Pharmacokinetic Comparison of Berberine in Rat Plasma after Oral Administration of Berberine Hydrochloride in Normal and Post Inflammation Irritable Bowel Syndrome Rats
Abstract
:1. Introduction
2. Results
2.1. Recording of Distal Colonic Motility and Calculation of Motility Index (MI)
2.2. The Number of the Fecal Pellet Output over Two Hours
2.3. The Time of the Glass Bead Output
2.4. Histological Features of Colonic Tissue
2.5. Mast Cell Count in Proximal Colon
2.6. Pharmacokinetic Analysis
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Animals
4.3. Induction of PI-IBS Rats
4.3.1. Recording of Distal Colonic Motility and Calculation of Motility Index
4.3.2. Measurement of the Number of Feces Defecated in 2 h and the Time of Glass Bead Output
4.3.3. Histological Examination of Inflammation and Mast Cells Counting
4.4. Drug Analysis
4.5. Pharmacokinetic Analysis
4.6. Pharmacokinetic Data Analysis
4.7. Data Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kong, D.X.; Li, X.J.; Tang, G.Y.; Zhang, H.Y. How many traditional Chinese medicine components have been recognized by modern Western medicine? A chemo informatic analysis and implications for finding multicomponent drugs. Chem. Med. Chem 2008, 3, 233–236. [Google Scholar]
- Liu, Q.; Jiang, H.; Liu, Z.; Wang, Y.; Zhao, M.; Hao, C.; Feng, S.; Guo, H.; Xu, B.; Yang, Q.; et al. Berberine radiosensitizes human esophageal cancer cells by down regulating homologous recombination repair protein RAD51. PLoS One 2011, 6, e23427. [Google Scholar]
- Yan, K.; Zhang, C.; Feng, J.; Hou, L.; Yan, L.; Zhou, Z.; Liu, Z.; Liu, C.; Fan, Y.; Zheng, B.; et al. Induction of G1 cell cycle arrest and apoptosis by berberine in bladder cancer cells. Eur. J. Pharmacol 2011, 661, 1–7. [Google Scholar]
- Sun, Y.; Xun, K.; Wang, Y.; Chen, X. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anticancer Drugs 2009, 20, 757–769. [Google Scholar]
- Imanshahidi, M.; Hosseinzadeh, H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother. Res 2008, 22, 999–1012. [Google Scholar]
- Zhou, H.; Mineshita, S. The effect of berberine chloride on experimental colitis in rats in vivo and in vitro. J. Pharmacol. Exp. Ther 2000, 294, 822–829. [Google Scholar]
- Lee, I.A.; Hyun, Y.J.; Kim, D.H. Berberine ameliorates TNBS-induced colitis by inhibiting lipid peroxidation, enterobacterial growth and NF-κB activation. Eur. J. Pharmacol 2010, 648, 162–170. [Google Scholar]
- Zhang, M.; Long, Y.; Sun, Y.; Wang, Y.; Li, Q.; Wu, H.; Guo, Z.; Li, Y.; Niu, Y.; Li, C.; et al. Evidence for the complementary and synergistic effects of the three-alkaloid combination regimen containing berberine, hypaconitine and skimmianine on the ulcerative colitis rats induced by trinitrobenzene-sulfonic acid. Eur. J. Pharmacol 2011, 651, 187–196. [Google Scholar]
- Pan, L.R.; Tang, Q.; Fu, Q.; Hu, B.R.; Xiang, J.Z.; Qian, J.Q. Roles of nitric oxide in protective effect of berberine in ethanol-induced gastric ulcer mice. Acta Pharmacol. Sin 2005, 26, 1334–1338. [Google Scholar]
- Zhang, J.; Zhou, F.; Lu, M.; Ji, W.; Niu, F.; Zha, W.; Wu, X.; Hao, H.; Wang, G. Pharmacokinetics-pharmacology disconnection of herbal medicines and its potential solutions with cellular pharmacokinetic-pharmacodynamic strategy. Curr. Drug. Metab 2012, 13, 558–576. [Google Scholar]
- Lu, T.; Liang, Y.; Song, J.; Xie, L.; Wang, G.J.; Liu, X.D. Simultaneous determination of berberine and palmatine in rat plasma by HPLC-ESI-MS after oral administration of traditional Chinese medicinal preparation Huang-Lian-Jie-Du decoction and the pharmacokinetic application of the method. J. Pharm. Biomed. Anal 2006, 40, 1218–1224. [Google Scholar]
- Chen, W.; Miao, Y.Q.; Fan, D.J.; Yang, S.S.; Lin, X.; Meng, L.K.; Tang, X. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS Pharm. Sci. Tech 2011, 12, 705–711. [Google Scholar]
- Battu, S.K.; Repka, M.A.; Maddineni, S.; Chittiboyina, A.G.; Avery, M.A.; Majumdar, S. Physicochemical characterization of berberine chloride: A perspective in the development of a solution dosage form for oral delivery. AAPS Pharm. Sci. Tech 2010, 11, 1466–1475. [Google Scholar]
- Li, Y.; You, X.F.; Jiang, J.D. Progress in pharmacokinetic researches of berberine. Chin. J. New Drugs 2008, 17, 733–738. [Google Scholar]
- Li, W.; Guo, J.; Tang, Y.; Wang, H.; Huang, M.; Qian, D.; Duan, J.A. Pharmacokinetic comparison of ferulic acid in normal and blood deficiency rats after oral administration of angelica sinensis, ligusticum chuanxiong and their combination. Int. J. Mol. Sci 2012, 13, 3583–3597. [Google Scholar]
- Yun, T.; Zhi, F.Y.; Yan, L.; Qiao, Y.; Yang, J.; Jia, Y.Y.; Wen, A.D. Pharmacokinetic comparisons of hydroxyl safflower yellow A in normal and blood stasis syndrome rats. J. Ethnopharmacol 2010, 129, 1–4. [Google Scholar]
- Fang, F.; Jia, B.W.; Yan, L.Z.; Jin, C.; Kong, W.J.; Zhao, H.P.; Wang, H.J.; Xiao, X.H. A comparative study on the tissue distributions of rhubarb anthraquinones in normal and CCl4-injured rats orally administered rhubarb extract. J. Ethnopharmacol 2011, 137, 1492–1497. [Google Scholar]
- Yuan, X.D.; Qun, Z.S.; Bo, C.; Zhang, X.J.; Liu, S.Z.; Qiu, X.M. Comparative pharmacokinetics of baicalin in normal and the type 2 diabetic rats after oral administration of the Radix scutellariae extract. Fitoterapia 2012, 83, 1435–1442. [Google Scholar]
- Ming, F.Z.; Lin, M.P.; Hua, X.Z.; Zhang, Q.C.; Guo, L.W. Comparative pharmacokinetics of baicalin in plasma after oral administration of Huang-Lian-Jie-Du-Tang or pure baicalin in MCAO and sham-operated rats. Fitoterapia 2010, 81, 490–496. [Google Scholar]
- Xian, L.Z.; Jin, X.; Mei, H.W.; Yu, Q.; Chen, W.W.; Chen, G.Y.; Tang, W.F. Effect of acute pancreatitis on the pharmacokinetics of Chinese herbal ointment Liu-He-Dan in anaesthetized rats. J. Ethnopharmacol 2013, 145, 94–99. [Google Scholar]
- Quimby, J.M.; Gustafson, D.L.; Lunn, K.F. The pharmacokinetics of mirtazapine in cats with chronic kidney disease and in age-matched control cats. J. Vet. Intern. Med 2011, 25, 985–989. [Google Scholar]
- Mearin, F.; Perez, O.M.; Perello, A.; Vinyet, J.; Ibanez, A.; Coderch, J.; Perona, M. Dyspepsia and irritable bowel syndrome after a salmonella gastroenteritis outbreak: One-year follow-up cohort study. Gastroenterology 2005, 129, 98–104. [Google Scholar]
- Jun, S.; Xiao, H.H. Current status of study on post-infectious irritable bowel syndrome. China J. Gastroenterol 2012, 17, 71. [Google Scholar]
- Tao, T.; Shan, X.U. Various animal model of irritable bowel syndrome and perfect idea of modeling. Gansu J. TCM 2007, 20, 16–19. [Google Scholar]
- Tao, H.; Tuan, M.H. Advances in understanding the relationship between mast cells and irritable bowel syndrome. World Chin. J. Digestol 2011, 19, 494–497. [Google Scholar]
- Philpott, H.; Gibson, P.; Thien, F. Irritable bowel syndrome-an inflammatory disease involving mast cells. Asia Pac. Allergy 2011, 1, 36–42. [Google Scholar]
- Kim, H.S.; Lim, J.H.; Park, H. Increased immunoendocrine cells in intestinal mucosa of post-infectious irritable bowel syndrome patients 3 years after acute Shigella infection—An observation in a small case control study. Yonsei Med. J 2010, 51, 45–51. [Google Scholar]
- Wang, Y.B.; Tang, X.S.; Yang, Z.X. Effects of berberine on patients with IBS. J. Capital. Uni. Med. Sci 2002, 23, 151–152. [Google Scholar]
- Projean, D.; Dautrey, S.; Vu, H.K.; Groblewski, T.; Brazier, J.L.; Ducharme, J. Selective downregulation of hepatic cytochrome P450 expression and activity in a rat model of inflammatory pain. Pharm. Res 2011, 28, 1561–1576. [Google Scholar]
- Uno, S.; Fujili, A.; Komura, H.; Kawase, A.; lwaki, M. Prediction of metabolic clearance of diclofenac in adjuvant-induced arthritis rats using a substrate depletion assay. Xenobiotica 2008, 38, 482–495. [Google Scholar]
- La, J.H.; Kim, T.W.; Sung, T.S.; Kang, J.W.; Kim, H.J.; Yang, I.S. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J. Gastroenterol 2003, 9, 2791–2795. [Google Scholar]
- Williams, C.L.; Villar, G.R.; Peterson, J.M.; Burks, T.F. Stress induced changes in intestinal transit in the rats: A model for irritable bowel syndrome. Gastroenterology 1988, 94, 611–621. [Google Scholar]
- Shu, Y.X. The Pharmacological Experimental Methods; People’s Medical Publishing House: Beijing, China, 2002; p. 1313. [Google Scholar]
- Iwa, M.; Matsushima, M.; Nakade, Y.; Pappas, T.N.; Fujimiya, M.; Takahashi, T. Electro-acupuncture at ST-36 accelerates colonic motility and transit in freely moving conscious rats. Am. J. Physiol. Gastrointest. Liver Physiol 2006, 290, 285–292. [Google Scholar]
- Ying, C.; Yu, J.L.; Ya, J.W.; Qing, Y.; Yu, D.; Xiao, G.W.; Xiao, X.Z.; Yi, W.W.; Zi, P.G.; Rui, J.Z. Comparative pharmacokinetics of active alkaloids after oral administration of Rhizoma Coptidis extract and Wuji Wan formulas in rat using a UPLC-MS/MS method. Eur. J. Drug. Metab. Pharmacokinet 2014, in press. [Google Scholar]
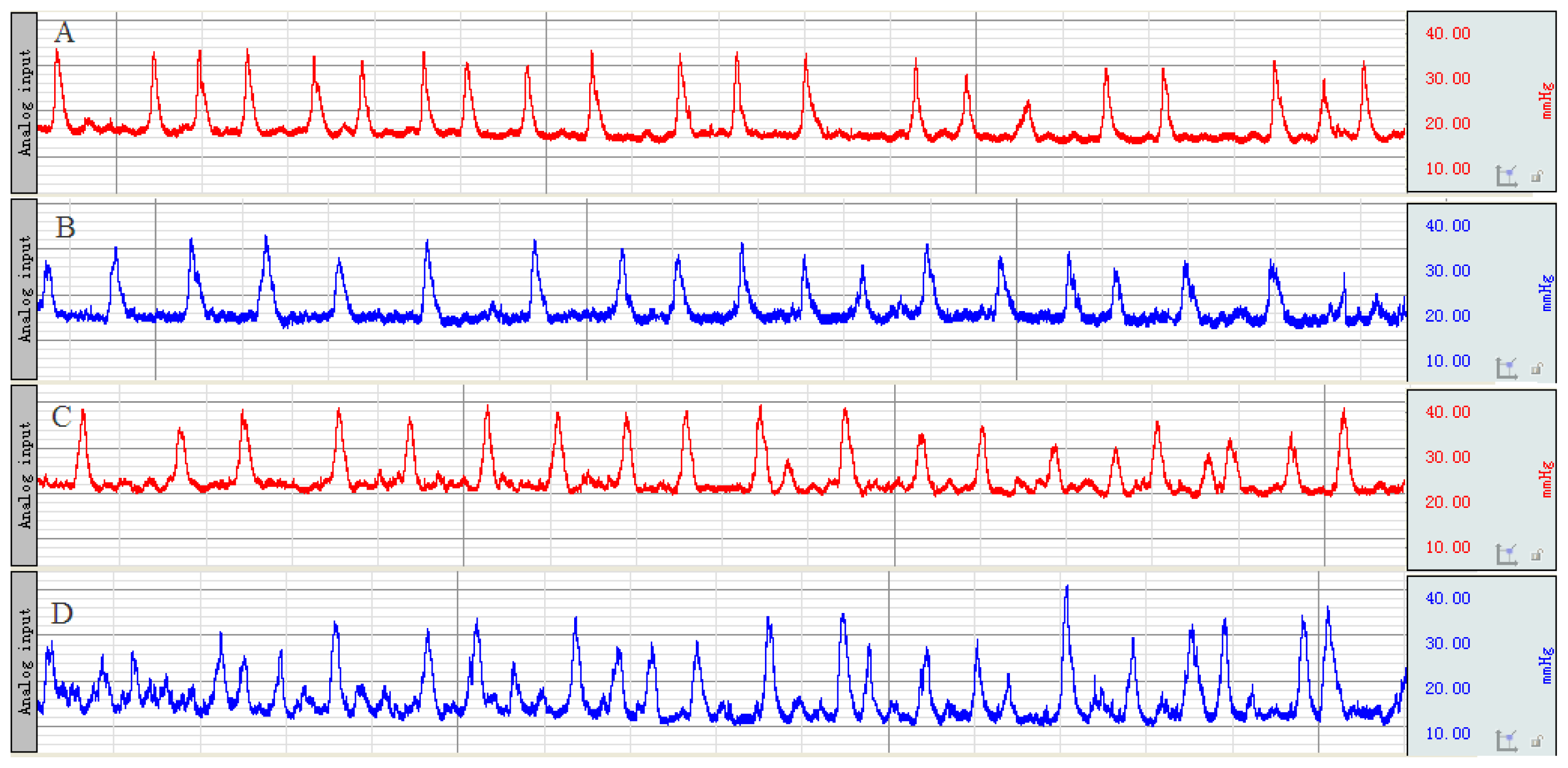
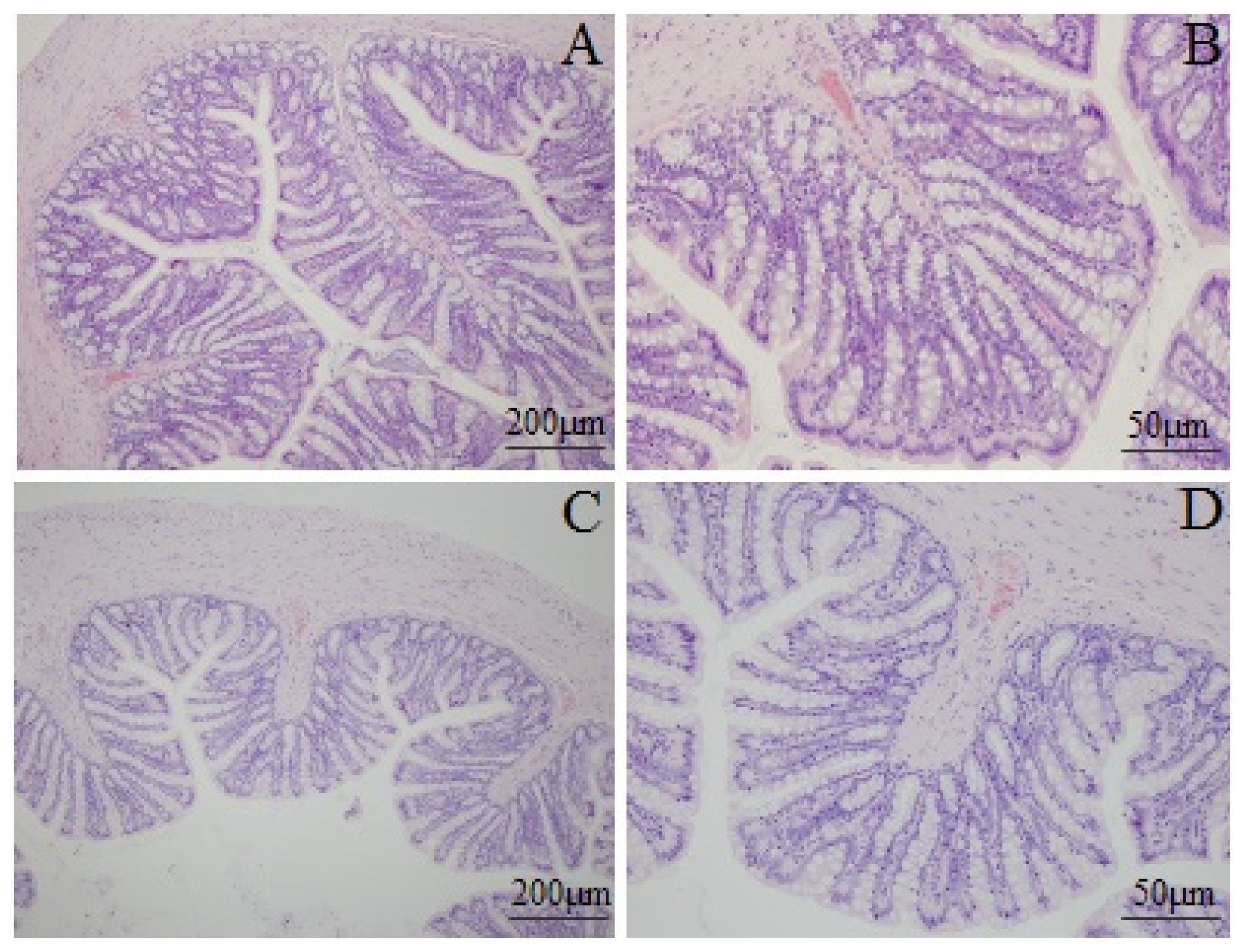

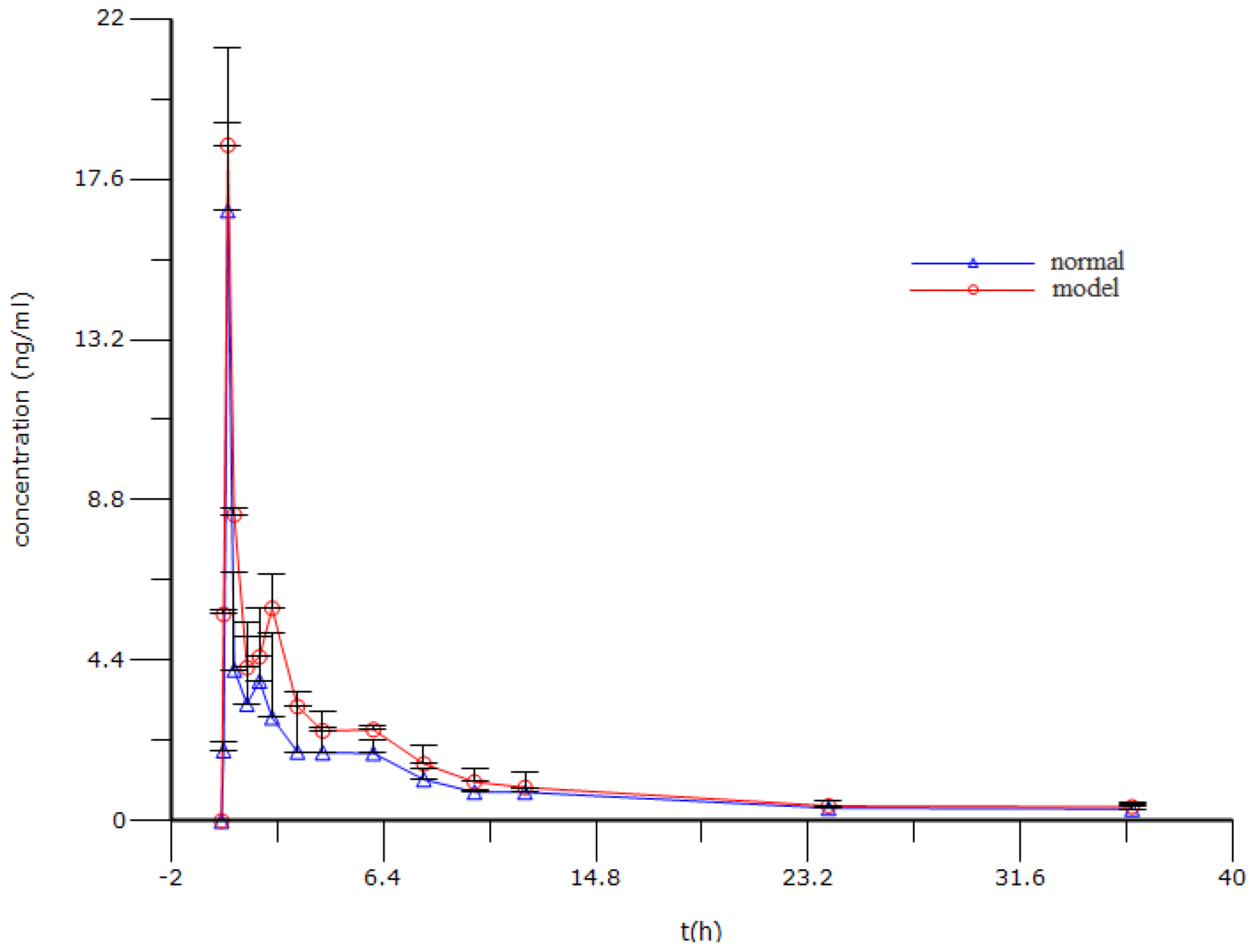
| Group | Before enema | After stress |
|---|---|---|
| Normal | 1085.57 ± 134.93 | 1096.29 ± 119.53 |
| Model | 1098.86 ± 150.18 | 2107.29 ± 270.30 ** |
| Group | Before enema | After stress |
|---|---|---|
| Normal | 4.43 ± 0.98 | 5.14 ± 1.07 |
| Model | 4.57 ± 1.13 | 8.29 ± 1.11 ** |
| Group | Before enema | After stress |
|---|---|---|
| Normal | 1837.71 ± 160.54 | 1859.14 ± 102.08 |
| Model | 1772.57 ± 227.97 | 1297.71 ± 139.76 ** |
| Group | Mast cell count after stress |
|---|---|
| Normal | 2.27 ± 1.05 |
| Model | 6.08 ± 2.28 ** |
| Parameters | Normal | Model |
|---|---|---|
| T1/2,λz (min) | 770.36 ± 65.01 | 941.45 ± 60.39 |
| Tmax (min) | 15.00 ± 0.00 | 15.00 ± 0.00 |
| Cmax (ng/mL) | 16.74 ± 4.47 | 18.53 ± 0.61 |
| AUC0–t (min ng/mL) | 2,039.49 ± 492.24 | 2,763.43 ± 203.14 * |
| Vd/Fλz (L/kg) | 60,036.51 ± 19,704.59 | 41,202.89 ± 4,112.68 |
| CL/F (L/h/kg) | 4,999.34 ± 1,198.79 | 3,270.57 ± 58.32 * |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gong, Z.; Chen, Y.; Zhang, R.; Wang, Y.; Guo, Y.; Yang, Q.; Zhang, H.; Dong, Y.; Weng, X.; Gao, S.; et al. Pharmacokinetic Comparison of Berberine in Rat Plasma after Oral Administration of Berberine Hydrochloride in Normal and Post Inflammation Irritable Bowel Syndrome Rats. Int. J. Mol. Sci. 2014, 15, 456-467. https://doi.org/10.3390/ijms15010456
Gong Z, Chen Y, Zhang R, Wang Y, Guo Y, Yang Q, Zhang H, Dong Y, Weng X, Gao S, et al. Pharmacokinetic Comparison of Berberine in Rat Plasma after Oral Administration of Berberine Hydrochloride in Normal and Post Inflammation Irritable Bowel Syndrome Rats. International Journal of Molecular Sciences. 2014; 15(1):456-467. https://doi.org/10.3390/ijms15010456
Chicago/Turabian StyleGong, Zipeng, Ying Chen, Ruijie Zhang, Yinghan Wang, Yan Guo, Qing Yang, Haixian Zhang, Yu Dong, Xiaogang Weng, Shuangrong Gao, and et al. 2014. "Pharmacokinetic Comparison of Berberine in Rat Plasma after Oral Administration of Berberine Hydrochloride in Normal and Post Inflammation Irritable Bowel Syndrome Rats" International Journal of Molecular Sciences 15, no. 1: 456-467. https://doi.org/10.3390/ijms15010456




