Overexpression of the CaTIP1-1 Pepper Gene in Tobacco Enhances Resistance to Osmotic Stresses
Abstract
:1. Introduction
2. Results and Discussion
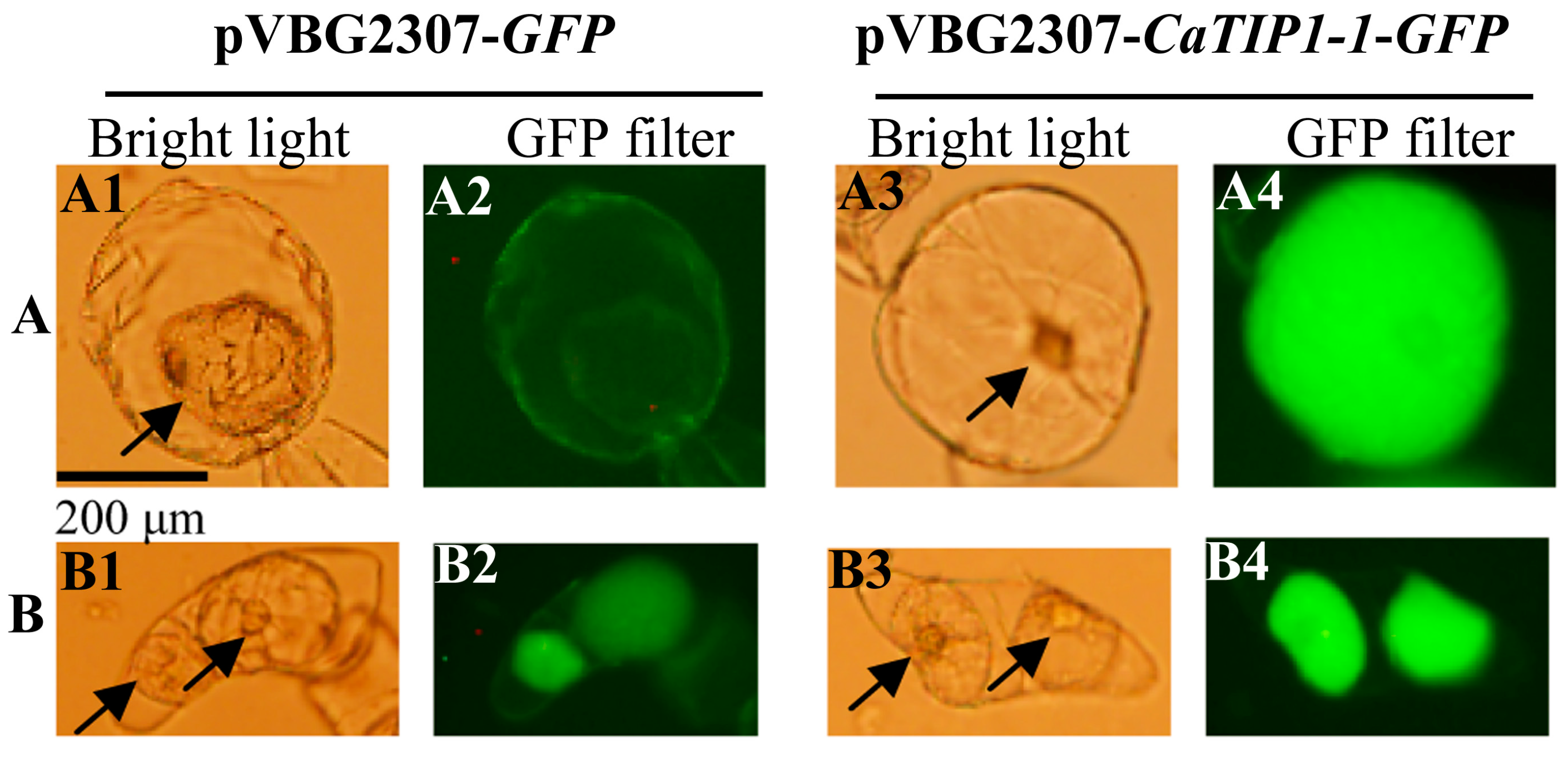
2.1. Subcellular Localization of CaTIP1-1
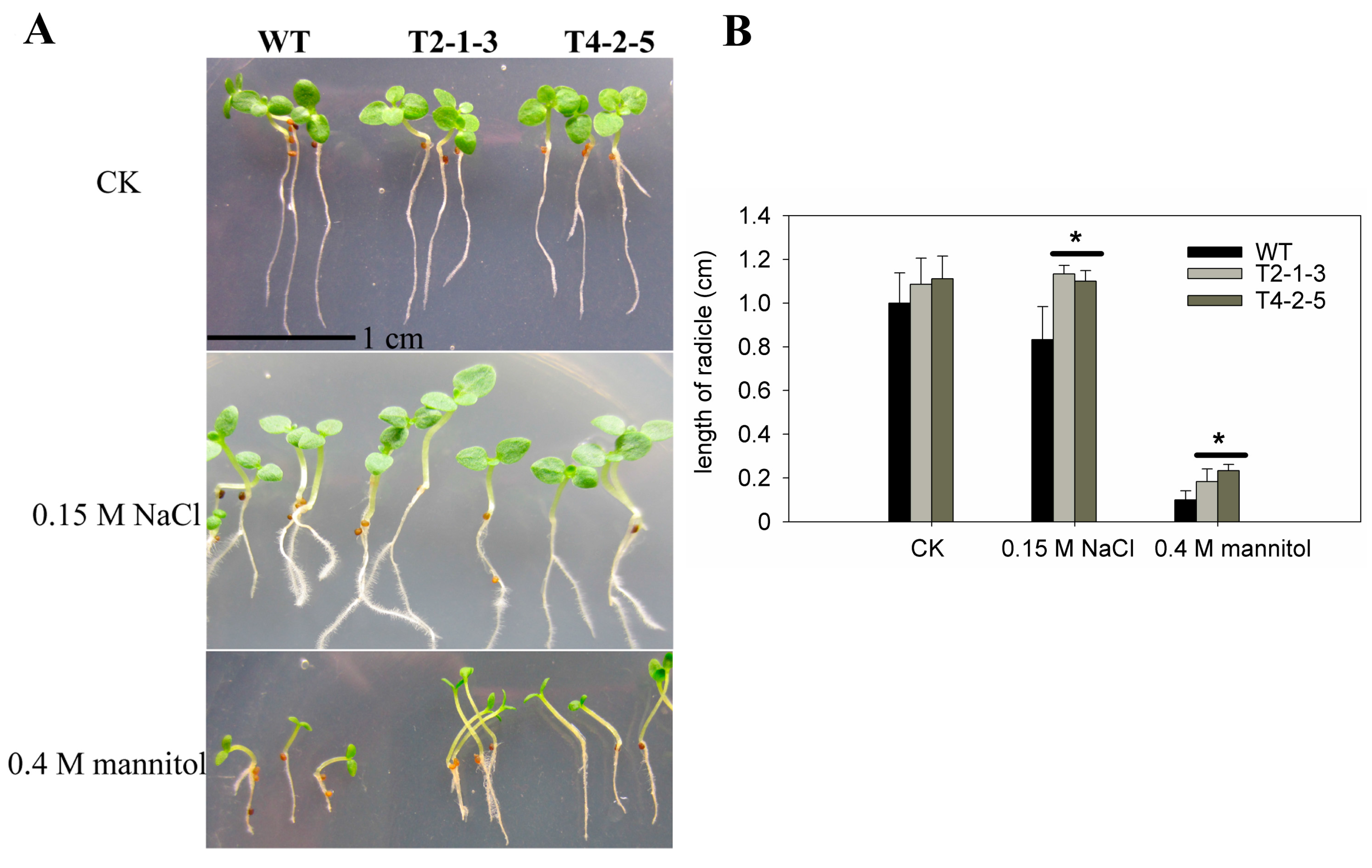
2.2. Radicle Growth of CaTIP1-1-Overexpressing Tobacco Plants
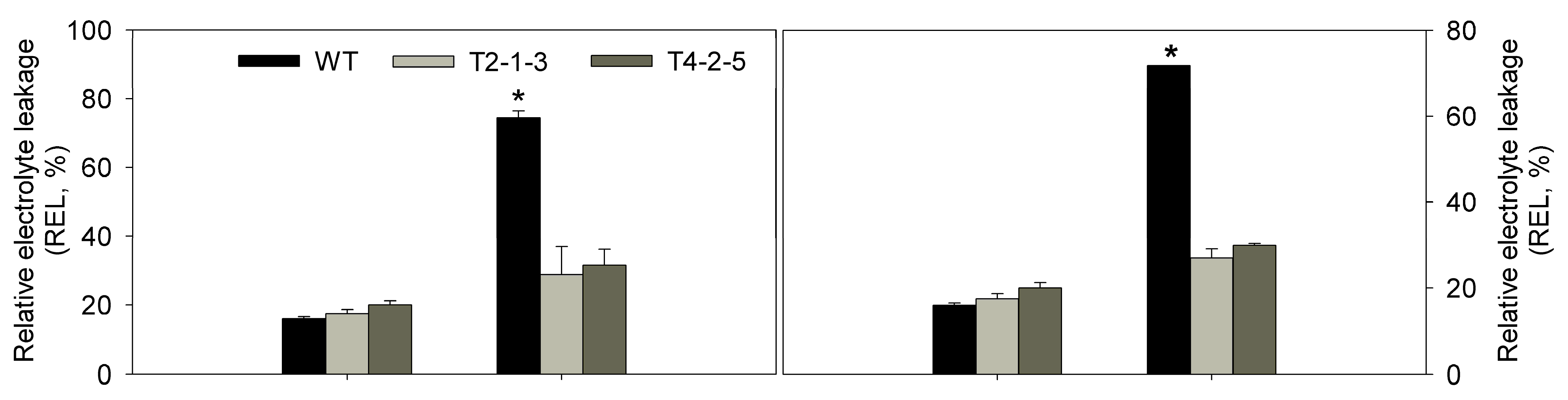
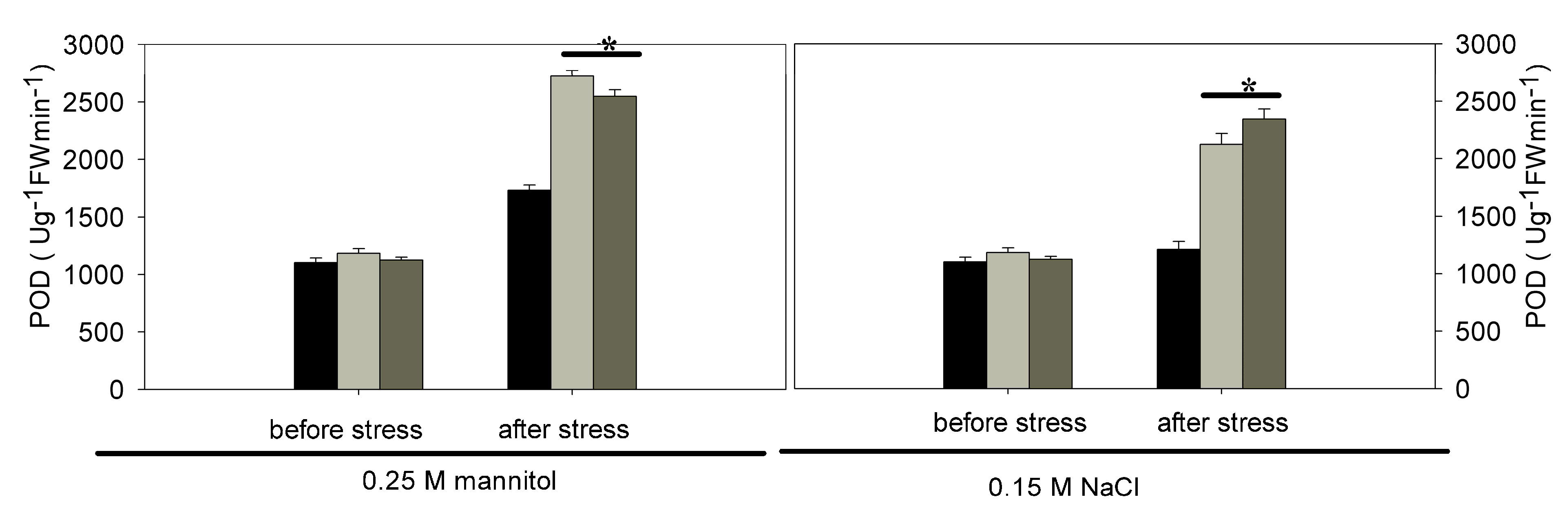
2.3. Analysis of Relative Water Content (RWC), Relative Electrolyte Leakage (REL), Malondialdehyde (MDA) and Antioxidant Enzyme Activities



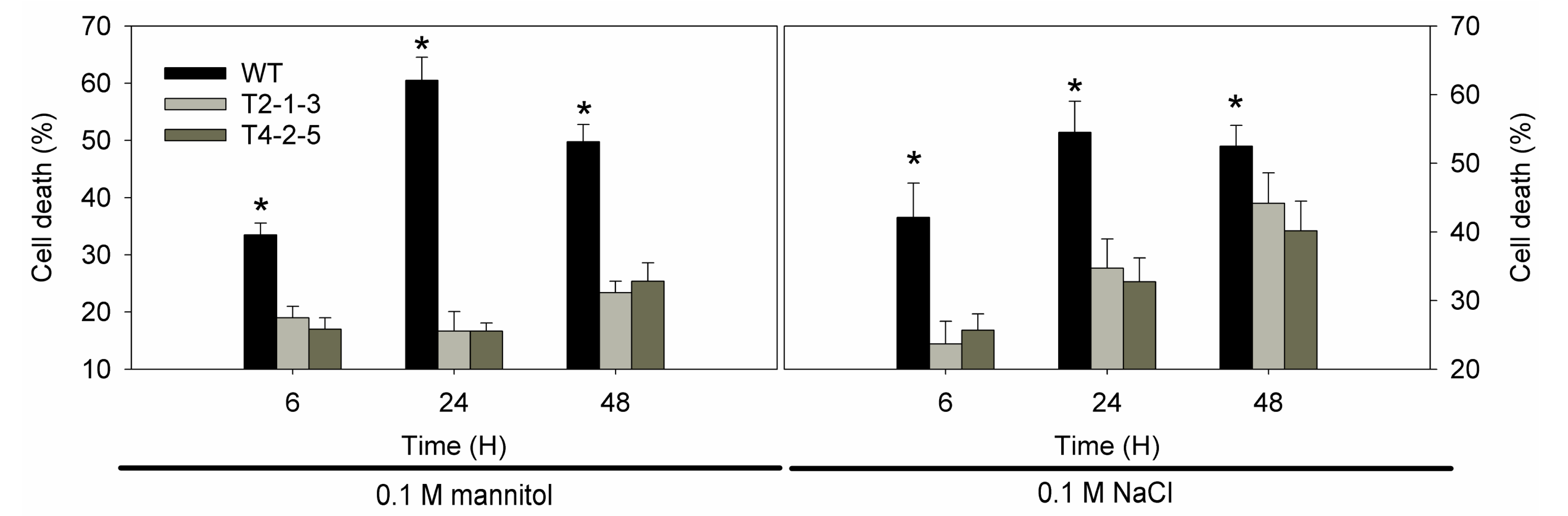
2.4. Cell Viability under Osmotic Stresses
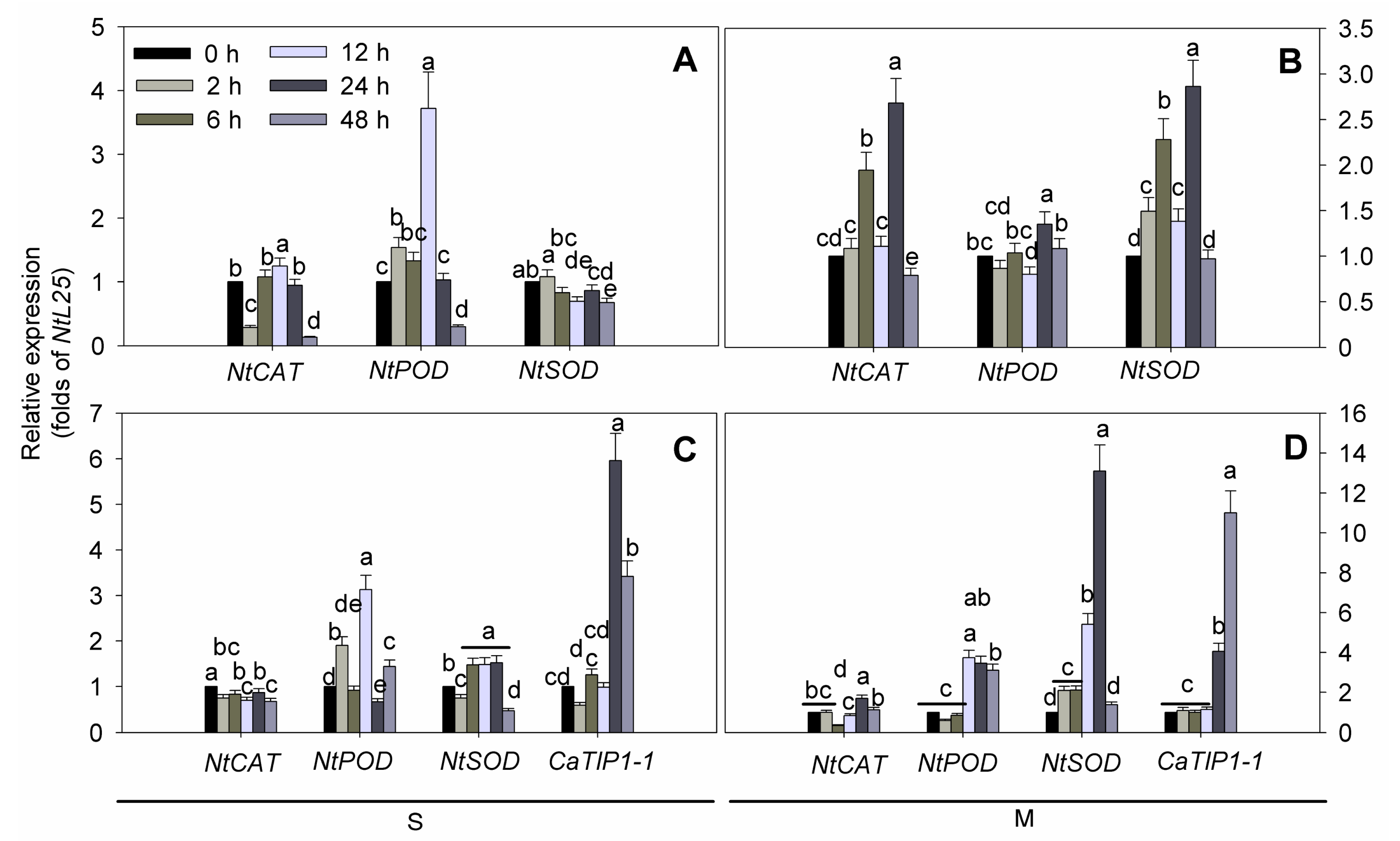
2.5. ROS-Related Genes Expression under Osmotic Stresses in Transgenic Tobacco
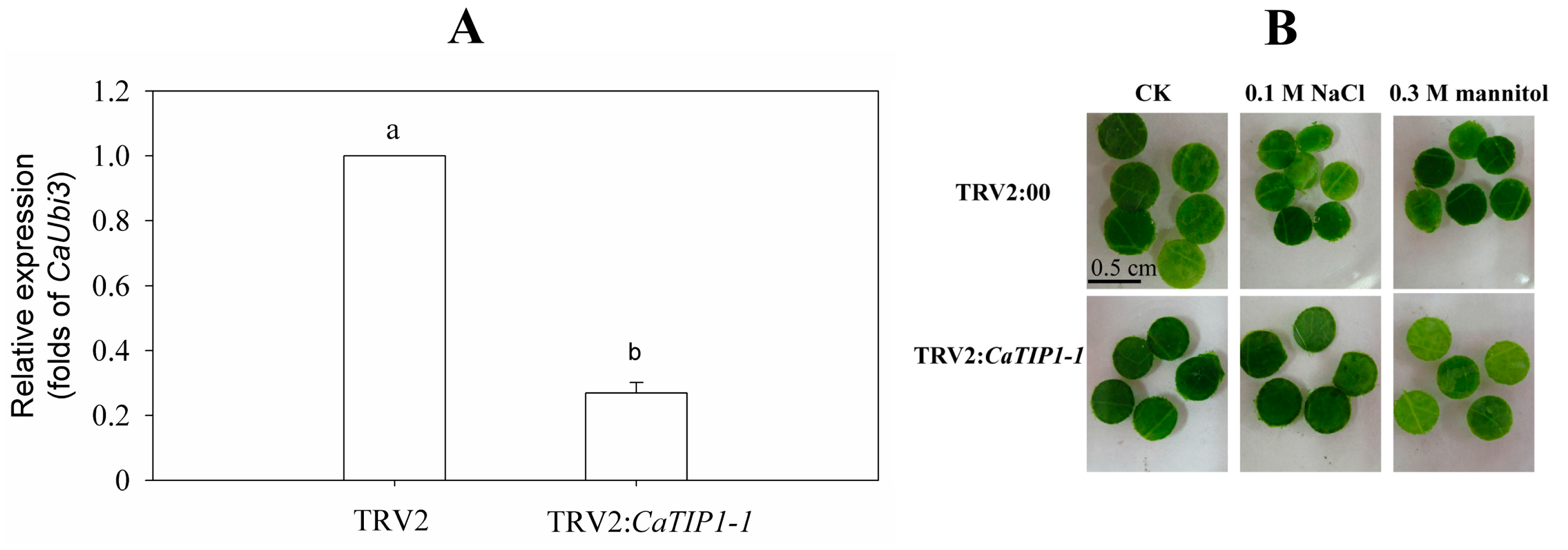
2.6. Leaf Discs Phenotype of Gene-Silenced Pepper in Response to Osmotic Stresses
2.7. Discussion
3. Experimental Section
3.1. Plant Materials, Cell Culture and Seedling Treatment
3.2. Subcellular Localization of CaTIP1-1 Protein
| Primers | Primer Sequence (5'→3') | Explanation |
|---|---|---|
| CaTIP1-1 | F1: CGATGGCGTCACTACTCCTC R1: TGATGTACAGAAGTCCCCTG | RT-PCR |
a F: GC  CTCTTCAGTTTGGTTGTAGGC CTCTTCAGTTTGGTTGTAGGCb R: CGG  GCACCGAAAGTAACAGCAG GCACCGAAAGTAACAGCAG | VIGS Vector construct | |
a F2: GC  ATGCCGATCCGCCAAATTG ATGCCGATCCGCCAAATTGb R2: CGG  AAAATCTCCTCCACTTGGGATTTGC AAAATCTCCTCCACTTGGGATTTGC | Subcellular localization | |
| GFP | F: GTAAGGGAGAAGAACTTTTCACTG R: TGTGGTCTCTCTTTTCGTTGG | |
| NtCAT | F: GTATTGCTTGAGGATTACCATTT R: CTTGACAGCAAACCCACG | RT-PCR |
| NtSOD | F: CTCCTACCGTCGCCAAAT R: GCCCAACCAAGAGAACCC | |
| NtPOD | F: GCTGTTCGACGAGTTGTTAACAG R: CTCTGGCTGAGTTGTTGTTGG | |
| NtL25 | F: CCTAAAGTATCCCCTCACCACAG R: CTTTCTTCGTCCCATCAGGC | |
| CaUbi3 | F: TGTCCATCTGCTCTCTGTTG R: CACCCCAAGCACAATAAGAC |
3.3. RWC, REL, MDA and Antioxidant Enzyme Assays in the Transgenic Tobacco Plants
3.4. RNA Isolation and Quantitative Real-Time PCR Analysis
3.5. Determination of Viability of Tobacco Suspension Cells under Osmotic Stresses
3.6. VIGS Assay of CaTIP1-1 in Pepper Plants
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maurel, C.; Verdoucq, L.; Luu, D.T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar]
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly regulated channels controlling plant water relations. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar]
- Boursiac, Y.; Chen, S.; Luu, D.T.; Sorieul, M.; van den Dries, N.; Maurel, C. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 2005, 139, 790–805. [Google Scholar]
- Li, G.W.; Peng, Y.H.; Yu, X.; Zhang, M.H.; Cai, W.M.; Sun, W.N.; Su, W.A. Transport functions and expression analysis of vacuolar membrane aquaporins in response to various stresses in rice. J. Plant Physiol. 2008, 165, 1879–1888. [Google Scholar]
- Wang, X.; Li, Y.; Ji, W.; Bai, X.; Cai, H.; Zhu, D.; Sun, X.L.; Chen, L.J.; Zhu, Y.M. A novel Glycine soja tonoplast intrinsic protein gene responds to abiotic stress and depresses salt and dehydration tolerance in transgenic Arabidopsis thaliana. J. Plant Physiol. 2011, 168, 1241–1248. [Google Scholar]
- Pou, A.; Medrano, H.; Flexas, J.; Tyerman, S.D. A putative role for TIP and PIP aquaporins in dynamics of leaf hydraulic and stomatal conductances in grapevine under water stress and re-watering. Plant Cell Environ. 2013, 36, 828–843. [Google Scholar]
- Yin, Y.X.; Guo, W.L.; Zhang, Y.L.; Ji, J.J.; Xiao, H.J.; Yan, F.; Zhao, Y.Y.; Zhu, W.C.; Chen, R.G.; Chai, W.G.; et al. Cloning and characterisation of a pepper aquaporin, CaAQP, which reduces chilling stress in transgenic tobacco plants. Plant Cell Tissue Organ Cult. 2014, 118, 431–444. [Google Scholar]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar]
- Venkatesh, J.; Yu, J.W.; Park, S.W. Genome-wide analysis and expression profiling of the Solanum tuberosum aquaporins. Plant Physiol. Biochem. 2013, 73, 392–404. [Google Scholar]
- Reuscher, S.; Akiyama, M.; Mori, C.; Aoki, K.; Shibata, D.; Shiratake, K. Genome-wide identification and expression analysis of aquaporins in tomato. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Danielson, J.A.H.; Johanson, U. Unexpected complexity of the aquaporin gene family in the moss Physcomitrella patens. BMC Plant Biol. 2008, 8. [Google Scholar] [CrossRef]
- Gattolin, S.; Sorieul, M.; Frigerio, L. Mapping of tonoplast intrinsic proteins in maturing and germinating Arabidopsis seeds reveals dual localization of embryonic TIPs to the tonoplast and plasma membrane. Mol. Plant 2011, 4, 180–189. [Google Scholar]
- Li, D.D.; Tai, F.J.; Zhang, Z.T.; Li, Y.; Zheng, Y.; Wu, Y.F.; Li, X.B. A cotton gene encodes a tonoplast aquaporin that is involved in cell tolerance to cold stress. Gene 2009, 438, 26–32. [Google Scholar]
- Sade, N.; Vinocur, B.J.; Diber, A.; Shatil, A.; Ronen, G.; Nissan, H.; Wallach, R.; Karchi, H.; Moshelion, M. Improving plant stress tolerance and yield production: Is the tonoplast aquaporin SlTIP2;2 a key to isohydric to anisohydric conversion? New Phytol. 2009, 181, 651–661. [Google Scholar]
- Liu, C.W.; Fukumoto, T.; Matsumoto, T.; Gena, P.; Frascaria, D.; Kaneko, T.; Katsuhara, M.; Zhong, S.H.; Sun, X.L.; Zhu, Y.M.; et al. Aquaporin OsPIP1;1 promotes rice salt resistance and seed germination. Plant Physiol. Biochem. 2013, 63, 151–158. [Google Scholar]
- Peng, Y.H.; Lin, W.L.; Cai, W.M.; Arora, R. Overexpression of a Panax ginseng tonoplast aquaporin alters salt tolerance, drought tolerance and cold acclimation ability in transgenic Arabidopsis plants. Planta 2007, 226, 729–740. [Google Scholar]
- Leitao, L.; Prista, C.; Moura, T.F.; Loureiro-Dias, M.C.; Soveral, G. Grapevine aquaporins: Gating of a tonoplast intrinsic protein (TIP2;1) by cytosolic pH. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Beebo, A.; Thomas, D.; Der, C.; Sanchez, L.; Leborgne-Castel, N.; Marty, F.; Schoefs, B.; Bouhidel, K. Life with and without AtTIP1;1, an Arabidopsis aquaporin preferentially localized in the apposing tonoplasts of adjacent vacuoles. Plant Mol. Biol. 2009, 70, 193–209. [Google Scholar]
- Aharon, R.; Shahak, Y.; Wininger, S.; Bendov, R.; Kapulnik, Y.; Galili, G. Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 2003, 15, 439–447. [Google Scholar]
- Hussain, S.S.; Iqbal, M.T.; Arif, M.A.; Amjad, M. Beyond osmolytes and transcription factors: Drought tolerance in plants via protective proteins and aquaporins. Biol. Plant. 2011, 55, 401–413. [Google Scholar]
- Hu, W.; Yuan, Q.Q.; Wang, Y.; Cai, R.; Deng, X.M.; Wang, J.; Zhou, S.Y.; Chen, M.J.; Chen, L.H.; Huang, C.; et al. Overexpression of a wheat aquaporin gene, TaAQP8, enhances salt stress tolerance in transgenic tobacco. Plant Cell Physiol. 2012, 53, 2127–2141. [Google Scholar]
- Zhou, S.Y.; Hu, W.; Deng, X.M.; Ma, Z.B.; Chen, L.H.; Huang, C.; Wang, C.; Wang, J.; He, Y.Z.; Yang, G.X.; et al. Overexpression of the wheat aquaporin gene, TaAQP7, enhances drought tolerance in transgenic tobacco. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, Z.; Chai, T.Y.; Wen, Z.S.; Zhang, H.M. Indian mustard aquaporin improves drought and heavy-metal resistance in tobacco. Mol. Biotechnol. 2008, 40, 280–292. [Google Scholar]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcon, C.; Aroca, R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: New challenges in physiological and molecular studies. J. Exp. Bot. 2012, 63, 4033–4044. [Google Scholar]
- Kothari, S.L.; Joshi, A.; Kachhwaha, S.; Ochoa-Alejo, N. Chilli peppers-A review on tissue culture and transgenesis. Biotechnol. Adv. 2010, 28, 35–48. [Google Scholar]
- Wang, J.E.; Li, D.W.; Gong, Z.H.; Zhang, Y.L. Optimization of virus-induced gene silencing in pepper (Capsicum annuum L.). Genet. Mol. Res. 2013, 12, 2492–2506. [Google Scholar]
- Xiao, H.J.; Yin, Y.X.; Chai, W.G.; Gong, Z.H. Silencing of the CaCP gene delays salt- and osmotic-induced leaf senescence in Capsicum annuum L. Int. J. Mol. Sci. 2014, 15, 8316–8334. [Google Scholar]
- Gattolin, S.; Sorieul, M.; Frigerio, L. Tonoplast intrinsic proteins and vacuolar identity. Biochem. Soc. Trans. 2010, 38, 769–773. [Google Scholar]
- Mitsuhashi, N.; Shimada, T.; Mano, S.; Nishimura, M.; Hara-Nishimura, I. Characterization of organelles in the vacuolar-sorting pathway by visualization with GFP in tobacco BY-2 cells. Plant Cell Physiol. 2000, 41, 993–1001. [Google Scholar]
- Lee, O.R.; Cho, H.T. Cytoplasm localization of aminopeptidase M1 and its functional activity in root hair cells and BY-2 cells. Mol. Biol. Rep. 2012, 39, 10211–10218. [Google Scholar]
- Johanson, U.; Karlsson, M.; Johansson, I.; Gustavsson, S.; Sjovall, S.; Fraysse, L.; Weig, A.R.; Kjellbom, P. The complete set of genes encoding major intrinsic proteins in arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 2001, 126, 1358–1369. [Google Scholar]
- An, G. High efficiency transformation of cultured tobacco cells. Plant Physiol. 1985, 79, 568–570. [Google Scholar]
- Schussler, M.D.; Alexandersson, E.; Bienert, G.P.; Kichey, T.; Laursen, K.H.; Johanson, U.; Kjellbom, P.; Schjoerring, J.K.; Jahn, T.P. The effects of the loss of TIP1;1 and TIP1;2 aquaporins in Arabidopsis thaliana. Plant J. 2008, 56, 756–767. [Google Scholar]
- Ben Rejeb, K.; Abdelly, C.; Savoure, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar]
- Wang, J.E.; Liu, K.K.; Li, D.W.; Zhang, Y.L.; Zhao, Q.; He, Y.M.; Gong, Z.H. A novel peroxidase CanPOD gene of pepper is involved in defense responses to phytophtora capsici infection as well as abiotic stress tolerance. Int. J. Mol. Sci. 2013, 14, 3158–3177. [Google Scholar]
- Jang, J.Y.; Kim, D.G.; Kim, Y.O.; Kim, J.S.; Kang, H. An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Mol. Biol. 2004, 54, 713–725. [Google Scholar]
- Mimura, T.; Kura-Hotta, M.; Tsujimura, T.; Ohnishi, M.; Miura, M.; Okazaki, Y.; Mimura, M.; Maeshima, M.; Washitani-Nemoto, S. Rapid increase of vacuolar volume in response to salt stress. Planta 2003, 216, 397–402. [Google Scholar]
- Del Amor, F.M.; Rubio, J.S. Effects of antitranspirant spray and potassium: calcium: magnesium ratio on photosynthesis, nutrient and water uptake, growth, and yield of sweet pepper. J. Plant Nutr. 2009, 32, 97–111. [Google Scholar]
- Ahmed, S.S.; Gong, Z.H.; Ji, J.J.; Yin, Y.X.; Xiao, H.J.; Khan, M.A.; Rehman, A.; Ahmad, I. Construction of the intermediate vector pVBG2307 by incorporating vital elements of expression vectors pBI121 and pBI221. Genet. Mol. Res. 2012, 11, 3091–3104. [Google Scholar]
- Guo, W.L.; Chen, R.G.; Gong, Z.H.; Yin, Y.X.; Ahmed, S.S.; He, Y.M. Exogenous abscisic acid increases antioxidant enzymes and related gene expression in pepper (Capsicum annuum) leaves subjected to chilling stress. Genet. Mol. Res. 2012, 11, 4063–4080. [Google Scholar]
- Schmidt, G.W.; Delaney, S.K. Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol. Genet. Genomics 2010, 283, 233–241. [Google Scholar]
- Wan, H.J.; Yuan, W.; Ruan, M.Y.; Ye, Q.J.; Wang, R.Q.; Li, Z.M.; Zhou, G.Z.; Yao, Z.P.; Zhao, J.; Liu, S.J.; et al. Identification of reference genes for reverse transcription quantitative real-time PCR normalization in pepper (Capsicum annuum L.). Biochem. Biophys. Res. Commun. 2011, 416, 24–30. [Google Scholar]
- Sun, J.A.; Li, L.S.; Liu, M.Q.; Wang, M.J.; Ding, M.Q.; Deng, S.R.; Lu, C.F.; Zhou, X.Y.; Shen, X.; Zheng, X.J.; et al. Hydrogen peroxide and nitric oxide mediate K+/Na+ homeostasis and antioxidant defense in NaCl-stressed callus cells of two contrasting poplars. Plant Cell Tissue Organ. Cult. 2010, 103, 205–215. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.-X.; Wang, S.-B.; Xiao, H.-J.; Zhang, H.-X.; Zhang, Z.; Jing, H.; Zhang, Y.-L.; Chen, R.-G.; Gong, Z.-H. Overexpression of the CaTIP1-1 Pepper Gene in Tobacco Enhances Resistance to Osmotic Stresses. Int. J. Mol. Sci. 2014, 15, 20101-20116. https://doi.org/10.3390/ijms151120101
Yin Y-X, Wang S-B, Xiao H-J, Zhang H-X, Zhang Z, Jing H, Zhang Y-L, Chen R-G, Gong Z-H. Overexpression of the CaTIP1-1 Pepper Gene in Tobacco Enhances Resistance to Osmotic Stresses. International Journal of Molecular Sciences. 2014; 15(11):20101-20116. https://doi.org/10.3390/ijms151120101
Chicago/Turabian StyleYin, Yan-Xu, Shu-Bin Wang, Huai-Juan Xiao, Huai-Xia Zhang, Zhen Zhang, Hua Jing, Ying-Li Zhang, Ru-Gang Chen, and Zhen-Hui Gong. 2014. "Overexpression of the CaTIP1-1 Pepper Gene in Tobacco Enhances Resistance to Osmotic Stresses" International Journal of Molecular Sciences 15, no. 11: 20101-20116. https://doi.org/10.3390/ijms151120101




