OCT4 Expression and Vasculogenic Mimicry Formation Positively Correlate with Poor Prognosis in Human Breast Cancer
Abstract
:1. Introduction
2. Results
2.1. OCT4 Expression and VM Formation in Breast Cancer Specimens
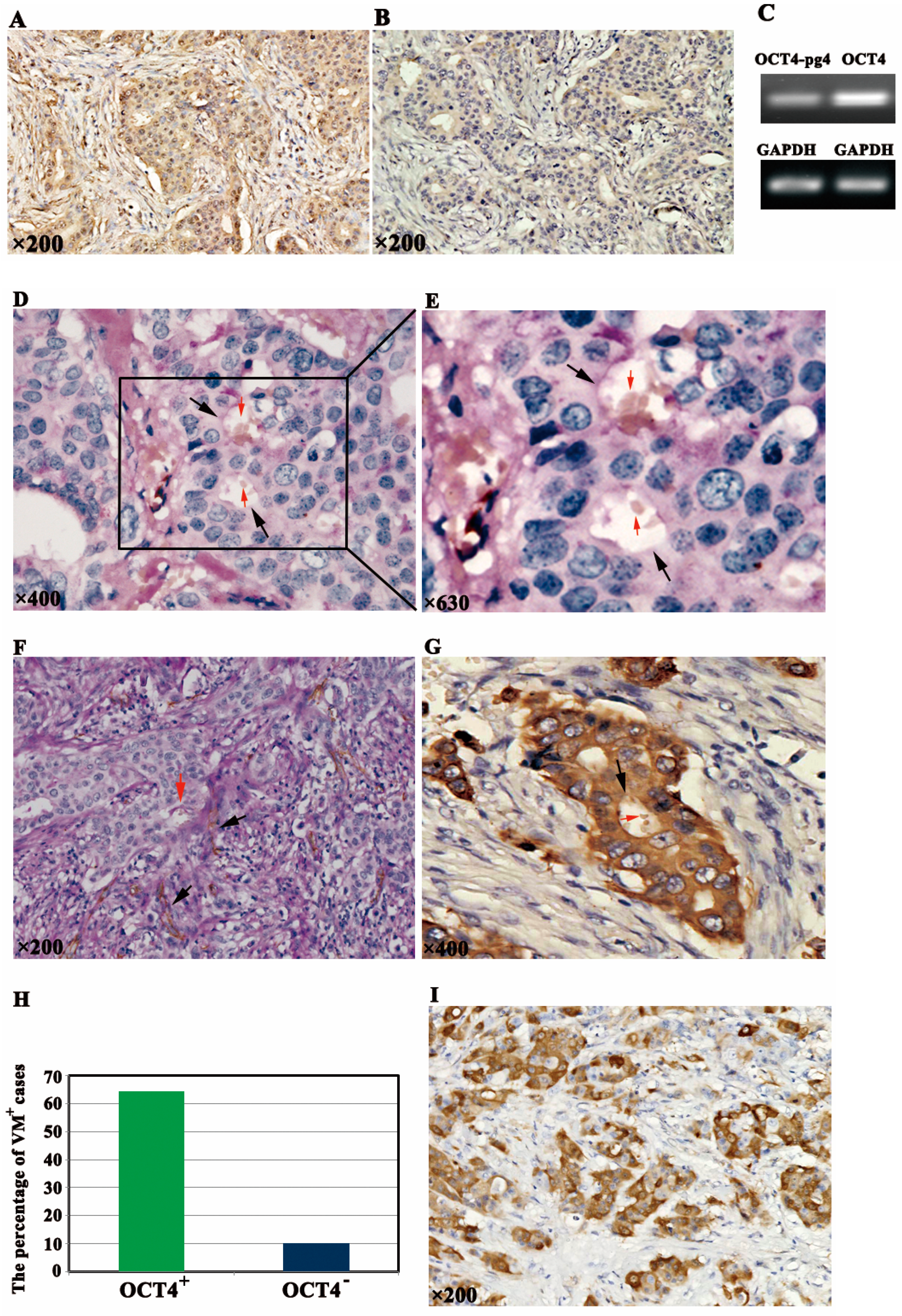
2.2. Association of OCT4-Positive Expression and VM with Clinicopathological Characteristics
| Variables | OCT4 Expression | VM Formation | |||||
|---|---|---|---|---|---|---|---|
| Cases (n) | + | − | p | + | − | p | |
| Age (years) | 0.212 | 0.508 | |||||
| <50 | 47 | 19 | 28 | 15 | 32 | ||
| ≥50 | 43 | 12 | 31 | 11 | 32 | ||
| Tumor size | 0.864 | 0.656 | |||||
| T1: <2 | 30 | 10 | 20 | 9 | 21 | ||
| T2: 2–5 cm | 56 | 19 | 37 | 15 | 41 | ||
| T3: >5 cm | 4 | 2 | 2 | 2 | 2 | ||
| Nodal status | 0.022 * | 0.004 * | |||||
| 0 | 49 | 11 | 38 | 8 | 41 | ||
| 1–3 | 17 | 7 | 10 | 5 | 12 | ||
| ≥4 | 24 | 13 | 11 | 13 | 11 | ||
| Grade | 0.038 * | 0.000 * | |||||
| I | 23 | 4 | 19 | 0 | 23 | ||
| II | 30 | 9 | 21 | 6 | 24 | ||
| III | 37 | 18 | 19 | 20 | 17 | ||
| NPI | 0.006 * | 0.000 * | |||||
| NPI < 3.4 | 26 | 4 | 22 | 1 | 25 | ||
| 3.4 ≤ NPI ≤ 5.4 | 42 | 14 | 28 | 9 | 33 | ||
| NPI > 5.4 | 22 | 13 | 9 | 16 | 6 | ||
| ER | 0.391 | 0.630 | |||||
| Positive | 52 | 16 | 36 | 14 | 38 | ||
| Negative | 38 | 15 | 23 | 12 | 26 | ||
| PR | 0.321 | 0.499 | |||||
| Positive | 50 | 15 | 35 | 13 | 37 | ||
| Negative | 40 | 16 | 24 | 13 | 27 | ||
| Her2 | 0.114 | 0.247 | |||||
| 0/+ | 46 | 18 | 28 | 14 | 32 | ||
| ++ | 20 | 3 | 17 | 3 | 17 | ||
| +++ | 24 | 10 | 14 | 9 | 15 | ||
2.3. Correlation of OCT4-Positive Expression and VM with Overall Survival (OS) and Disease-Free Survival (DFS)

| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR ** | 95% CI | p | HR ** | 95% CI | p | |
| Age (years) | ||||||
| <50, ≥50 | 0.956 | 0.575–1.587 | 0.861 | |||
| Tumor size | ||||||
| T1, T2, T3 | 0.931 | 0.575–1.507 | 0.771 | |||
| Nodal status | ||||||
| 0, 1–3, ≥4 | 1.625 | 1.178–2.241 | 0.003 * | |||
| Grade | ||||||
| I, II, III | 2.013 | 1.427–2.840 | 0.000 * | |||
| NPI | ||||||
| Good, Moderate, Worse | 2.837 | 1.870–4.303 | 0.000 * | 1.903 | 1.187–3.050 | 0.008 * |
| ER | ||||||
| Positive, Negative | 0.640 | 0.386–1.061 | 0.083 | |||
| PR | ||||||
| Positive, Negative | 0.724 | 0.438–1.197 | 0.208 | |||
| Her2 | ||||||
| 0/+, ++, +++ | 1.210 | 0.897–1.632 | 0.211 | 1.398 | 1.031–1.894 | 0.031 * |
| OCT4 | ||||||
| Positive, Negative | 3.459 | 1.974–6.061 | 0.000 * | |||
| VM | ||||||
| Positive, Negative | 10.750 | 5.404–21.383 | 0.000 * | 7.744 | 3.514–17.069 | 0.000 * |
| OCT4/VM | ||||||
| OCT4+/VM+, Non-OCT4+/VM+ | 8.907 | 4.565–17.378 | 0.000 * | |||
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR ** | 95% CI | p | HR ** | 95% CI | p | |
| Age (years) | ||||||
| <50, ≥50 | 0.997 | 0.634–1.568 | 0.989 | |||
| Tumor size | ||||||
| T1, T2, T3 | 1.034 | 0.671–1.592 | 0.881 | |||
| Nodal status | ||||||
| 0, 1–3, ≥4 | 1.517 | 1.137–2.025 | 0.005 * | |||
| Grade | ||||||
| I, II, III | 1.786 | 1.314–2.426 | 0.000 * | |||
| NPI | ||||||
| Good, Moderate, Worse | 2.339 | 1.642–3.331 | 0.000 * | 1.895 | 1.288–2.789 | 0.001 * |
| ER | ||||||
| Positive, Negative | 0.753 | 0.476–1.192 | 0.227 | |||
| PR | ||||||
| Positive, Negative | 0.673 | 0.426–1.062 | 0.089 | |||
| Her2 | ||||||
| 0/+, ++, +++ | 1.228 | 0.935–1.612 | 0.139 | 1.424 | 1.078–1.880 | 0.013 * |
| OCT4 | ||||||
| Positive, Negative | 4.097 | 2.407–6.973 | 0.000 * | |||
| VM | ||||||
| Positive, Negative | 8.557 | 4.509–16.238 | 0.000 * | |||
| OCT4/VM | ||||||
| OCT4+/VM+, Non-OCT4+/VM+ | 10.011 | 5.228–19.170 | 0.000 * | 7.880 | 3.864–16.067 | 0.000 * |
3. Discussion
4. Materials and Methods
4.1. Tissue Specimens
4.2. Immunohistochemical and Histochemical Double-Staining Methods
4.3. Reverse-Transcription Polymerase Chain Reaction (RT-PCR) Analysis
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Niu, Y.; Liu, T.; Tse, G.M.; Sun, B.; Niu, R.; Li, H.M.; Wang, H.; Yang, Y.; Ye, X.; Wang, Y.; et al. Increased expression of centrosomal α, γ-tubulin in atypical ductal hyperplasia and carcinoma of the breast. Cancer Sci. 2009, 100, 580–587. [Google Scholar] [PubMed]
- Loh, Y.H.; Wu, Q.; Chew, J.L.; Vega, V.B.; Zhang, W.; Chen, X.; Bourque, G.; George, J.; Leong, B.; Liu, J.; et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 2006, 38, 431–440. [Google Scholar] [PubMed]
- Adjaye, J.; Huntriss, J.; Herwig, R.; BenKahla, A.; Brink, T.C.; Wierling, C.; Hultschig, C.; Groth, D.; Yaspo, M.L.; Picton, H.M.; et al. Primary differentiation in the human blastocyst: comparative molecular portraits of inner cell mass and trophectoderm cells. Stem Cells 2005, 23, 1514–1525. [Google Scholar] [CrossRef] [PubMed]
- Babaie, Y.; Herwig, R.; Greber, B.; Brink, T.C.; Wruck, W.; Groth, D.; Lehrach, H.; Burdon, T.; Adjaye, J. Analysis of Oct4-dependent transcriptional networks regulating self-renewal and pluripotency in human embryonic stem cells. Stem Cells 2007, 25, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gidekel, S.; Pizov, G.; Bergman, Y.; Pikarsky, E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell 2003, 4, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Huang, G.R.; Hu, P. Over-expression of Oct4 in human esophageal squamous cell carcinoma. Mol. Cells 2011, 32, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Yang, K.; Liu, R.L.; Meng, C.; Dang, R.F.; Xu, Y. Formation of vasculogenic mimicry in bone metastasis of prostate cancer: Correlation with cell apoptosis and senescence regulation pathways. Pathol. Res. Pract. 2014, 210, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Greber, B.; Arauzo-Bravo, M.J.; Meyer, J.; Park, K.I.; Zaehres, H.; Scholer, H.R. Direct reprogramming of human neural stem cells by OCT4. Nature 2009, 461, 649–643. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Liu, S.; Breiter, D.R.; Wang, F.; Tang, Y.; Sun, S. Octamer 4 small interfering RNA results in cancer stem cell-like cell apoptosis. Cancer Res. 2008, 68, 6533–6540. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Shieh, G.S.; Wu, P.; Lin, C.C.; Shiau, A.L.; Wu, C.L. Oct-3/4 expression reflects tumor progression and regulates motility of bladder cancer cells. Cancer Res. 2008, 68, 6281–6291. [Google Scholar] [CrossRef] [PubMed]
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.; Pe’er, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Kirschmann, D.A.; Seftor, E.A.; Hardy, K.M.; Seftor, R.E.; Hendrix, M.J. Molecular pathways: Vasculogenic mimicry in tumor cells: Diagnostic and therapeutic implications. Clin. Cancer Res. 2012, 18, 2726–2732. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.H.; Ping, Y.F.; Bian, X.W. Contribution of cancer stem cells to tumor vasculogenic mimicry. Protein Cell 2011, 2, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.J.; Sun, B.C.; Zhao, X.L.; Zhao, X.M.; Sun, T.; Gu, Q.; Yao, Z.; Dong, X.Y.; Zhao, N.; Liu, N. CD133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene 2013, 32, 544–553. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, K.; Wang, F.; Qin, Y.R.; Fan, Q.X. Expression of OCT4 in human esophageal squamous cell carcinoma is significantly associated with poorer prognosis. World J. Gastroenterol. 2012, 18, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, Z.Y.; Zhang, R.; Xin, B.; Chen, R.; Zhao, J.; Wang, T.; Wen, W.H.; Jia, L.T.; Yao, L.B.; et al. Pseudogene OCT4-pg4 functions as a natural micro RNA sponge to regulate OCT4 expression by competing for miR-145 in hepatocellular carcinoma. Carcinogenesis 2013, 34, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Albergaria, A.; Ricardo, S.; Milanezi, F.; Carneiro, V.; Amendoeira, I.; Vieira, D.; Cameselle-Teijeiro, J.; Schmitt, F. Nottingham Prognostic Index in triple-negative breast cancer: A reliable prognostic tool? BMC Cancer 2011, 11, 299. [Google Scholar] [CrossRef]
- Liu, N.; Yu, Q.; Liu, T.J.; Gebreamlak, E.P.; Wang, S.L.; Zhang, R.J.; Zhang, J.; Niu, Y. P-cadherin expression and basal-like subtype in breast cancers. Med. Oncol. 2012, 29, 2606–2612. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, B.; Zhao, X.; Gu, Q.; Dong, X.; Yao, Z.; Zhao, N.; Chi, J.; Liu, N.; Sun, R.; Ma, Y. HER2/neu expression correlates with vasculogenic mimicry in invasive breast carcinoma. J. Cell Mol. Med. 2013, 17, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Seftor, R.E.; Hess, A.R.; Seftor, E.A.; Kirschmann, D.A.; Hardy, K.M.; Margaryan, N.V.; Hendrix, M.J. Tumor cell vasculogenic mimicry: From controversy to therapeutic promise. Am. J. Pathol. 2012, 181, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- De Resende, M.F.; Chinen, L.T.; Vieira, S.; Jampietro, J.; da Fonseca, F.P.; Vassallo, J.; Campos, L.C.; Guimaraes, G.C.; Soares, F.A.; Rocha, R.M. Prognostication of OCT4 isoform expression in prostate cancer. Tumour Biol. 2013, 34, 2665–2673. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Sun, B.C.; Zhao, X.L.; Zhao, N.; Dong, X.Y.; Che, N.; Yao, Z.; Ma, Y.M.; Gu, Q.; Zong, W.K.; et al. Promotion of tumor cell metastasis and vasculogenic mimicry by way of transcription coactivation by Bcl-2 and Twist1: A study of hepatocellular carcinoma. Hepatology 2011, 54, 1690–1706. [Google Scholar] [CrossRef] [PubMed]
- Iki, K.; Pour, P.M. Expression of Oct4, a stem cell marker, in the hamster pancreatic cancer model. Pancreatology 2006, 6, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Ezeh, U.I.; Turek, P.J.; Reijo, R.A.; Clark, A.T. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer 2005, 104, 2255–2265. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Chien, Y.; Lu, K.H.; Chang, S.C.; Chou, Y.C.; Huang, Y.T. Oct4-related cytokine effects regulate tumorigenic properties of colorectal cancer cells. Biochem. Biophys. Res. Commun. 2011, 415, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, S.; Tanaka, K.; Toiyama, Y.; Yokoe, T.; Okugawa, Y.; Ioue, Y.; Miki, C.; Kusunoki, M. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann. Surg. Oncol. 2009, 16, 3488–3498. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Cai, N.; Wu, X.L.; Cao, H.Z.; Xie, L.L.; Zheng, P.S. OCT4 promotes tumorigenesis and inhibits apoptosis of cervical cancer cells by miR-125b/BAK1 pathway. Cell Death Dis. 2013, 4, e760. [Google Scholar]
- Tsai, L.L.; Hu, F.W.; Lee, S.S.; Yu, C.H.; Yu, C.C.; Chang, Y.C. OCT4 mediates tumor initiating properties in oral squamous cell carcinomas through the regulation of epithelial-mesenchymal transition. PLoS One 2014, 9, e87207. [Google Scholar]
- Kim, R.J.; Nam, J.S. OCT4 expression enhances features of cancer stem cells in a mouse model of breast cancer. Lab. Anim. Res. 2011, 27, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Ge, J.; Wang, X.; Qian, X.; Zhang, C.; Li, X. OCT4 regulates epithelial-mesenchymal transition and its knockdown inhibits colorectal cancer cell migration and invasion. Oncol. Rep. 2013, 29, 155–160. [Google Scholar] [PubMed]
- Wang, W.; Lin, P.; Han, C.; Cai, W.; Zhao, X.; Sun, B. Vasculogenic mimicry contributes to lymph node metastasis of laryngeal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2010, 29, 60. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, K.; Wakasugi, H.; Heike, Y.; Watanabe, I.; Yamada, S.; Saito, K.; Konishi, F. Vasculogenic mimicry and pseudo-comedo formation in breast cancer. Int. J. Cancer 2002, 99, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.H.; Park, N.R.; Shim, J.K.; Kim, B.K.; Shin, H.J.; Lee, J.H.; Huh, Y.M.; Lee, S.J.; Kim, S.H.; Kim, E.H.; et al. Isolation of glioma cancer stem cells in relation to histological grades in glioma specimens. Childs Nerv. Syst. 2013, 29, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, S.; Zhang, D.; Du, J.; Guo, H.; Zhao, X.; Zhang, W.; Hao, X. Vasculogenic mimicry is associated with high tumor grade, invasion and metastasis, and short survival in patients with hepatocellular carcinoma. Oncol. Rep. 2006, 16, 693–698. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Sun, B.; Zhao, X.; Li, Y.; Gu, Q.; Dong, X.; Liu, F. OCT4 Expression and Vasculogenic Mimicry Formation Positively Correlate with Poor Prognosis in Human Breast Cancer. Int. J. Mol. Sci. 2014, 15, 19634-19649. https://doi.org/10.3390/ijms151119634
Liu T, Sun B, Zhao X, Li Y, Gu Q, Dong X, Liu F. OCT4 Expression and Vasculogenic Mimicry Formation Positively Correlate with Poor Prognosis in Human Breast Cancer. International Journal of Molecular Sciences. 2014; 15(11):19634-19649. https://doi.org/10.3390/ijms151119634
Chicago/Turabian StyleLiu, Tieju, Baocun Sun, Xiulan Zhao, Yanlei Li, Qiang Gu, Xueyi Dong, and Fang Liu. 2014. "OCT4 Expression and Vasculogenic Mimicry Formation Positively Correlate with Poor Prognosis in Human Breast Cancer" International Journal of Molecular Sciences 15, no. 11: 19634-19649. https://doi.org/10.3390/ijms151119634
APA StyleLiu, T., Sun, B., Zhao, X., Li, Y., Gu, Q., Dong, X., & Liu, F. (2014). OCT4 Expression and Vasculogenic Mimicry Formation Positively Correlate with Poor Prognosis in Human Breast Cancer. International Journal of Molecular Sciences, 15(11), 19634-19649. https://doi.org/10.3390/ijms151119634




