Nicotinic Acid Increases Adiponectin Secretion from Differentiated Bovine Preadipocytes through G-Protein Coupled Receptor Signaling
Abstract
:1. Introduction
2. Results
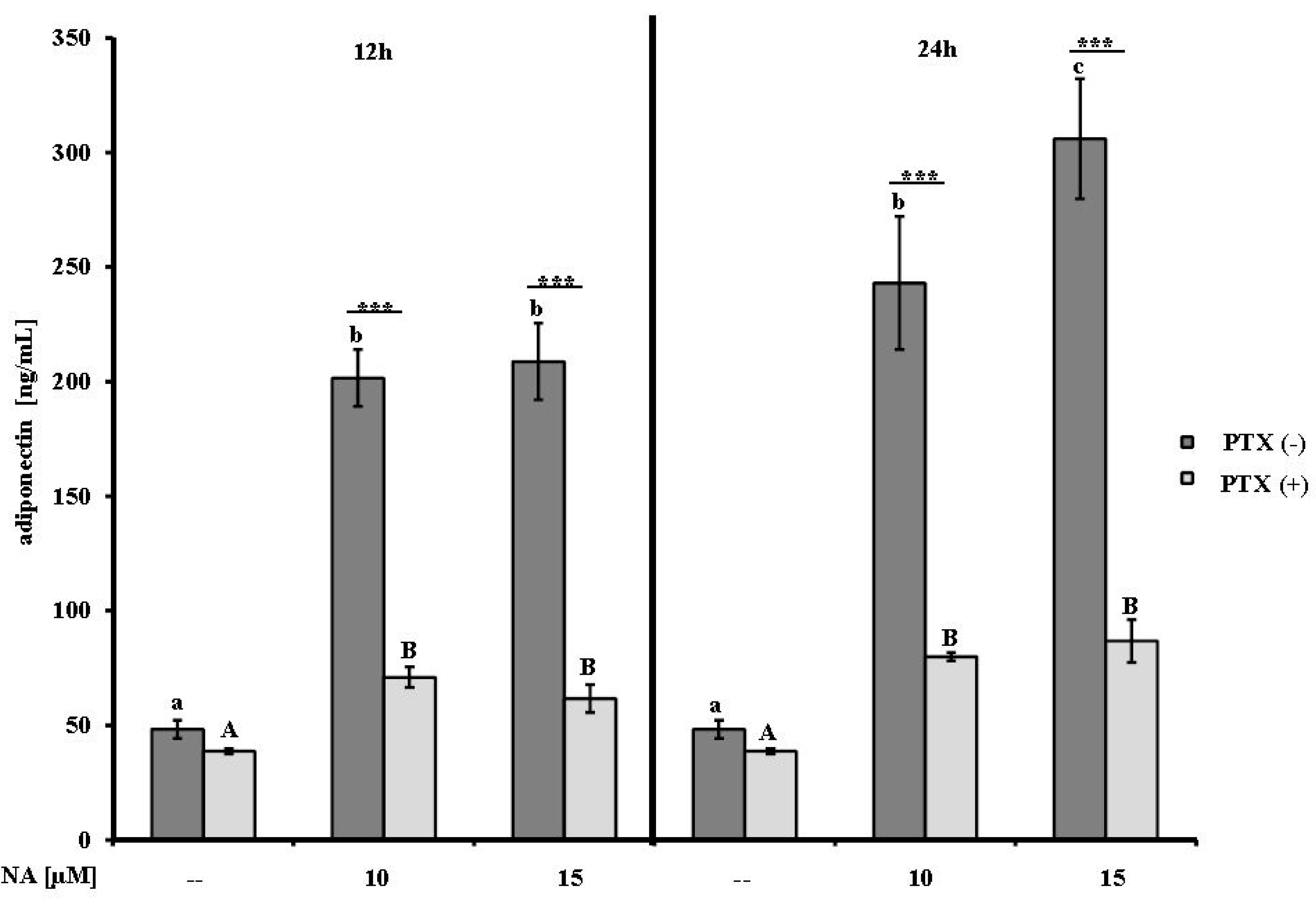
2.1. Effect of NA on Adiponectin Secretion
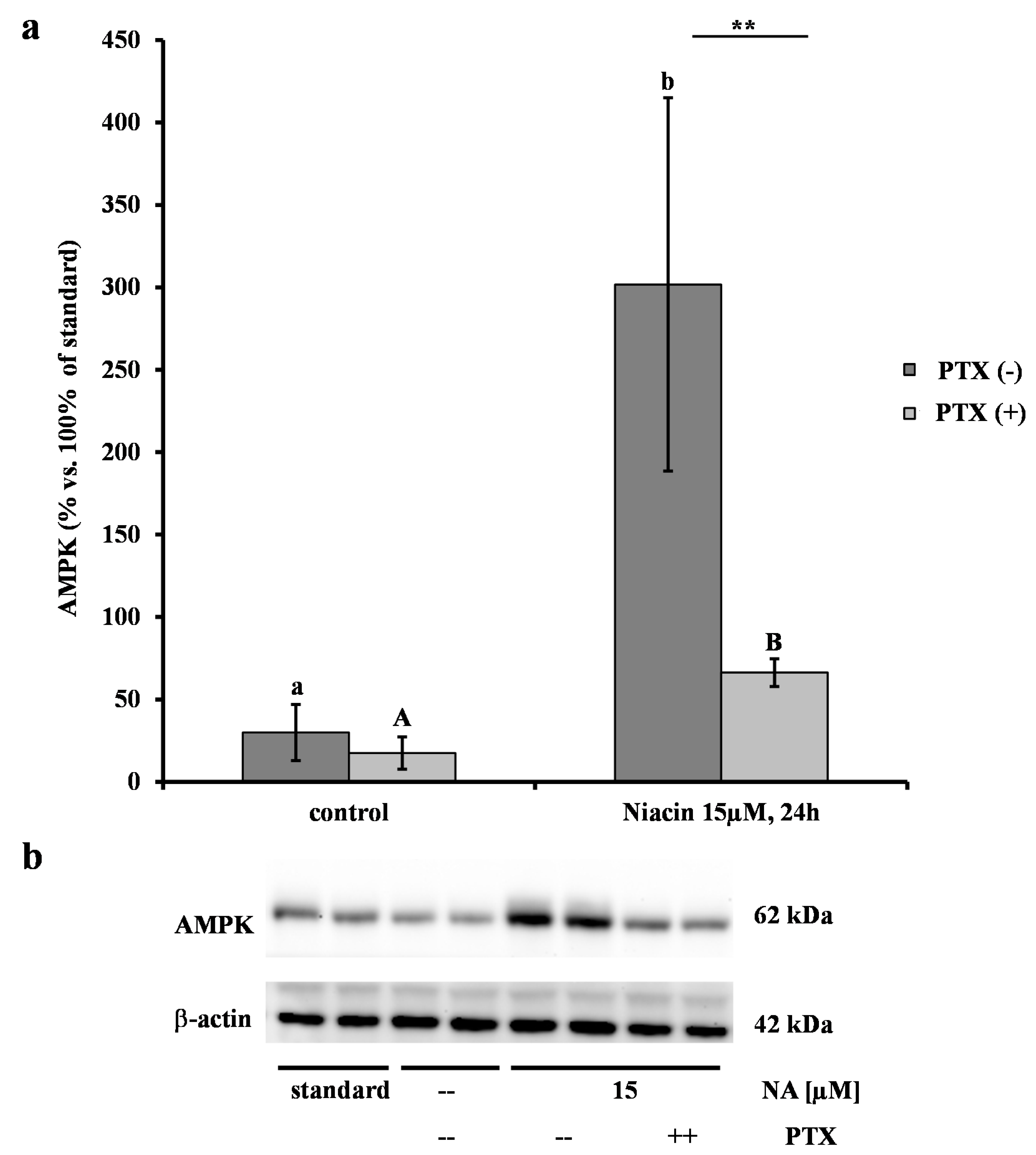
2.2. Effect of NA on AMPK
2.3. Effects of NA on the Abundances of AdipoR1/2, FABP4, and GPR109A mRNAs

| Treatment/Genes | 12 h | 24 h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NA a [μM] | - | 10 | p | 15 | p | - | 10 | p | 15 | p |
| AdipoR1 b | 1.1 ± 0.4 | 1.1 ± 0.5 | n.s. | 1.1 ± 0.4 | n.s. | 1 ± 0.3 | 1.1 ± 0.3 | n.s. | 1.4 ± 0.5 | n.s. |
| AdipoR2 c | 1 ± 0.1 | 1 ± 0.2 | n.s. | 1 ± 0.1 | n.s. | 1 ± 0.2 | 1 ± 0.2 | n.s. | 1 ± 0.1 | n.s. |
| GPR109A d | 1.2 ± 0.01 | 3.13 ± 0.7 | n.s. | 3.27 ± 1.25 | n.s. | 1.6 ± 0.05 | 3.48 ± 0.46 | 0.07 | 3.9 ± 0.1 | 0.02 |
| FABP4 e | 1.2 ± 0.3 | 1.2 ± 0.6 | n.s. | 1.1 ± 0.6 | n.s. | 1.1 ± 0.5 | 0.6 ± 0.5 | n.s. | 0.8 ± 0.5 | n.s. |
| Chemerin | 0.71 ± 0.19 | 0.76 ± 0.16 | n.s. | 0.67 ± 0.38 | n.s. | 0.76 ± 0.46 | 2.89 ± 1.42 | 0.006 | 1.59 ± 1.18 | n.s. |
2.4. Effect of NA on the mRNA Abundance of Chemerin
3. Discussion
4. Experimental Section
4.1. Isolation of Bovine Preadipocytes
4.2. Differentiation of Bovine Preadipocytes
4.3. Treatment of the Cells with NA
4.4. RNA Extraction, cDNA Synthesis and mRNA Quantification
| Gene a | Forward Primer Sequence (5'-3') Reverse Primer Sequence (5'-3') | Acc. No.d | Base Pairs | Con. (nM) e | Mean Cq f | Annealing (s/°C) g | Efficiency |
|---|---|---|---|---|---|---|---|
| AdipoR1 b | GCTGAAGTGAGAGGAAGAGTC GAGGGAATGGAGTTTATTGCC | NM_001034055 | 118 | 800 | 23.9 | 35/61 | 99.9 |
| AdipoR2 b | GGCAACATCTGGACACATC CTGGAGACCCCTTCTGAG | NM_001040499 | 200 | 400 | 24.2 | 45/60 | 90.7 |
| GPR109A c | GGACAGCGGGCATCATCTC CCAGCGGAAGGCATCACAG | XR_028237 | 140 | 200 | 31.9 | 30/61 | 86.5 |
| FABP4 b | CATCTTGCTGAAAGCTGCAC AGCCACTTTCCTGGTAGCAA | X89244 | 160 | 800 | 22.9 | 30/60 | 120.4 |
| Chemerin b | GAAGAAAGACTGGAGGAAAG TTGAACCTGAGTCTGTATGG | FJ594406 | 139 | 200/100 | 23.2 | 60/60 | 89.1 |
| MARVELDI c | GGCCAGCTGTAAGATCATCACA TCTGATCACAGACAGAGCACCAT | NM_001101262 | 100 | 400 | 23.4 | 45/59 | 101.2 |
| EMD b | GCCCTCAGCTTCACTCTCAGA GAGGCGTTCCCGATCCTT | NM_203361 | 100 | 400 | 23.4 | 45/59 | 101.7 |
| LRP10 b | CCAGAGGATGAGGACGATGT ATAGGGTTGCTGTCCCTGTG | Bc149232 | 139 | 400 | 22.7 | 30/61 | 101.1 |
| EIF3K b | CCAGGCCCACCAAGAAGAA TTATACCTTCCAGGAGGTCCATGT | NM_001034489 | 125 | 400 | 23.4 | 45/59 | 97.5 |
| POLII b | GAAGGGGGAGAGACAAACTG GGGAGGAAGAAGAAAAAGGG | X63564 | 86 | 800 | 23.1 | 60/60 | 97.4 |
4.5. Protein Extraction from the Cell Lysate
4.6. Western Blot
4.7. Measurement of Adiponectin Secreted from Bovine Adipocytes
4.8. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grummer, R.R. Impact of changes in organic nutrient metabolism on feeding the transition dairy cow. J. Anim. Sci. 1995, 73, 2820–2833. [Google Scholar] [PubMed]
- McNamara, J.P. Regulation of bovine adipose tissue metabolism during lactation. 5. relationships of lipid synthesis and lipolysis with energy intake and utilization. J. Dairy Sci. 1989, 72, 407–418. [Google Scholar]
- Grummer, R.R. Etiology of lipid-related metabolic disorders in periparturient dairy cows. J. Dairy Sci. 1993, 76, 3882–3896. [Google Scholar] [CrossRef] [PubMed]
- Drackely, J.K. Biology of dairy cows during the transition period: the final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S. Monitoring metabolic health of dairy cattle in the transition period. J. Reprod. Dev. 2010, 56, S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Schmidt, R.J.; Foxworthy, P.; Emkey, R.; Oler, J.K.; Large, T.H.; Wang, H.; Su, E.W.; Mosior, M.K.; Eacho, P.I.; et al. Niacin mediates lipolysis in adipose tissue throughits G-protein coupled receptor HM74A. Biochem. Biophys. Res. Commun. 2005, 334, 729–732. [Google Scholar]
- Tunaru, S.; Kero, J.; Schaub, A.; Wufka, C.; Blaukat, A.; Pfeffer, K.; Offermanns, S. Puma-G and HM74 are receptors for nicotinic acid and mediate its anti-lypolytic effect. Nat. Med. 2003, 9, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Wise, A.; Foord, S.M.; Fraser, N.J.; Barnes, A.A.; Elshourbagy, N.; Eilert, M.; Ignar, D.M.; Murdock, P.R.; Steplewski, K.; Green, A.; et al. Molecular identification of high and low affinity receptors for nicotinic acid. J. Biol. Chem. 2003, 278, 9869–9874. [Google Scholar]
- Bogan, K.L.; Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [PubMed]
- Offermanns, S.; Colletti, S.L.; Lovenberg, T.W.; Semple, G.; Wise, A.; Ijzerman, A.P. International union of basic and clinical pharmacology. LXXXII: Nomenclature and classification of hydroxy-carboxylic acid receptors (GPR81, GPR109A, and GPR109B). Pharmacol. Rev. 2011, 63, 269–290. [Google Scholar] [PubMed]
- Taggart, A.K.; Kero, J.; Gan, X.; Cai, T.-Q.; Cheng, K.; Ippolito, M.; Ren, N.; Kaplan, R.; Wu, K.; Wu, T.-J.; et al. (D)-β-hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005, 280, 26649–26652. [Google Scholar]
- Tunaru, S.; Lätting, J.; Kero, J.; Krause, G.; Offermanns, S. Characterization of determinants of ligand binding to the nicotinic acid receptor GPR109A (HM74A/PUMA-G). Mol. Pharm. 2005, 65, 1271–1280. [Google Scholar] [CrossRef]
- Titgemeyer, E.C.; Mamedova, L.K.; Spivey, K.S.; Farney, J.K.; Bradford, B.J. An unusual distribution of the niacin receptor in cattle. J. Dairy Sci. 2011, 94, 4962–4967. [Google Scholar] [CrossRef] [PubMed]
- Fronk, T.J.; Schultz, L.H. Oral nicotinic acid as a treatment for ketosis. J. Dairy Sci. 1979, 62, 1804–1807. [Google Scholar] [CrossRef] [PubMed]
- Jaster, E.H.; Ward, N.E. Supplemental nicotinic acid or nicotinamide for lactating dairy cows. J. Dairy Sci. 1990, 73, 2880–2887. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.A.A.; Grummer, R.R. The use of nicotinic acid to induce sustained low plasma nonesterified fatty acids in feed-restricted Holstein cows. J. Dairy Sci. 2007, 90, 4635–4642. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.A.A.; Pescara, J.B.; Grummer, R.R. Reduction of plasma NEFA concentration by nicotinic acid enhances the response to insulin in feed-restricted Holstein cows. J. Dairy Sci. 2007, 90, 3725–3732. [Google Scholar] [CrossRef] [PubMed]
- Plaisance, E.P.; Lukasova, M.; Offermanns, S.; Zhang, Y.; Cao, G.; Judd, R.L. Niacin stimulates adiponectin secretion through the GPR109A receptor. Am. J. Physiol. Endocrinol. Metab. 2009, 296, 549–558. [Google Scholar] [CrossRef]
- Wanders, D.; Graff, E.C.; White, B.D.; Judd, R.L. Niacin increases adiponectin and decreases adipose tissue inflammation in high fat diet-fed mice. PLoS One 2013, 8, e71285. [Google Scholar] [CrossRef] [PubMed]
- Digby, J.E.; McNeill, E.; Dyar, O.J.; Lam, V.; Greaves, D.R.; Choudhury, R.P. Anti-inflammatory effects of nicotinic acid in adipocytes demonstrated by suppression of fractalkine, RANTES, and MCP-1 and upregulation of adiponectin. Atherosclerosis 2010, 209, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Westphal, S.; Borucki, K.; Taneva, E.; Makarova, R.; Luley, C. Extended-release niacin raises adiponectin and leptin. Atherosclerosis 2007, 193, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Brochu-Gaudreau, K.; Rehfeldt, C.; Blouin, R.; Bordignon, V.; Murphy, B.D.; Palin, M.F. Adiponectin action from head to toe. Endocrine 2010, 37, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T. Adiponectin and Adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.; Snowden, M.A.; Carling, D. Identification of a novel AMP-activated protein kinase b subunit isoform that is highly expressed in skeletal muscle. J. Biol. Chem. 1998, 273, 12443–12450. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Oppenheim, J.J. Chemerin reveals its chimeric nature. J. Exp. Med. 2008, 205, 2187–2190. [Google Scholar] [CrossRef] [PubMed]
- Cash, J.L.; Hart, R.; Russ, A.; Dixon, J.P.; Colledge, W.H.; Doran, J.; Hendrick, A.G.; Carlton, M.B.; Greaves, D.R. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J. Exp. Med. 2008, 205, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Takahashi, Y.; Takahashi, K.; Zolotaryov, F.N.; Hong, K.S.; Kitazawa, R.; Iida, K.; Okimura, Y.; Kaji, H.; Kitazawa, S.; et al. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett. 2008, 582, 573–578. [Google Scholar]
- Song, S.H.; Fukui, K.; Nakajima, K.; Kozakai, T.; Sasaki, S.; Roh, S.G.; Katoh, K. Cloning, expression analysis, and regulatory mechanisms of bovine chemerin and chemerin receptor. Domest. Anim. Endocrinol. 2010, 39, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kenéz, A.; Locher, L.; Rehage, J.; Dänicke, S.; Huber, K. Agonists of the G protein-coupled receptor 109A-mediated pathway promote antilipolysis by reducing serine residue 563 phosphorylation of hormone-sensitive lipase in bovine adipose tissue explants. J. Dairy Sci. 2014, 97, 3626–3634. [Google Scholar] [CrossRef] [PubMed]
- Guerre-Millo, M. Adiponectin: An update. Diabetes Metab. 2008, 34, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, L.; Li, X.; Li, X.; Sun, G.; Yuan, X.; Lei, L.; Liu, J.; Yin, L.; Deng, Q.; et al. Adiponectin activates the AMPK signaling pathway to regulate lipid metabolism in bovine hepatocytes. J. Steroid Biochem. Mol. Biol. 2013, 138, 445–454. [Google Scholar]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 2005, 46, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Mu, J.; Birnbaum, M.J. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis in 3T3-L1 adipocytes. J. Biol. Chem. 2003, 278, 43074–43080. [Google Scholar] [CrossRef] [PubMed]
- Daval, M.; Foufelle, F.; Ferré, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Locher, L.F.; Rehage, J.; Khraim, N.; Meyer, U.; Dänicke, S.; Hansen, K.; Huber, K. Lipolysis in early lactation is associated with an increase in phosphorylation of adenosine monophosphate-activated protein kinase (AMPK)alpha1 in adipose tissue of dairy cows. J. Dairy Sci. 2012, 95, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Takekoshi, K.; Fukuhara, M.; Quin, Z.; Nissato, S.; Isobe, K.; Kawakami, Y.; Ohmori, H. Long-term exercise stimulates adenosine monophosphate-activated protein kinase activity and subunit expression in rat visceral adipose tissue and liver. Metabolism 2006, 55, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Durante, P.E.; Mustard, K.J.; Park, S.H.; Winder, W.W.; Hardie, D.G. Effects of endurance training on activity and expression of AMP-activated protein kinase isoforms in rat muscles. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E178–E186. [Google Scholar] [PubMed]
- Nielsen, J.N.; Mustard, K.J.; Graham, D.A.; Yu, H.; MacDonald, C.S.; Pilegaard, H.; Goodyear, L.J.; Hardie, D.G.; Richter, E.A.; Wojtaszewski, J.F. 5'-AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J. Appl. Physiol. 2003, 94, 631–641. [Google Scholar] [PubMed]
- Steinberg, G.R.; Rush, J.W.; Dyck, D.J. AMPK expression and phosphorylation are increased in rodent muscle after chronic leptin treatment. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E648–E654. [Google Scholar] [PubMed]
- Martínez-Agustin, O.; Hernández-Morante, J.J.; Martínez-Plata, E.; Sánchez de Medina, F.; Garaulet, M. Differences in AMPK expression between subcutaneous and visceral adipose tissue in morbid obesity. Regul. Pept. 2010, 163, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Motoshima, H.; Mahadev, K.; Stalker, T.J.; Scalia, R.; Goldstein, B.J. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes 2003, 52, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Sell, H.; Dietze-Schroeder, D.; Eckardt, K.; Eckel, J. Cytokine secretion by human adipocytes is differentially regulated by adiponectin, AICAR, and troglitazone. Biochem. Biophys. Res. Commun. 2006, 343, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H.; Zhao, S.P. Niacin promotes cholesterol efflux through stimulation of the PPARgamma-LXRalpha-ABCA1 pathway in 3T3-L1 adipocytes. Pharmacology 2009, 84, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.T.; Digby, J.E.; Ruparelia, N.; Jefferson, A.; Handa, A.; Choudhury, R.P. Nicotinic acid receptor GPR109A is down-regulated in human macrophage-derived foam cells. PLoS One 2013, 8, e62934. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Choung, S.Y. Antihyperglycemic and Antihyperlipidemic action of Cinnamomi Cassiae (Cinnamon Bark) extract in C57BL/Ks db/db mice. Arch. Pharm. Res. 2010, 33, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, M.; Matsuda, M.; Maeda, N.; Funahashi, T.; Matsuzawa, Y.; Makishima, M.; Shimomura, I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 2003, 52, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, F.; Di, M.D. Peroxisome proliferator-activated receptor-gamma agonists and diabetes: Current evidence and future perspectives. Vasc. Health Risk Manag. 2008, 4, 297–304. [Google Scholar] [PubMed]
- Rafaeloff-Phail, R.; Ding, L.; Conner, L.; Yeh, W.Y.; McClure, D.; Guo, H.; Emerson, K.; Brooks, H. Biochemical regulation of mammalian AMP-activated protein kinase activity by NAD and NADH. J. Biol. Chem. 2004, 279, 52934–52939. [Google Scholar] [CrossRef]
- Maiese, K.; Chong, Z.Z.; Hou, J.; Shang, Y.C. The vitamin nicotinamide: Translating nutrition into clinical care. Molecules 2009, 14, 3446–3485. [Google Scholar] [CrossRef] [PubMed]
- Kralisch, S.; Fasshauer, M. Adipocyte fatty acid binding protein: A novel adipokine involved in the pathogenesis of metabolic and vascular disease? Diabetologia 2013, 56, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, C.; Ewen, E.; Hosseini, A.; Sauerwein, H.; Mielenz, M. Expression profile of the mRNAs of the adiponectin system as well as of the niacin-binding receptor GPR109A during differentiation of bovine preadipocytes in vitro. In Proceedings of the Book of Abstracts of the 7th International Conference on Farmer Animal Endocrinology, Bern, Switzerland, 24–26 August 2011.
- Wanders, D.; Graff, E.C.; Judd, R.L. Effects of high fat diet on GPR109A and GPR81 gene expression. Biochem. Biophys. Res. Commun. 2012, 425, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Jeninga, E.H.; Bugge, A.; Nielsen, R.; Kersten, S.; Hamers, N.; Dani, C.; Wabitsch, M.; Berger, R.; Stunnenberg, H.G.; Mandrup, S.; et al. Peroxisome proliferator-activated receptor gamma regulates expression of the anti-lipolytic G-protein-coupled receptor 81 (GPR81/Gpr81). J. Biol. Chem. 2009, 284, 26385–26393. [Google Scholar] [PubMed]
- Friedrichs, P.; Saremi, B.; Winand, S.; Rehage, J.; Däncke, S.; Sauerwein, H.; Mielenz, M. Energy and metabolic sensing G protein-coupled receptors during lactation-induced changes in energy balance. Domest. Anim. Endocrinol. 2014, 48, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Bondue, B.; De Henau, O.; Luangsay, S.; Devosse, T.; de Nadaï, P.; Springael, J.Y.; Parmentier, M.; Vosters, O. The chemerin/ChemR23 system does not affect the pro-inflammatory response of mouse and human macrophages ex vivo. PLoS One 2012, 7, e40043. [Google Scholar] [PubMed]
- Roh, S.; Song, S.H.; Cjoi, K.C.; Katoh, K.; Wittamer, V.; Parmentier, M.; Sasaki, S. Chemerin-a new adipokine that modulates adipogenesis via its own receptor. Biochem. Biophys. Res. Commun. 2007, 362, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.C.; Ortiz-Colon, G.; Doumit, M.E.; Buskirk, D.D. Optimization of in vitro conditions for bovine subcutaneous and intramuscular preadipocytes differentiation. J. Anim. Sci. 2008, 86, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.; Kimura, K.; Ahmeda, M.; Yamaji, D.; Matsushita, Y.; Okamatsu-Ogura, Y.; Makondo, K.; Saito, M. Inverse regulation of leptin mRNA expression by short- and long-chain fatty acids in cultured bovine adipocytes. Domest. Anim. Endocrinol. 2007, 33, 400–409. [Google Scholar] [PubMed]
- Saremi, B.; Sauerwein, H.; Danicke, S.; Mielenz, M. Technical note: Identification of reference genes for gene expression studies in different bovine tissues focusing on different fat depots. J. Dairy. Sci. 2012, 95, 3131–3138. [Google Scholar]
- Lemor, A.; Hosseini, A.; Sauerwein, H.; Mielenz, M. Transition period-related changes in the abundance of the mRNAs of adiponectin and its receptors, of visfatin, and of fatty acid binding receptors in adipose tissue of high-yielding dairy cows. Domest. Anim. Endocrinol. 2009, 37, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Behrendt, C.; Regenhard, P.; Sauerwein, H.; Mielenz, M. Differential effects of propionate and β-hydroxybutyrate on genes related to energy balance and insulin sensitivity in bovine adipose white tissue explants from a subcutaneous and a visceral depot. J. Anim. Physiol. Anim. Nutr. 2011, 96, 570–580. [Google Scholar] [CrossRef]
- Kadegowda, A.K.G.; Bionaz, M.; Thering, B.; Piperova, L.S.; Erdman, R.A.; Loor, J.J. Identification of internal control genes for quantitative polymerase chain reaction in mammary tissue of lactating cows receiving lipid supplements. J. Dairy Sci. 2009, 92, 2007–2019. [Google Scholar] [CrossRef] [PubMed]
- Chey, S.; Claus, C.; Liebert, G. Improved method for simultaneous isolation of proteins and nucleic acids. Anal. Biochem. 2011, 411, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Mielenz, M.; Mielenz, B.; Singh, S.P.; Kopp, C.; Heinz, J.; Häussler, S.; Sauerwein, H. Development, validation, and pilot application of a semiquantitative Western blot analysis and an ELISA for bovine adiponectin. Domest. Anim. Endocrinol. 2013, 44, 121–130. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopp, C.; Hosseini, A.; Singh, S.P.; Regenhard, P.; Khalilvandi-Behroozyar, H.; Sauerwein, H.; Mielenz, M. Nicotinic Acid Increases Adiponectin Secretion from Differentiated Bovine Preadipocytes through G-Protein Coupled Receptor Signaling. Int. J. Mol. Sci. 2014, 15, 21401-21418. https://doi.org/10.3390/ijms151121401
Kopp C, Hosseini A, Singh SP, Regenhard P, Khalilvandi-Behroozyar H, Sauerwein H, Mielenz M. Nicotinic Acid Increases Adiponectin Secretion from Differentiated Bovine Preadipocytes through G-Protein Coupled Receptor Signaling. International Journal of Molecular Sciences. 2014; 15(11):21401-21418. https://doi.org/10.3390/ijms151121401
Chicago/Turabian StyleKopp, Christina, Afshin Hosseini, Shiva P. Singh, Petra Regenhard, Hamed Khalilvandi-Behroozyar, Helga Sauerwein, and Manfred Mielenz. 2014. "Nicotinic Acid Increases Adiponectin Secretion from Differentiated Bovine Preadipocytes through G-Protein Coupled Receptor Signaling" International Journal of Molecular Sciences 15, no. 11: 21401-21418. https://doi.org/10.3390/ijms151121401
APA StyleKopp, C., Hosseini, A., Singh, S. P., Regenhard, P., Khalilvandi-Behroozyar, H., Sauerwein, H., & Mielenz, M. (2014). Nicotinic Acid Increases Adiponectin Secretion from Differentiated Bovine Preadipocytes through G-Protein Coupled Receptor Signaling. International Journal of Molecular Sciences, 15(11), 21401-21418. https://doi.org/10.3390/ijms151121401





