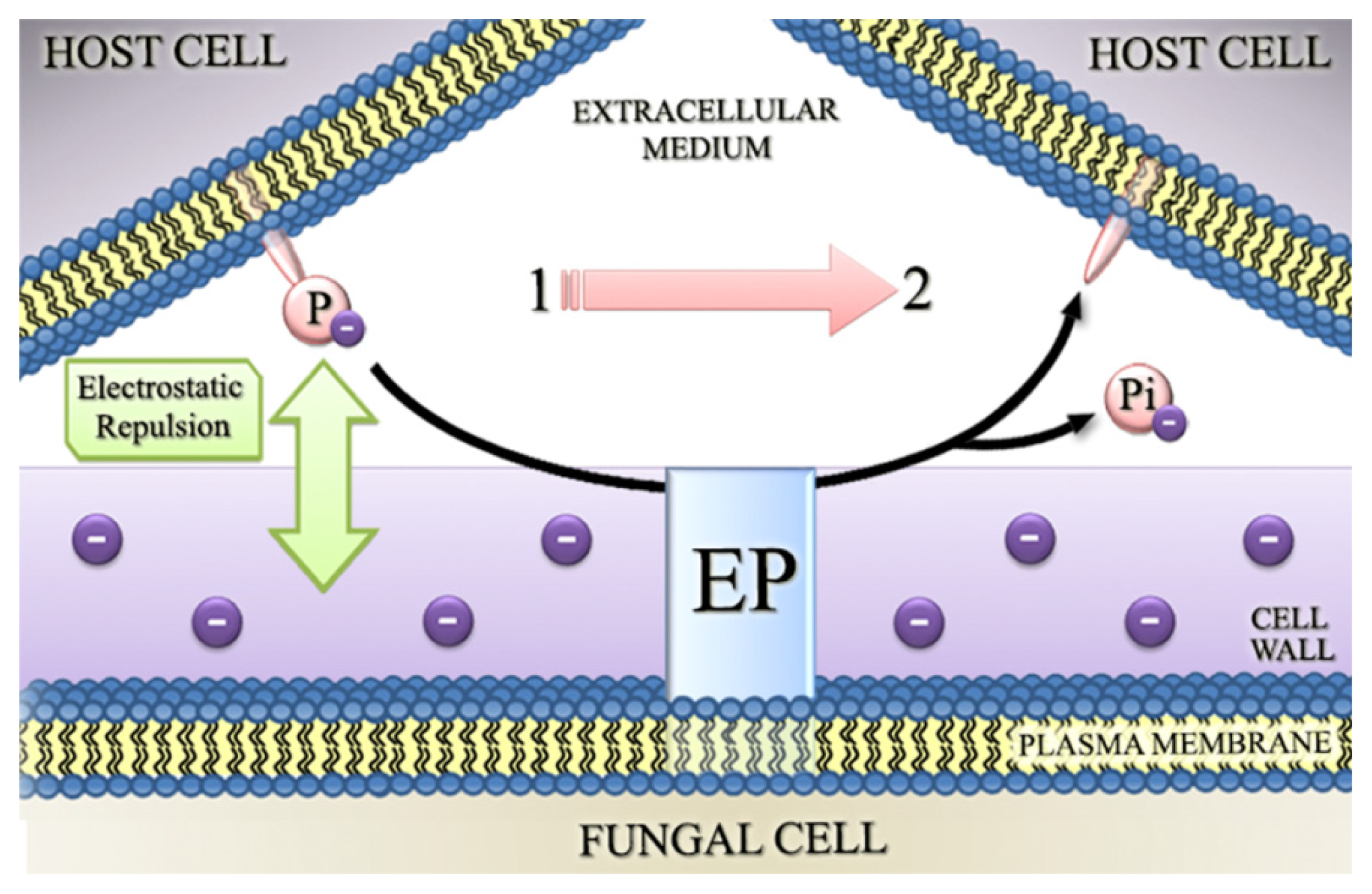
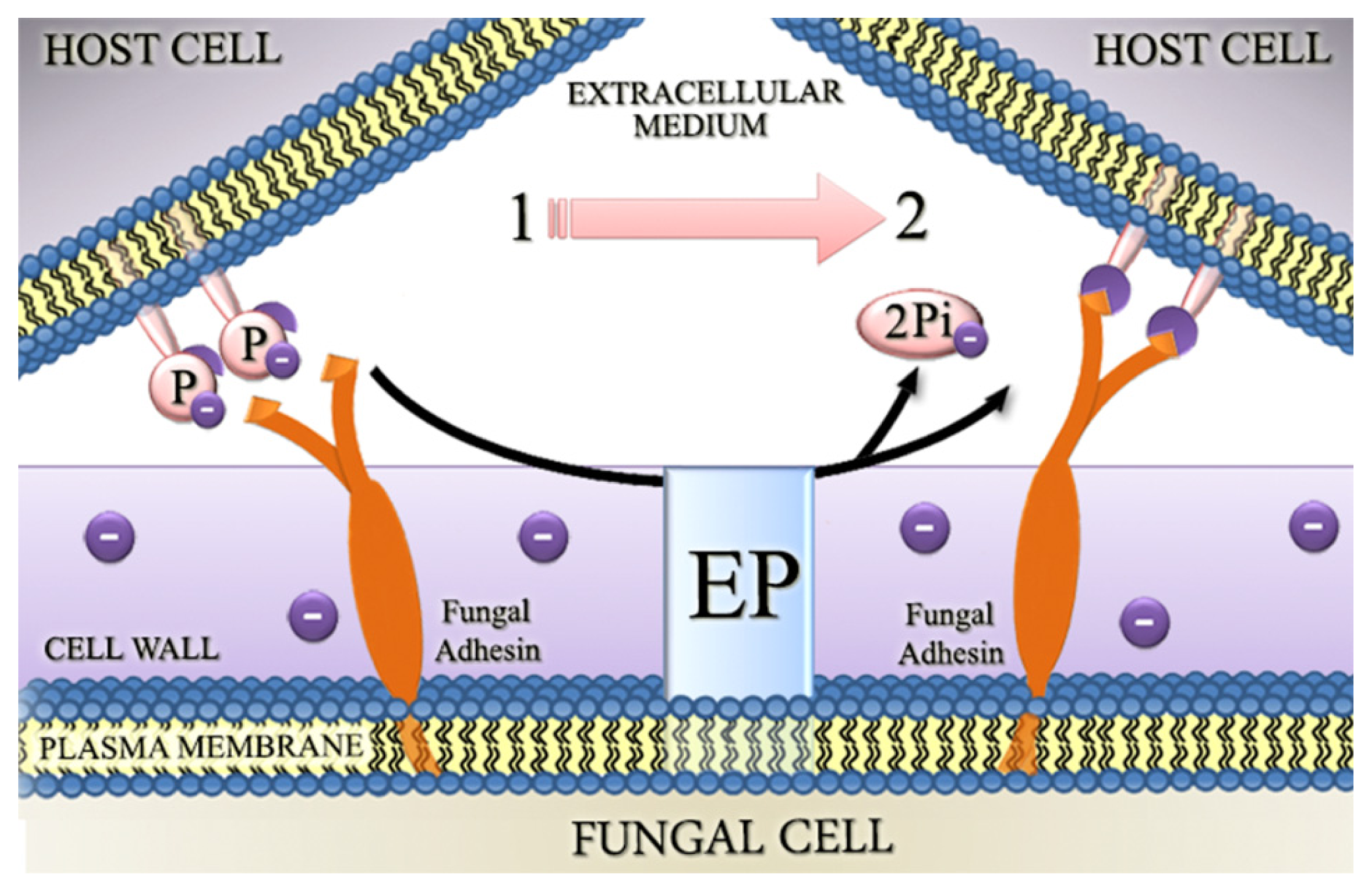
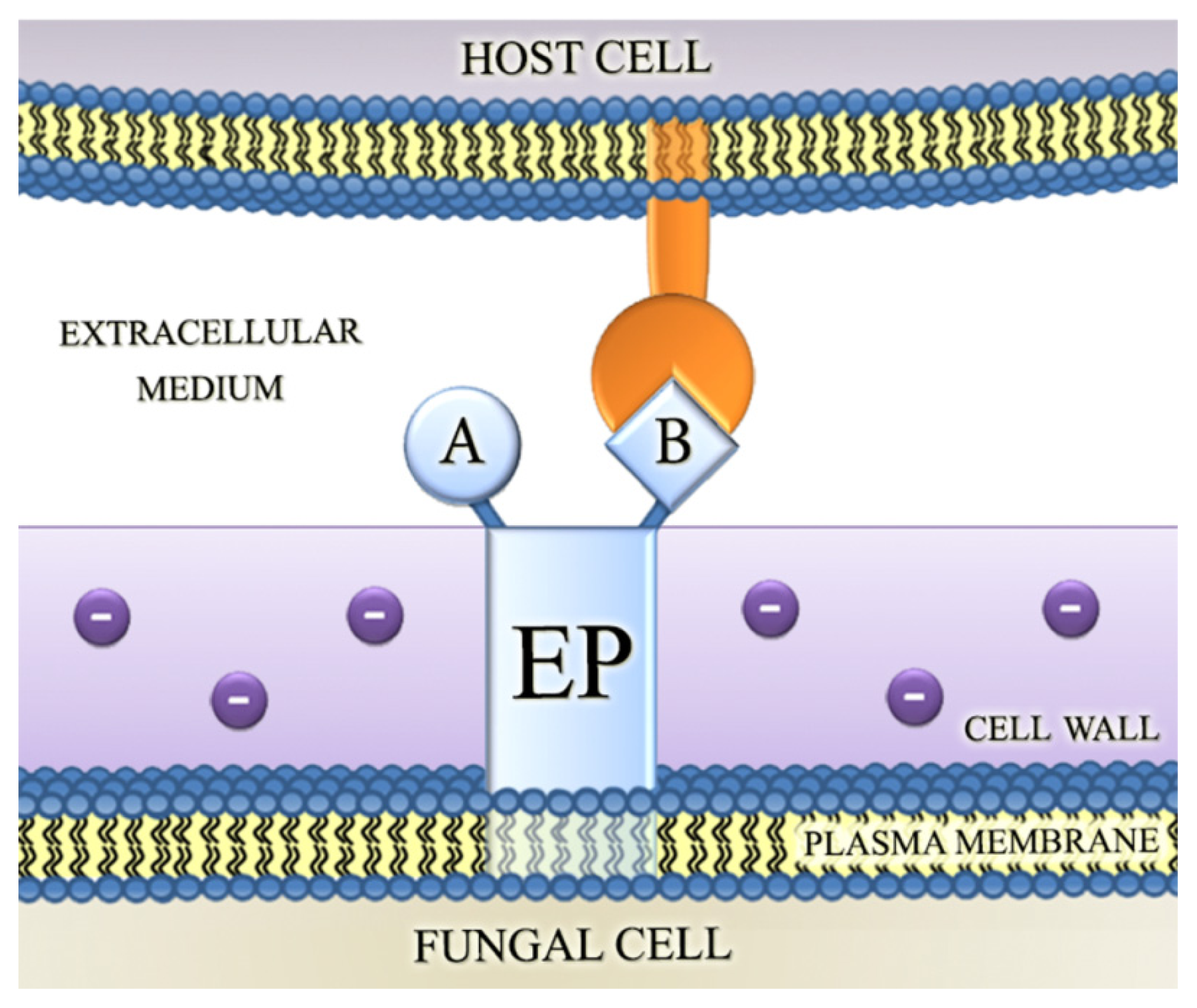
4.2. Involvement of Fungal Ectophosphatase Activities in Host-Parasite Interactions
5. Conclusion
Acknowledgments
Conflicts of Interest
References
- Andreeva, A.V.; Kutuzov, M.A. Protozoan protein tyrosine phosphatases. Int. J. Parasitol 2008, 38, 1279–1295. [Google Scholar]
- Szöör, B. Trypanosomatid protein phosphatases. Mol. Biochem. Parasitol 2010, 173, 53–63. [Google Scholar]
- Cohen, P. The structure and regulation of protein phosphatases. Annu. Rev. Biochem 1989, 58, 453–508. [Google Scholar]
- Zhan, X.L.; Hong, Y.; Zhu, T.; Mitchell, A.P.; Deschenes, R.J.; Guan, K.L. Essential functions of protein tyrosine phosphatase Ptp2 and Ptp3 and Rim11 tyrosine phosphorylation in Saccharomyces cerevisiae meiosis and sporulation. Mol. Biol. Cell 2000, 11, 663–676. [Google Scholar]
- Da-Silva, A.M.; Zapella, P.D.; Andrioli, L.P.; Campanhã, R.B.; Fiorini, L.C.; Etchebehere, L.C.; Da-Costa-Maia, J.C.; Terenzi, H.F. Searching for the role of protein phosphatases in eukaryotic microorganisms. Braz. J. Med. Biol. Res 1999, 32, 835–839. [Google Scholar]
- Madhani, H.D.; Fink, G.R. The control of filamentous differentiation and virulence in fungi. Trends Cell Biol 1998, 8, 348–353. [Google Scholar]
- Suresh, K.; Subramanyam, C. A putative role for calmodulin in the activation of Neurospora crassa chitin synthase. FEMS Microbiol. Lett 1997, 150, 95–100. [Google Scholar]
- Yatzkan, E.; Szöor, B.; Fehér, Z.; Dombrádi, V.; Yarden, O. Protein phosphatase 2A is involved in hyphal growth of Neurospora crassa. Mol. Genet. Genomics 1998, 259, 523–531. [Google Scholar]
- Borgia, P.T. Roles of orlA, tseE and bimG genes of Aspergillus nidulans in chitin synthesis. J. Bacteriol 1992, 174, 384–389. [Google Scholar]
- Csank, C.; Makris, C.; Meloche, S.; Schröppel, K.; Röllinghoff, M.; Dignard, D.; Thomas, D.Y.; Whiteway, M. Derepressed hyphal growth and reduced virulence in VH1 family-related protein phosphatase mutant of the human pathogen Candida albicans. Mol. Biol. Cell 1997, 8, 2539–2551. [Google Scholar]
- Winkler, A.; Arkind, C.; Mattison, C.P.; Burkholder, A.; Knoche, K.; Ota, I. Heat stress activated the yeast high-osmolarity glycerol mitogen-activated protein-kinase pathway, and protein tyrosine phosphatases are essential under heat stress. Eukaryot. Cell 2002, 1, 163–173. [Google Scholar]
- Andrews, P.D.; Stark, M.J.R. Type 1 protein phosphatase is required for maintenance of cell wall integrity, morphogenesis and cell cycle progression in Saccharomyces cerevisiae. J. Cell Sci 2000, 113, 507–520. [Google Scholar]
- De Jesus, J.B.; Ferreira, M.A.; Cuervo, P.; Britto, C.; Silva-Filho, F.C.; Meyer-Fernandes, J.R. Iron modulates ecto-phosphohydrolase activities in pathogenic trichomonads. Parasitol. Int 2006, 55, 285–290. [Google Scholar]
- Dutra, P.M.; Dias, F.A.; Rodrigues, C.O.; Romeiro, A.; Attias, M.; de Souza, W.; Lopes, A.H.; Meyer-Fernandes, J.R. Platelet-activating factor modulates a secreted phosphatase activity of the trypanosomatid parasite Herpetomonas muscarum muscarum. Curr. Microbiol 2001, 43, 288–292. [Google Scholar]
- Dutra, P.M.; Dias, F.A.; Santos, M.A.A.; Rodrigues, C.O.; Romeiro, A.; Attias, M.; de Souza, W.; Lopes, A.H.; Meyer-Fernandes, J.R. Secreted phosphatase activities in trypanossomatid parasites of plants modulated by patelet-activating factor. Phytophathology 2001, 1, 408–414. [Google Scholar]
- Fernandes, A.C.; Soares, D.C.; Saraiva, E.M.; Meyer-Fernandes, J.R.; Souto-Padrón, T. Different secreted phosphatase activities in Leishmania amazonensis. FEMS Microbiol. Lett 2013, 340, 117–128. [Google Scholar]
- Joliviet, P.; Queiroz-Claret, C.; Bergeron, E.; Meunier, J.C. Characterization of an exocellular protein phosphatase with dual substrate specificity from the yeast Yarrowia lipolytica. Int. J. Biochem. Cell Biol 1998, 30, 783–796. [Google Scholar]
- Justino, A.; Nosowa, S.R.; Maccheroni, W.; May, G.S.; Martinez-Rossi, N.M.; Rossi, A. The Aspergillus nidulans pyrG89 mutation alters glycosylation of secreted acid phosphatase. Fungal Genet. Biol 2001, 32, 113–120. [Google Scholar]
- Rodrigues, C.O.; Dutra, P.M.; Barros, F.S.; Souto-Padrón, T.; Meyer-Fernandes, J.R.; Lopes, A.H.C.S. Platelet-activating factor induction of secreted phosphatase activity in Trypanosoma cruzi. Biochem. Biophys. Res. Commun 1999, 266, 36–42. [Google Scholar]
- Santos, A.L.S.; Souto-Padrón, T.; Alviano, C.S.; Lopes, A.H.; Soares, R.M.; Meyer-Fernandes, J.R. Secreted phosphatase activity induced by dimethylsulfoxide in Herpetomonas samuelpessoai. Arch. Biochem. Biophys 2002, 405, 191–198. [Google Scholar]
- Fernandes, E.C.; Meyer-Fernandes, F.R.; Silva-Neto, M.A.C.; Vercesi, A.E. Trypanosoma brucei: Ecto-phosphatase activity on the surface of intact procyclic forms. Z. Naturforschung 1997, 52C, 351–358. [Google Scholar]
- Meyer-Fernandes, J.R.; da Silva-Neto, M.A.; Dos Santo Soares, M.; Fernandes, E.; Vercesi, A.E.; de Oliveira, M.M. Ecto-phosphatase activities on the cell surface of the amastigote forms of Trypanosoma cruzi. Z. Naturforschung 1999, 54, 977–984. [Google Scholar]
- Remaley, A.T.; Glew, R.H.; Kuhns, D.B.; Basford, R.E.; Waggoner, A.S.; Ernst, L.A.; Pope, M. Leishmania donovani: Surface membrane acid phosphatase blocks neutrophils oxidative metabolite production. Exp. Parasitol 1985, 60, 331–341. [Google Scholar]
- Campo-Aasen, I.; Albornoz, M.C. Alkaline phosphatase at the cell wall of the yeast phase of Paracoccidioides brasiliensis. Mycopathologia 1994, 127, 69–71. [Google Scholar]
- Mildner, P.; Ries, B.; Barbaric, S. Acid phosphatase and adenosine triphosphatase activities in the cell wall of baker’s yeast. Biochim. Biophys. Acta 1975, 391, 67–74. [Google Scholar]
- Furuya, T.; Zhong, L.; Meyer-Fernandes, J.R.; Lu, H.G.; Moreno, S.N.; Docampo, R. Ecto-protein tyrosine phosphatase activity in Trypanosoma cruzi infective stages. Mol. Biochem. Parasitol 1998, 92, 339–348. [Google Scholar]
- Meyer-Fernandes, J.R. Ecto-ATPases in protozoa parasites: Looking for a function. Parasitol. Int 2002, 51, 299–303. [Google Scholar]
- Meyer-Fernandes, J.R.; Lanz-Mendoza, H.; Gondim, K.C.; Willott, E.; Wells, M.A. Ectonucleotide diphosphohydrolase activities in hemocytes of larval Manduca sexta. Arch. Biochem. Biophys 2000, 382, 152–159. [Google Scholar]
- De Almeida-Amaral, E.E.; Belmont-Firpo, R.; Vannier-Santos, M.A.; Meyer-Fernandes, J.R. Leishmania amazonensis: Characterization of an ecto-phosphatase activity. Exp. Parasitol 2006, 114, 334–340. [Google Scholar]
- Dos-Santos, A.L.; Dick, C.F.; Alves-Bezerra, M.; Silveira, T.S.; Paes, L.S.; Gondim, K.C.; Meyer-Fernandes, J.R. Interaction between Trypanosoma rangeli and the Rhodnius prolixus salivary gland depends on the phosphotyrosine ecto-phosphatase activity of the parasite. Int. J. Parasitol 2012, 42, 819–827. [Google Scholar]
- Braibant, M.; Content, J. The cell surface associated phosphatase activity of Mycobacterium bovis BCG is not regulated by enviromental inorganis phosphate. FEMS Microbiol. Lett 2001, 195, 121–126. [Google Scholar]
- Arnold, W.N.; Mann, L.C.; Sakai, K.H.; Garrison, R.G.; Coleman, P.D. Acid phosphatases of Sporothrix schenckii. J. Gen. Microbiol 1986, 132, 3421–3432. [Google Scholar]
- Fernanado, P.H.; Panagoda, G.J.; Samaranayake, L.P. The relationship between the acid and alkaline phosphatase activity and the adherence of clinical isolates of Candida parapsilosis to human buccal epithelial cells. APMIS 1999, 107, 1034–1042. [Google Scholar]
- Kiffer-Moreira, T.; de Sá Pinheiro, A.A.; Alviano, W.S.; Barbosa, F.M.; Souto-Padrón, T.; Nimrichter, L.; Rodrigues, M.L.; Alviano, C.S.; Meyer-Fernandes, J.R. An ectophosphatase activity in Candida parapsilosis influences the interaction of fungi with epithelial cells. FEMS Yeast Res 2007, 7, 621–628. [Google Scholar]
- Yoda, K.; Ko, J.H.; Nagamatsu, T.; Lin, Y.; Kaibara, C.; Kawada, T.; Tomishige, N.; Hashimoto, H.; Noda, Y.; Yamasaki, M. Molecular characterization of a novel yeast cell-wall acid phosphatase cloned from Kluyveromyces marxianus. Biosci. Biotechnol. Biochem 2000, 64, 142–148. [Google Scholar]
- Bernard, M.; Mouyna, I.; Dubreucq, G.; Debeaupuis, J.P.; Fontaine, T.; Vorgias, C.; Fuglsang, C.; Latgé, J.P. Characterization of a cell-wall acid phosphatase (PhoAp) in Aspergillus fumigatus. Microbiology 2002, 148, 2819–2829. [Google Scholar]
- Kneipp, L.F.; Palmeira, V.F.; Pinheiro, A.A.; Alviano, C.S.; Rozental, S.; Travassos, L.R.; Meyer-Fernandes, J.R. Phosphatase activity on the cell wall of Fonsecaea pedrosoi. Med. Mycol 2003, 41, 469–477. [Google Scholar]
- Kneipp, L.F.; Rodrigues, M.L.; Holandino, C.; Esteves, F.F.; Souto-Padrón, T.; Alviano, C.S.; Travassos, L.R.; Meyer-Fernandes, J.R. Ectophosphatase activity in conidial forms of Fonsecaea pedrosoi is modulated by exogenous phosphate and influences fungal adhesion to mammalian cells. Microbiology 2004, 150, 3355–3362. [Google Scholar]
- Collopy-Junir, I.; Esteves, F.F.; Nimrichter, L.; Rodrigues, M.L.; Alviano, C.S.; Meyer-Fernandes, J.R. An ectophosphatase activity in Cryptococcus neoformans. FEMS Yeast Res 2006, 6, 1010–1017. [Google Scholar]
- Kiffer-Moreira, T.; Pinheiro, A.A.; Pinto, M.R.; Esteves, F.F.; Souto-Padrón, T.; Barreto-Bergter, E.; Meyer-Fernandes, J.R. Mycelial forms of Pseudallescheria boydii present ectophosphatase activities. Arch. Microbiol 2007, 188, 159–166. [Google Scholar]
- Portela, M.B.; Kneipp, L.F.; Ribeiro de Souza, I.P.; Holandino, C.; Alviano, C.S.; Meyer-Fernandes, J.R.; de Araújo Soares, R.M. Ectophosphatase activity in Candida albicans influences fungal adhesion: study between HIV-positive and HIV-negative isolates. Oral Dis 2010, 16, 431–437. [Google Scholar]
- Kneipp, L.F.; Magalhães, A.S.; Abi-Chacra, E.A.; Souza, L.O.; Alviano, C.S.; Santos, A.L.; Meyer-Fernandes, J.R. Surface phosphatase in Rhinocladiella aquaspersa: biochemical properties and its involvement with adhesion. Med. Mycol 2012, 50, 570–578. [Google Scholar]
- Cosentino-Gomes, D.; Rocco-Machado, N.; Santi, L.; Broetto, L.; Vainstein, M.H.; Meyer-Fernandes, J.R.; Schrank, A.; Beyes-da-Silva, W.O. Inhibition of ecto-phosphatase activity in conidia reduces adhesion and virulence of Metarhizium anisopliae on the host insect Dysdercus peruvianus. Curr. Microbiol 2013, 66, 467–474. [Google Scholar]
- Li, Z.; Wang, Z.; Peng, G.; Yin, Y.; Zhao, H.; Cao, Y.; Xia, Y. Purification and characterization of a novel thermostable extracellular protein tyrosine phosphatase from Metarhizium anisopliae strain CQMa102. Biosci. Biotechnol. Biochem 2006, 70, 1961–1968. [Google Scholar]
- Gomes, M.T.; Lopes, A.H.; Meyer-Fernandes, J.R. Possible roles of ectophosphatases in host-parasite interactions. J. Parasitol. Res 2011, 2011, 1–7. [Google Scholar]
- Egloff, M.P.; Cohen, P.T.; Reinemer, P.; Barford, D. Crystal structure of the catalytic subunit of human protein phosphatase 1 and its complex with tugstate. J. Mol. Biol 1995, 254, 942–959. [Google Scholar]
- Denu, J.M.; Stuckey, J.A.; Saper, M.A.; Dixon, J.E. Form and function in protein dephosphorylation. Cell 1996, 87, 361–364. [Google Scholar]
- Alviano, D.S.; Kneipp, L.F.; Lopes, A.H.; Travassos, L.R.; Meyer-Fernandes, J.R.; Rodrigues, M.L.; Alviano, C.S. Differentiation of Fonsecaea pedrosoi mycelial forms into sclerotic cells is induced by platelet-activating factor. Res. Microbiol 2003, 154, 689–695. [Google Scholar]
- Arnold, W.N. Enzymes. In Yeast Cell Envelopes: Biochemistry, Biophysics and Ultrastructure; Arnold, W.N., Ed.; CRC Press: Boca Raton, FL, USA, 1981; Volume 2, pp. 1–46. [Google Scholar]
- Onishi, M.R.; Tkacz, J.S.; Lampen, J.O. Glycoprotein nature of yeast alkaline phosphatase: Formation of active enzyme in the presence of tunicamycin. J. Biol. Chem 1979, 254, 11943–11952. [Google Scholar]
- Schurr, A.; Yagil, E. Regulation and characterization of acid and alkaline phosphatase in yeast. J. Gen. Microbiol 1971, 65, 291–303. [Google Scholar]
- Cosentino-Gomes, D.; Meyer-Fernandes, J.R. Ecto-phosphatases in protozoan parasites: Possible roles in nutrition, growth and ROS sensing. J. Bioenerg. Biomembr 2011, 43, 89–92. [Google Scholar]
- Fernandes, E.C.; Granjeiro, J.M.; Aoyama, H.; Fonseca, F.V.; Meyer-Fernandes, J.R.; Vercesi, A.E. A metallo phosphatase activity present on the surface of Trypanosoma brucei procyclic forms. Vet. Parasitol 2003, 118, 19–28. [Google Scholar]
- Spencer, D.B.; Chen, C.P.; Hulett, F.M. Effect of cobalt on synthesis and activation of Bacillus licheniformis alkaline phosphatase. J. Bacteriol 1981, 145, 926–933. [Google Scholar]
- De Sá Pinheiro, A.A.; Amazonas, J.N.; Barros, F.S.; de Menezes, L.F.; Batista, E.J.; Silva, E.F.; de Souza, W.; Meyer-Fernandes, J.R. Entamoeba histolytica: An ectophosphatase activity regulated by oxidation-reduction reactions. Exp. Parasitol 2007, 115, 352–358. [Google Scholar]
- Huyer, G.; Liu, S.; Kelly, J.; Moffat, J.; Payette, P.; Kennedy, B.; Tsaprailis, G.; Gresser, M.J.; Ramachandran, C. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J. Biol. Chem 1997, 272, 843–851. [Google Scholar]
- Klarlund, J. Transformation of cells by an inhibitor of phosphatases acting on phosphotyrosine in proteins. Cell 1985, 41, 707–717. [Google Scholar]
- Cantley, L.C., Jr.; Aisen, P. The fate of cytoplasmic vanadium. Implications on (Na,K)-ATPase inhibition. J. Biol. Chem 1979, 254, 1781–1784. [Google Scholar]
- Dutra, P.M.; Rodrigues, C.O.; Jesus, J.B.; Lopes, A.H.; Souto-Padrón, T.; Meyer-Fernandes, J.R. A novel ecto-phosphatase activity of Herpetomonas muscarum muscarum inhibited by platelet-activating factor. Biochem. Biophys. Res. Commun 1998, 253, 164–169. [Google Scholar]
- Van Belle, H. Alkaline phosphatase. I. Kinetics and inhibition by levamisole of purified isoenzymes from humans. Clin. Chem 1976, 22, 972–976. [Google Scholar]
- Dick, C.F.; Dos-Santos, A.L.; Meyer-Fernandes, J.R. Inorganic phosphate as an important regulator of phosphatases. Enzym. Res 2011, 2011, 1–7. [Google Scholar]
- Hulett, F.M. The signal-transduction network for PHO regulation in Bacillus subtilis. Mol. Microbiol 1996, 19, 933–939. [Google Scholar]
- Torriani-Gorini, A.; Silver, S.; Yagil, E. Phosphate in Microorganisms: Cellular and Molecular Biology; ASM Press: Washington D.C., WA, USA, 1994. [Google Scholar]
- Dick, C.F.; Dos-Santos, A.L.; Fonseca-de-Souza, A.L.; Rocha-Ferreira, J.; Meyer-Fernandes, J.R. Trypanosoma rangeli: Differential expression of ecto-phosphatase activities in response to inorganic phosphate starvation. Exp. Parasitol 2010, 124, 386–393. [Google Scholar]
- Fonseca-de-Souza, A.L.; Dick, C.F.; Dos Santos, A.L.; Meyer-Fernandes, J.R. A Mg2+-dependent ecto-phosphatase activity on the external surface of Trypanosoma rangeli modulated by exogenous inorganic phosphate. Acta Trop 2008, 107, 153–158. [Google Scholar]
- Jacob, M.M.; Nyc, J.F.; Brown, D.M. Isolation and chemical properties of a repressible acid phosphatase in Neurospora crassa. J. Biol. Chem 1971, 246, 1419–1425. [Google Scholar]
- MacRae, W.D.; Buxton, F.P.; Sibley, S.; Garven, S.; Gwynne, D.I.; Davies, R.W.; Arst, H.N. A phosphate-repressible acid phosphatase gene from Aspergillus niger: Its cloning, sequencing and transcriptional analysis. Gene 1988, 71, 339–348. [Google Scholar]
- González, F.J.; Fauste, C.; Burguillo, F.J.; Domínguez, A. Kinetic behaviour of a repressible acid phosphatase from yeast Yarrowia lipolytica: A comparative study between the solubilized enzyme, the enzyme bound to cell-wall fragments and the enzyme bound to intact cells. Biochim. Biophys. Acta 1993, 1162, 17–27. [Google Scholar]
- Payne, W.E.; Gannon, P.M.; Kaiser, C.A. An inducible acid phosphatase from the yeast Pichia pastoris: Characterization of the gene and its product. Gene 1995, 163, 19–26. [Google Scholar]
- Haas, H.; Redl, B.; Friedlin, E.; Stoöffler, G. Isolation and analysis of the Penicillium chrysogenum phoA gene encoding a secreted phosphate-repressible acid phosphatase. Gene 1992, 113, 129–133. [Google Scholar]
- Haas, H.; Redl, B.; Leitner, E.; Stoöffler, G. Penicillium chrysogenum extracellular acid phosphatase: Purification and biochemical characterization. Biochim. Biophys. Acta 1991, 1074, 392–397. [Google Scholar]
- Ogawa, N.; DeRisi, J.; Brown, P.O. New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol. Biol. Cell 2000, 11, 4309–4321. [Google Scholar]
- Auesukaree, C.; Homma, T.; Tochio, H.; Shirakawa, M.; Kaneko, Y.; Harashima, S. Intracellular phosphate serves as a signal for the regulation of the PHO pathway in Saccharomyces cerevisiae. J. Biol. Chem 2004, 279, 17289–17294. [Google Scholar]
- Oshima, Y.; Ogawa, N.; Harashima, S. Regulation of phosphatase synthesis in Saccharomyces cerevisiae— A review. Gene 1996, 179, 171–177. [Google Scholar]
- Metzenberg, R.L. Implications of some genetic control mechanisms in Neurospora. Microbiol. Rev 1979, 43, 361–383. [Google Scholar]
- Jennings, D.H. Phosphorus. In The Physiology of Fungal Nutrition; Jennings, D.H., Ed.; Cambridge University Press: Cambridge, MA, USA, 1995; pp. 252–287. [Google Scholar]
- Toh-e, A. Phosphorus Regulation in Yeast. In Yeast Genetic Engineering; Barr, P.J., Brake, A.J., Valenzuela, P., Eds.; Butterworths: Boston, MA, USA, 1989; pp. 41–52. [Google Scholar]
- Schenkman, S.; Robbins, E.S.; Nussenzweig, V. Attachment of Trypanosoma cruzi to mammalian cells requires parasite energy, and invasion can be independent of the target cell cytoskeleton. Infect. Immun 1991, 59, 645–654. [Google Scholar]
- Manners, J.G. Assessment of Germination. In The fungus spore; Madelin, M.F., Ed.; Butterworths: London, UK, 1966; pp. 165–173. [Google Scholar]
- Touati, E.; Dassa, E.; Dassa, J.; Boquet, P.L. Acid phosphatase (pH 2.5) of Escherichia coli: regulatory characteristics. In Phosphate Metabolism and Cellular Regulation in Microorganisms; Torriani-Gorini, A., Rothman, F.G., Silver, S., Wright, A., Yagil, E., Eds.; ASM Press: Washington D.C., WA, USA, 1987; pp. 31–40. [Google Scholar]
- Novick, P.; Ferro, S.; Schekman, R. Order of events in the yeast secretory pathway. Cell 1981, 25, 461–469. [Google Scholar]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A. Emerging opportunistic yeast infections. Lancet. Infect. Dis 2011, 11, 142–151. [Google Scholar]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev 2003, 67, 400–428. [Google Scholar]
- Yang, Y.L. Virulence factors of Candida species. J. Microbiol. Immunol. Infect 2003, 36, 223–228. [Google Scholar]
- Catta-Preta, C.M.; Nascimento, M.T.; Garcia, M.C.; Saraiva, E.M.; Motta, M.C.; Meyer-Fernandes, J.R. The presence of a symbiotic bacterium in Strigomonas culicis is related to differential ecto-phosphatase activity and influences the mosquito-protozoa interaction. Int. J. Parasitol 2013, 43, 571–577. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Freitas-Mesquita, A.L.; Meyer-Fernandes, J.R. Biochemical Properties and Possible Roles of Ectophosphatase Activities in Fungi. Int. J. Mol. Sci. 2014, 15, 2289-2304. https://doi.org/10.3390/ijms15022289
Freitas-Mesquita AL, Meyer-Fernandes JR. Biochemical Properties and Possible Roles of Ectophosphatase Activities in Fungi. International Journal of Molecular Sciences. 2014; 15(2):2289-2304. https://doi.org/10.3390/ijms15022289
Chicago/Turabian StyleFreitas-Mesquita, Anita Leocadio, and José Roberto Meyer-Fernandes. 2014. "Biochemical Properties and Possible Roles of Ectophosphatase Activities in Fungi" International Journal of Molecular Sciences 15, no. 2: 2289-2304. https://doi.org/10.3390/ijms15022289



