Spectroscopic Studies of R(+)-α-Lipoic Acid—Cyclodextrin Complexes
Abstract
:1. Introduction
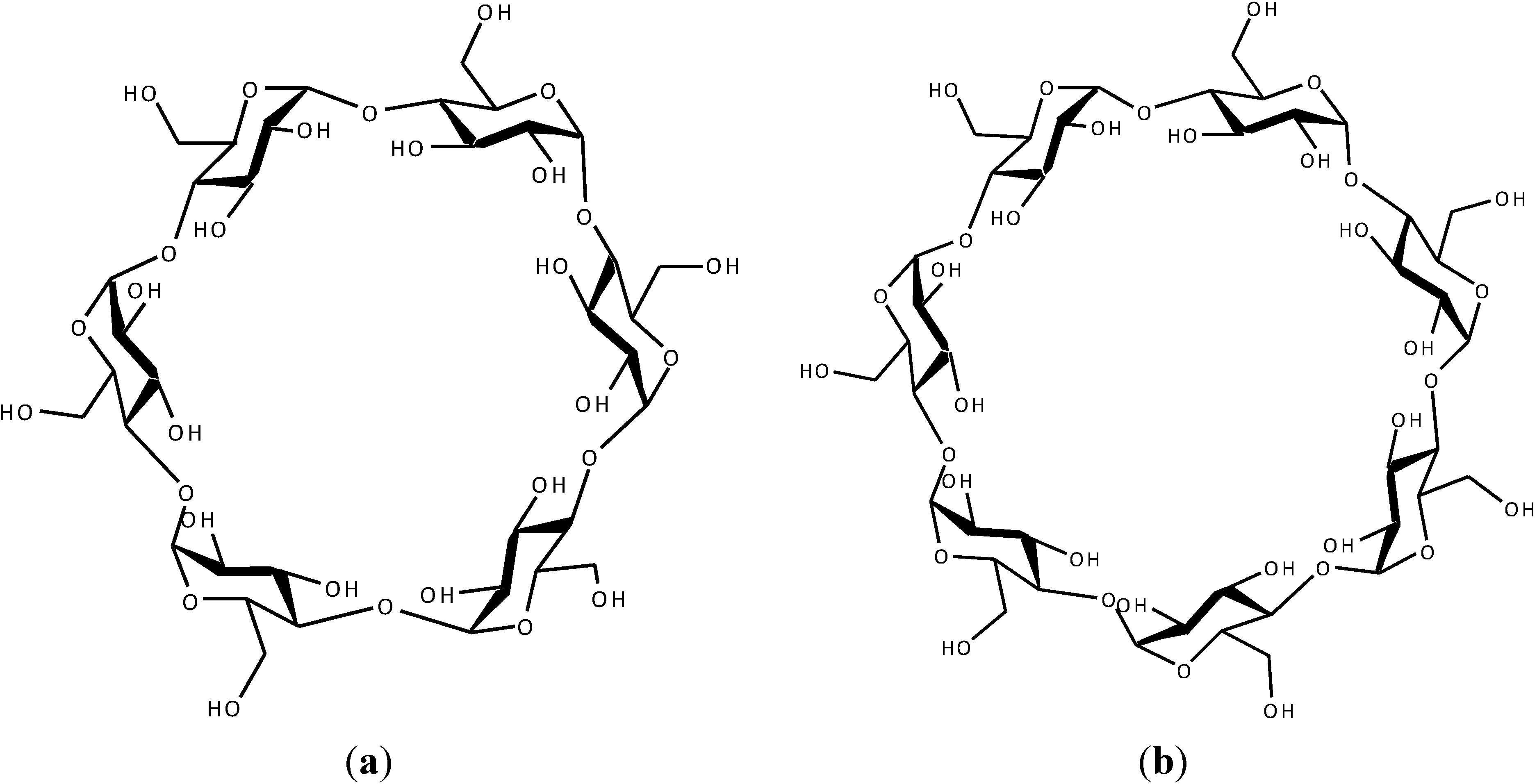
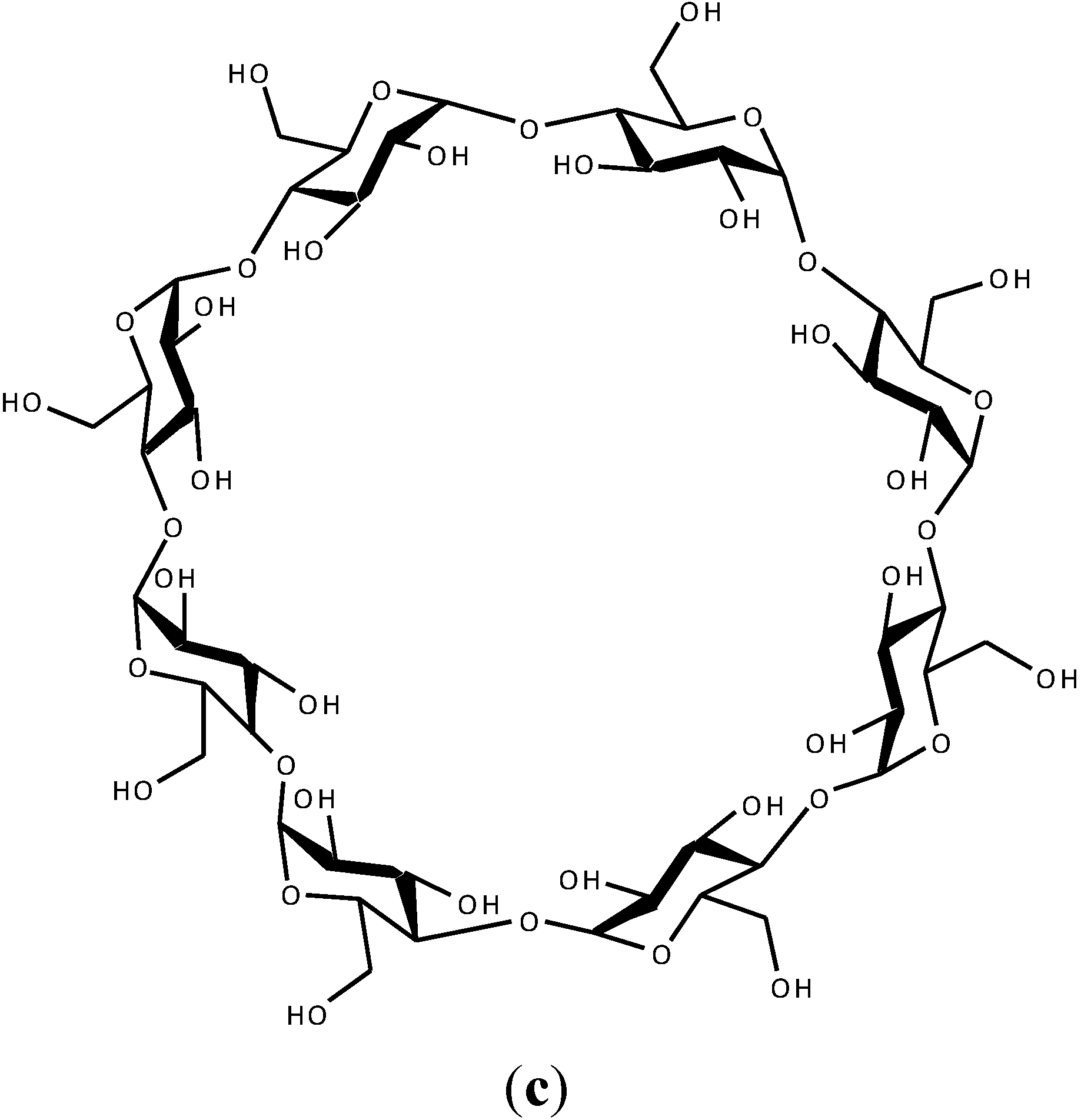
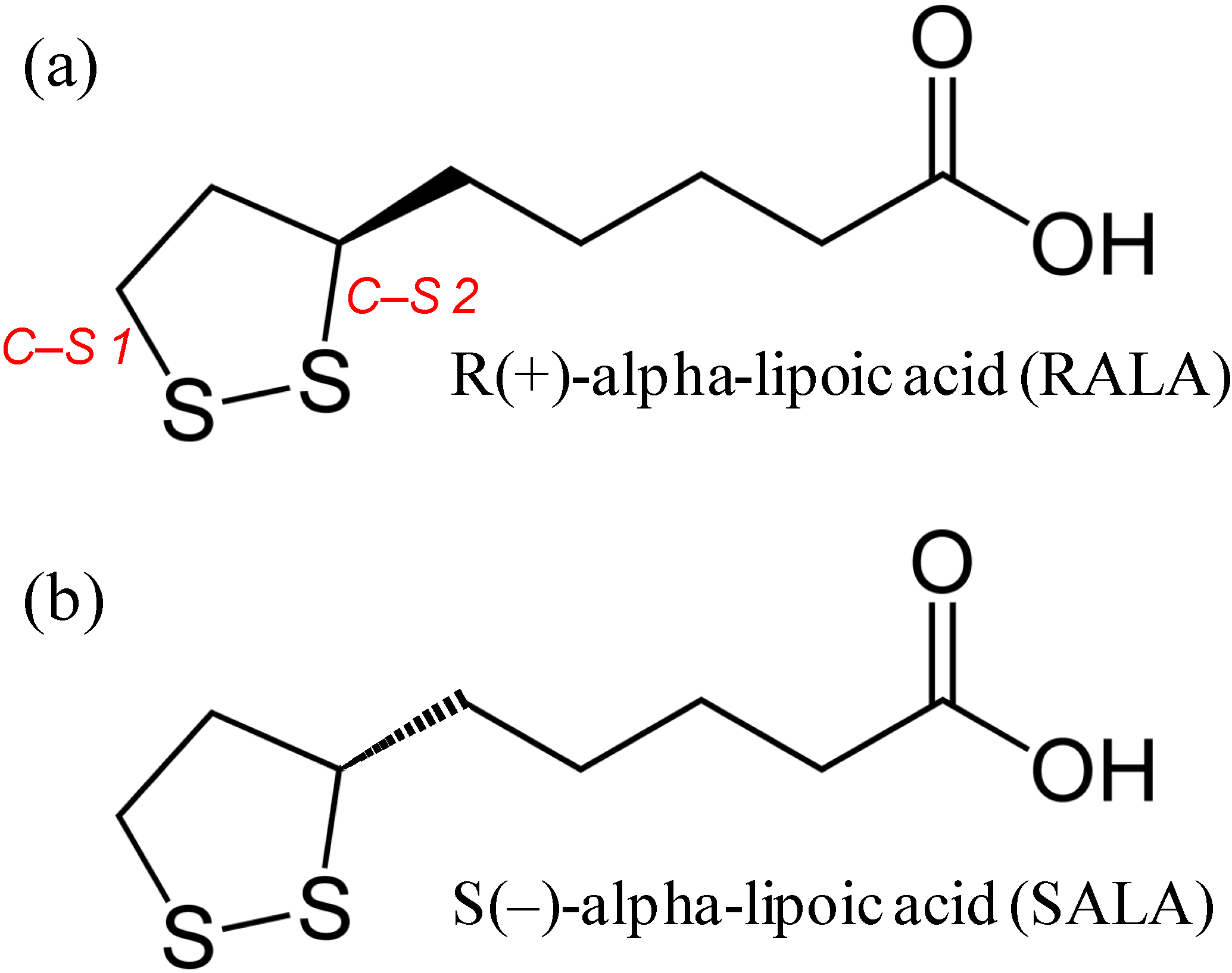
2. Results and Discussion
2.1. Microscopic Fourier Transform Infrared (FT-IR) Spectroscopy

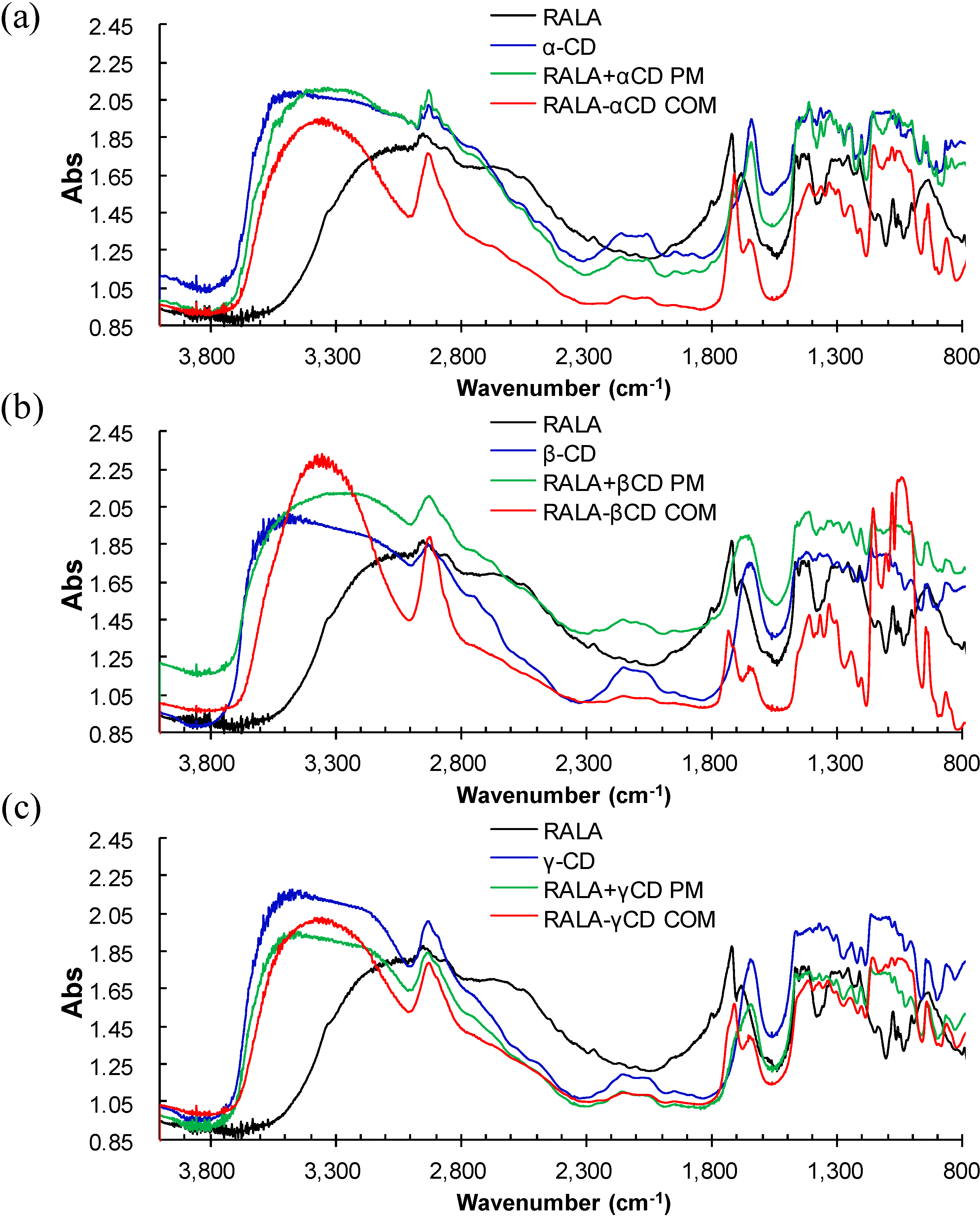
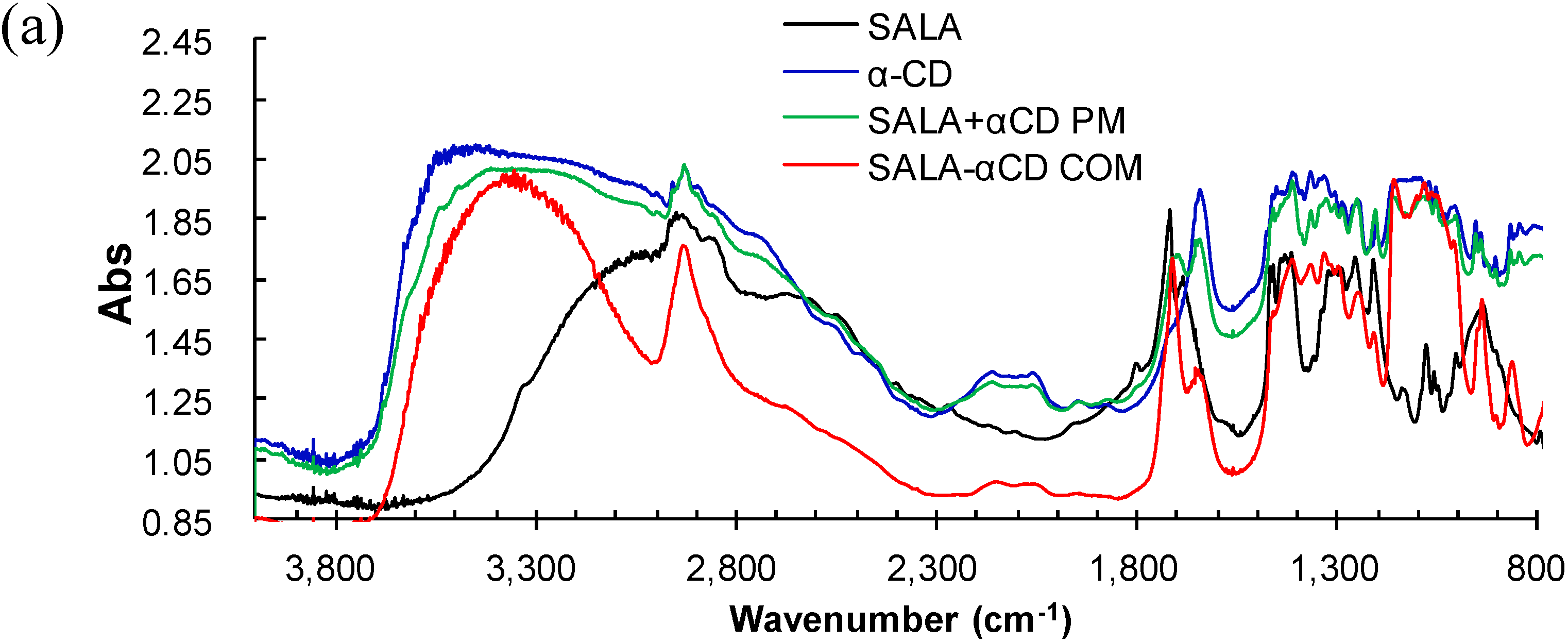

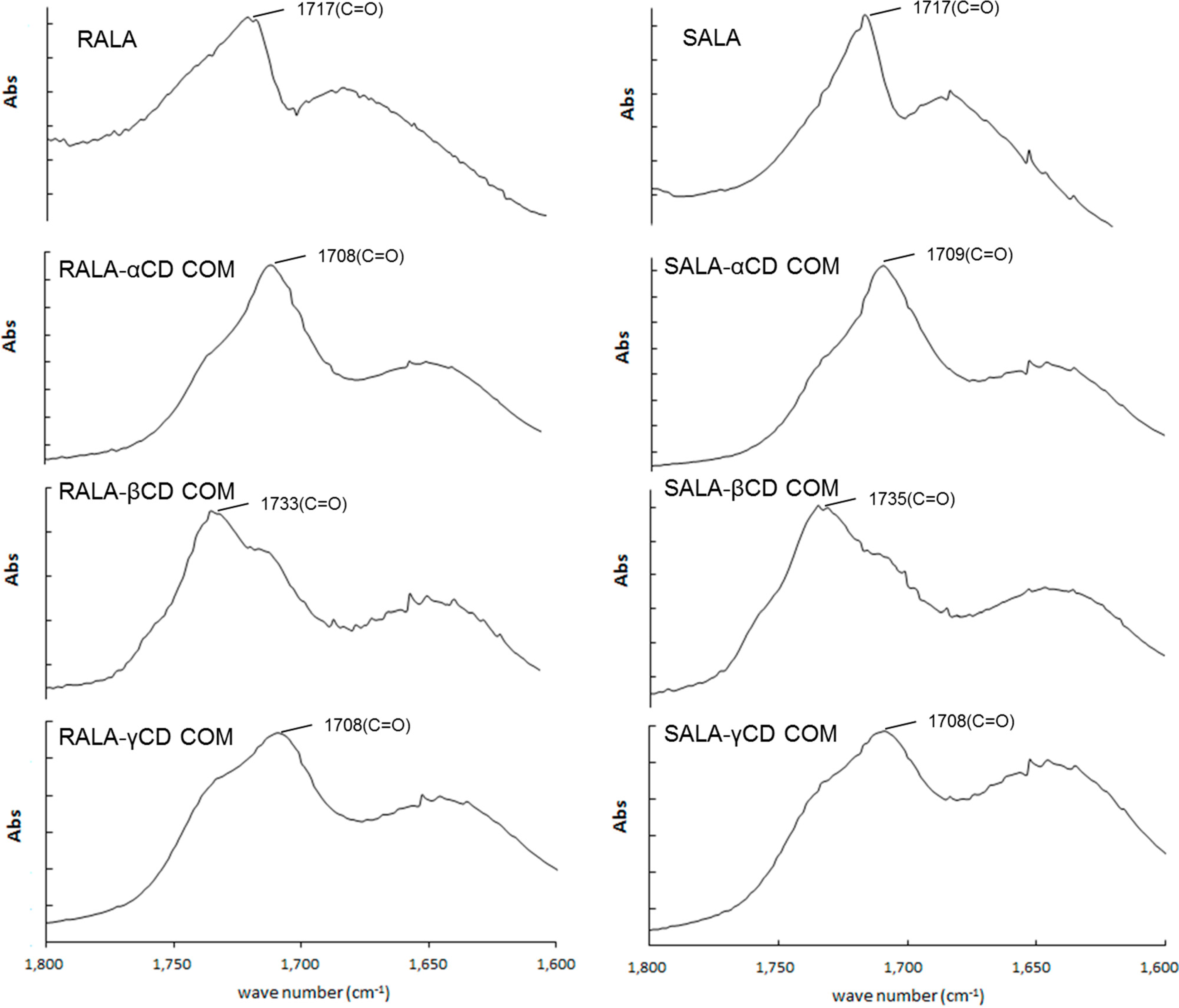
2.2. Microscopic Raman Spectroscopy and Raman Mapping Analysis
2.2.1. Microscopic Raman Spectroscopy


2.2.2. Raman Spectroscopic Imaging

3. Experimental Section
3.1. Chemicals
3.2. Equipments
3.3. Microscopic Fourier Transform Infrared Spectroscopy (Micro FT-IR)
3.4. Microscopic-Raman Spectroscopy and Raman Chemical Imaging
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Terao, K.; Nakata, D.; Fukumi, H.; Schmid, S.; Arima, H.; Hirayama, F.; Uekama, K. Enhancement of oral bioavailability of coenzyme Q10 by complexation with γ-cyclodextrin in healthy adults. Nutr. Res. 2006, 26, 503–508. [Google Scholar]
- Yadav, V.R.; Suresh, S.; Devi, K.; Yadav, S. Effect of cyclodextrin complexation of curcumin on its solubility and antiangiogenic and anti-inflammatory activity in rat colitis model. AAPS Pharm. Sci. Technol. 2009, 10, 752–762. [Google Scholar]
- Ikeda, S.; Uchida, T.; Ichikawa, T.; Watanabe, T.; Uekaji, Y.; Nakata, D.; Terao, K.; Yano, T. Complexation of tocotrienol with γ-cyclodextrin enhances intestinal absorption of tocotrienol in rats. Biosci. Biotechnol. Biochem. 2010, 74, 1452–1457. [Google Scholar]
- Takahashi, H.; Bungo, Y.; Mikuni, K. The aqueous solubility and thermal stability of α-lipoic acid are enhanced by cyclodextrin. Biosci. Biotechnol. Biochem. 2011, 75, 633–637. [Google Scholar]
- Tabushi, I. Cyclodextrin catalysis as a model for enzyme action. Acc. Chem. Res. 1982, 15, 66–72. [Google Scholar]
- Turro, N.J.; Okubo, T.; Chung, C.J. Analysis of static and dynamic host-guest associations of detergents with cyclodextrins via photoluminescence methods. J. Am. Chem. Soc. 1982, 104, 1789–1794. [Google Scholar]
- Yoshida, N.; Seiyama, A.; Fujimoto, M. Dynamic aspects in host-guest interactions: Mechanism for molecular recognition by α-cyclodextrin of alkyl-substituted hydroxyphenylazo derivatives of sulfanilic acid. J. Phys. Chem. 1990, 94, 4246–4253. [Google Scholar]
- Mock, W.L.; Shih, N.Y. Structure and selectivity in host-guest complexes of cucurbituril. J. Org. Chem. 1986, 51, 4440–4446. [Google Scholar]
- Breslow, R.; Zhang, B. Cholesterol recognition and binding bycyclodextrin dimers. J. Am. Chem. Soc. 1996, 118, 8495–8496. [Google Scholar]
- Matsugo, S.; Yan, L.J.; Konishi, T.; Youn, H.D.; Lodge, J.K.; Ulrich, H.; Packer, L. The lipoic acid analogue 1,2-diselenolane-3-pentanoic acid protects human low density lipoprotein against oxidative modification mediated by copper ion. Biochem. Biophys. Res. Commun. 1997, 240, 819–824. [Google Scholar]
- Trentin, M.; Carofiglio, T.; Fornasier, R.; Tonellato, U. Capillary zone electrophoresis study of cyclodextrin—Lipoic acid host-guest interaction. Electrophoresis 2002, 23, 4117–4122. [Google Scholar]
- Tong, L.H.; Pang, Z.Z.; Yi, Y. Inclusion complexes of α- and β-cyclodextrin with α-lipoic acid. J. Incl. Phenom. Mol. Recognit. Chem. 1995, 23, 119–126. [Google Scholar]
- Maeda, H.; Onodera, T.; Nakayama, H. Inclusion complex of α-lipoic acid and modified cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 201–206. [Google Scholar]
- Racz, C.P.; Santa, S.; Tomoaia-Cotisel, M.; Borodi, G.; Kacso, I.; Pirnau, A.; Bratu, I. Inclusion of α-lipoic acid in β-cyclodextrin. Physical–chemical and structural characterization. J. Incl. Phenom. Macrocycl. Chem. 2013, 76, 193–199. [Google Scholar]
- Packer, L.; Cadenas, E. Lipoic acid: Energy metabolism and redox regulation of transcription and cell signaling. J. Clin. Biochem. Nutr. 2011, 48, 26–32. [Google Scholar]
- Nikolai, S.; Huebbe, P.; Metges, C.C.; Schloesser, A.; Dose, J.; Ikuta, N.; Terao, K.; Matsugo, S.; Rimbach, G. R-α lipoic acid γ-cyclodextrin complex increases energy expenditure: A 4-month feeding study in mice. Nutrition 2014, 30, 228–233. [Google Scholar]
- Packer, L.; Kraemer, K.; Rimbach, G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 2001, 17, 888–895. [Google Scholar]
- Carofiglio, T.; Fornasier, R.; Jicsinszky, L.; Saielli, G.; Tonellato, U.; Vetta, R. Capillary electrophoresis, ROESY NMR and molecular modeling study of the inclusion complex β-cyclodextrin/lipoic acid. Eur. J. Org. Chem. 2002, 7, 1191–1196. [Google Scholar]
- Takahashi, H.; Bungo, Y.; Mikuni, K. Effect of cyclodextrin on the pungent taste of α-lipoic acid. J. Jpn. Soc. Food Sci. Technol.-Nippon Shokuhin Kagaku Kogaku Kaishi 2011, 58, 583–590. [Google Scholar]
- Racz, C.P.; Borodi, G.; Pop, M.M.; Kacso, I.; Santa, S.; Tomoaia-Cotisel, M. Structure of the inclusion complex of β-cyclodextrin with lipoic acid from laboratory powder diffraction data. Acta Crystallogr. Sect. B-Struct. Sci. 2012, 68, 164–170. [Google Scholar]
- Matsugo, S.; Han, D.; Tritschler, H.J.; Packer, L. Decomposition of α-lipoic acid derivatives by photoirradiation-formation of dihydrolipoic acid from α-lipoic acid. Biochem. Mol. Biol. Int. 1996, 38, 51–59. [Google Scholar]
- Ikuta, N.; Sugiyama, H.; Shimosegawa, H.; Nakane, R.; Ishida, Y.; Uekaji, Y.; Nakata, D.; Pallauf, K.; Rimbach, G.; Terao, K.; et al. Analysis of the enhanced stability of R(+)-α lipoic acid by the complex formation with cyclodextrins. Int. J. Mol. Sci. 2013, 14, 3639–3655. [Google Scholar]
- Ikeda, H.; Ikuta, N.; Nakata, D.; Fukumi, H.; Terao, K. An NMR study of inclusion complexes formed by α-cyclodextrin and (R)- or (S)-α-lipoic acid. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 443–447. [Google Scholar]
- Ficarra, R.; Tommasini, S.; Raneri, D.; Calabrò, M.L.; di Bella, M.R.; Rustichelli, C.; Gamberini, M.C.; Ficarra, P. Study of flavonoids/β-cyclodextrins inclusion complexes by NMR, FT-IR, DSC, X-ray investigation. J. Pharm. Biomed. Anal. 2002, 29, 1005–1014. [Google Scholar]
- Moyano, M.A.; Broussalis, A.M.; Segall, A.I. Thermal analysis of lipoic acid and evaluation of the compatibility with excipients. J. Therm. Anal. Calorim. 2010, 99, 631–637. [Google Scholar]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar]
- Crupi, V.; Majolino, D.; Venuti, V.; Guella, G.; Mancini, I.; Rossi, B.; Verrocchio, P.; Viliani, G.; Stancanelli, R. Temperature effect on the vibrational dynamics of cyclodextrin inclusion complexes: Investigation by FT-IR-ATR spectroscopy and numerical simulation. J. Phys. Chem. A 2010, 114, 6811–6817. [Google Scholar]
- Kasiouli, S.; Stasio, F.D.; McDonnell, S.O.; Constantinides, C.P.; Anderson, H.L.; Cacialli, F.; Hayes, S.C. Resonance Raman investigation of β-cyclodextrin-encapsulated π-conjugated polymers. J. Phys. Chem. B 2013, 117, 5737–5747. [Google Scholar]
- De Oliveira, V.E.; Almeida, E.W.; Castro, H.V.; Edwards, H.G.; dos Santos, H.F.; de Oliveira, L.F. Carotenoids and β-cyclodextrin inclusion complexes: Raman spectroscopy and theoretical investigation. J. Phys. Chem. A 2011, 115, 8511–8519. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Kodama, S.; Taga, A.; Aizawa, S.; Kemmei, T.; Honda, Y.; Suzuki, K.; Yamamoto, A. Direct enantioseparation of lipoic acid in dietary supplements by capillary electrophoresis using trimethyl-β-cyclodextrin as a chiral selector. Electrophoresis 2012, 33, 2441–2445. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikuta, N.; Tanaka, A.; Otsubo, A.; Ogawa, N.; Yamamoto, H.; Mizukami, T.; Arai, S.; Okuno, M.; Terao, K.; Matsugo, S. Spectroscopic Studies of R(+)-α-Lipoic Acid—Cyclodextrin Complexes. Int. J. Mol. Sci. 2014, 15, 20469-20485. https://doi.org/10.3390/ijms151120469
Ikuta N, Tanaka A, Otsubo A, Ogawa N, Yamamoto H, Mizukami T, Arai S, Okuno M, Terao K, Matsugo S. Spectroscopic Studies of R(+)-α-Lipoic Acid—Cyclodextrin Complexes. International Journal of Molecular Sciences. 2014; 15(11):20469-20485. https://doi.org/10.3390/ijms151120469
Chicago/Turabian StyleIkuta, Naoko, Akira Tanaka, Ayako Otsubo, Noriko Ogawa, Hiromitsu Yamamoto, Tomoyuki Mizukami, Shoji Arai, Masayuki Okuno, Keiji Terao, and Seiichi Matsugo. 2014. "Spectroscopic Studies of R(+)-α-Lipoic Acid—Cyclodextrin Complexes" International Journal of Molecular Sciences 15, no. 11: 20469-20485. https://doi.org/10.3390/ijms151120469





