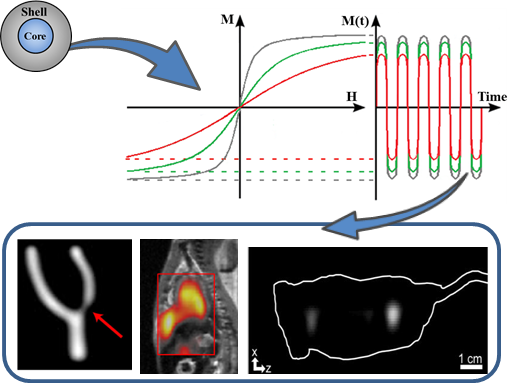
Design of Superparamagnetic Nanoparticles for Magnetic Particle Imaging (MPI)
Abstract
:1. Introduction
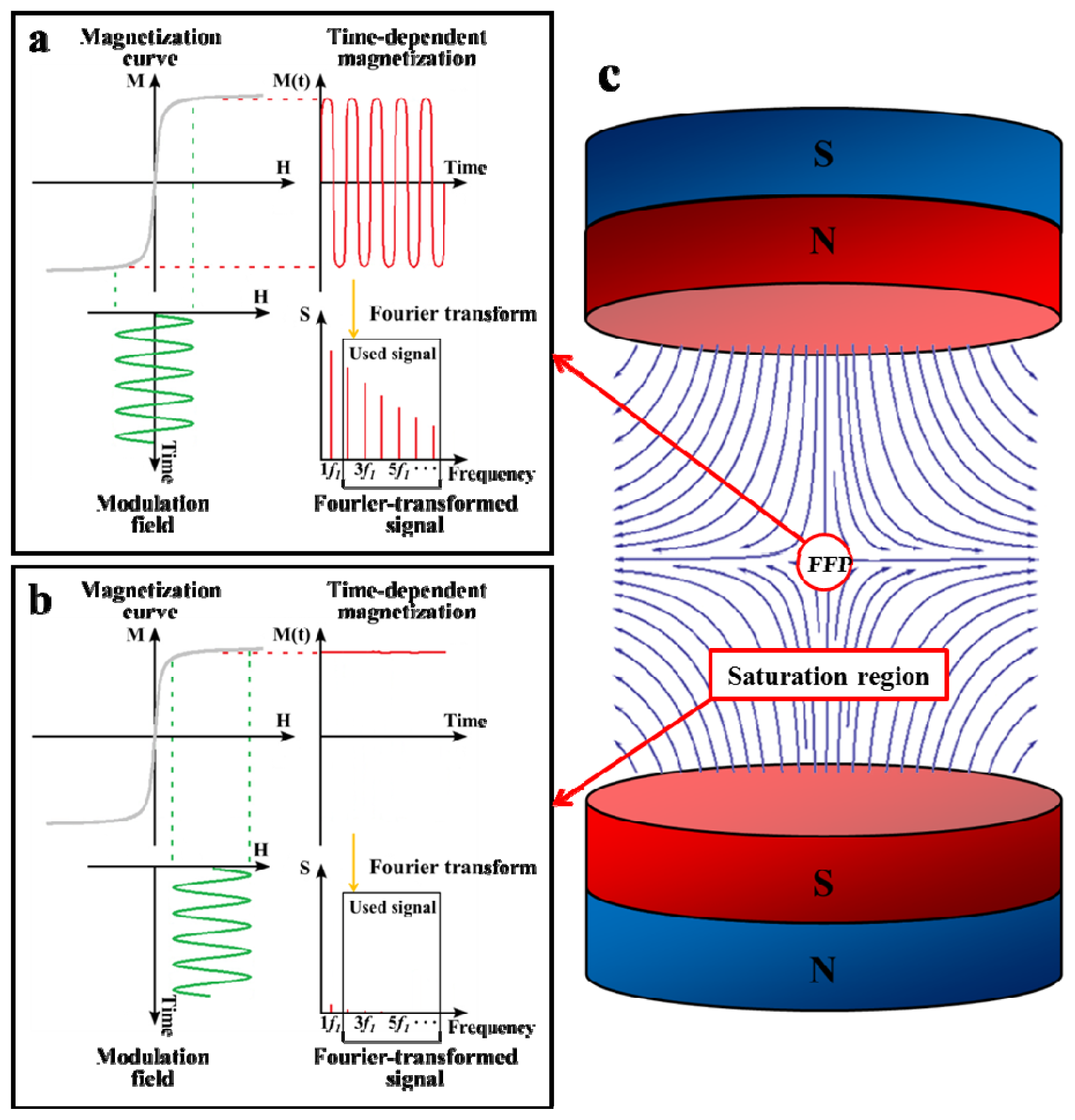
2. Principle of MPI
3. Design of Superparamagnetic Nanoparticles for Improvement of MPI Performance
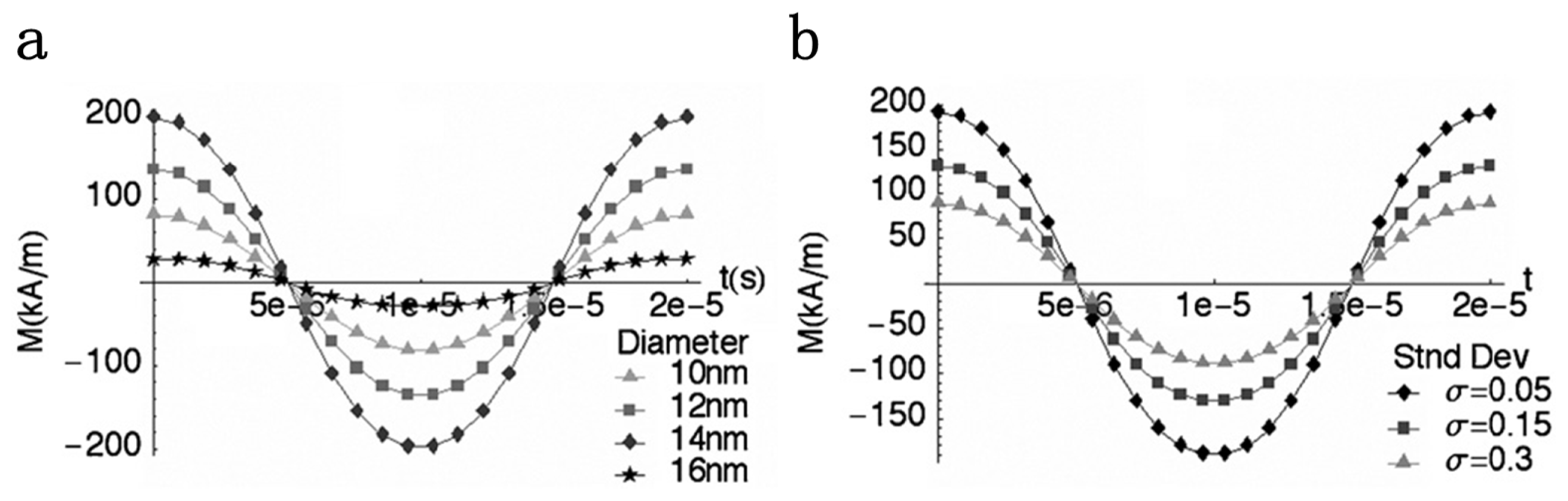
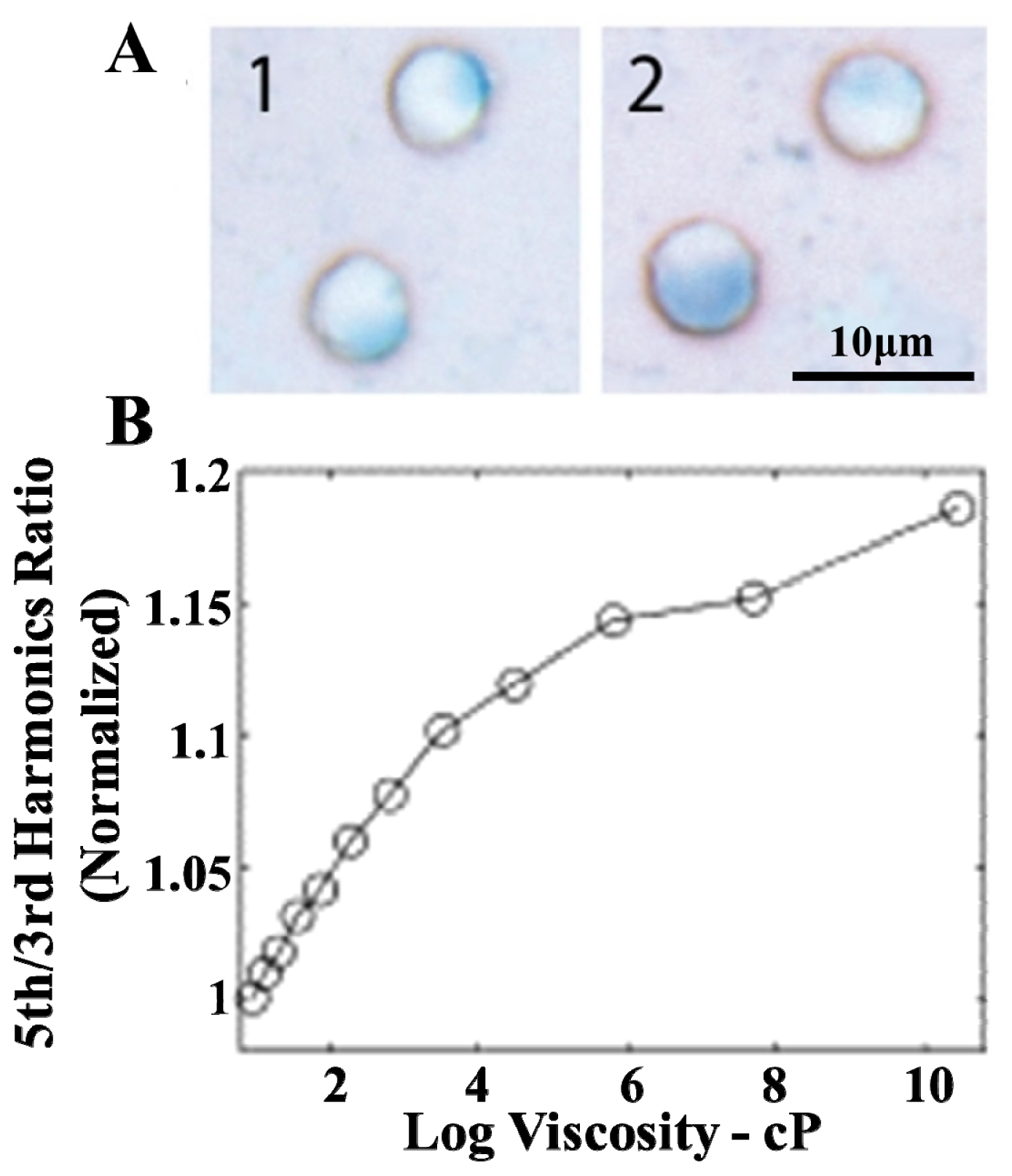
3.1. Magnetization Curve of MPI Tracer Materials
3.2. MPI Imaging Quality and Nanoparticle Properties
4. Design of Nanoparticles for Medical Applications of MPI
4.1. Cardiovascular System
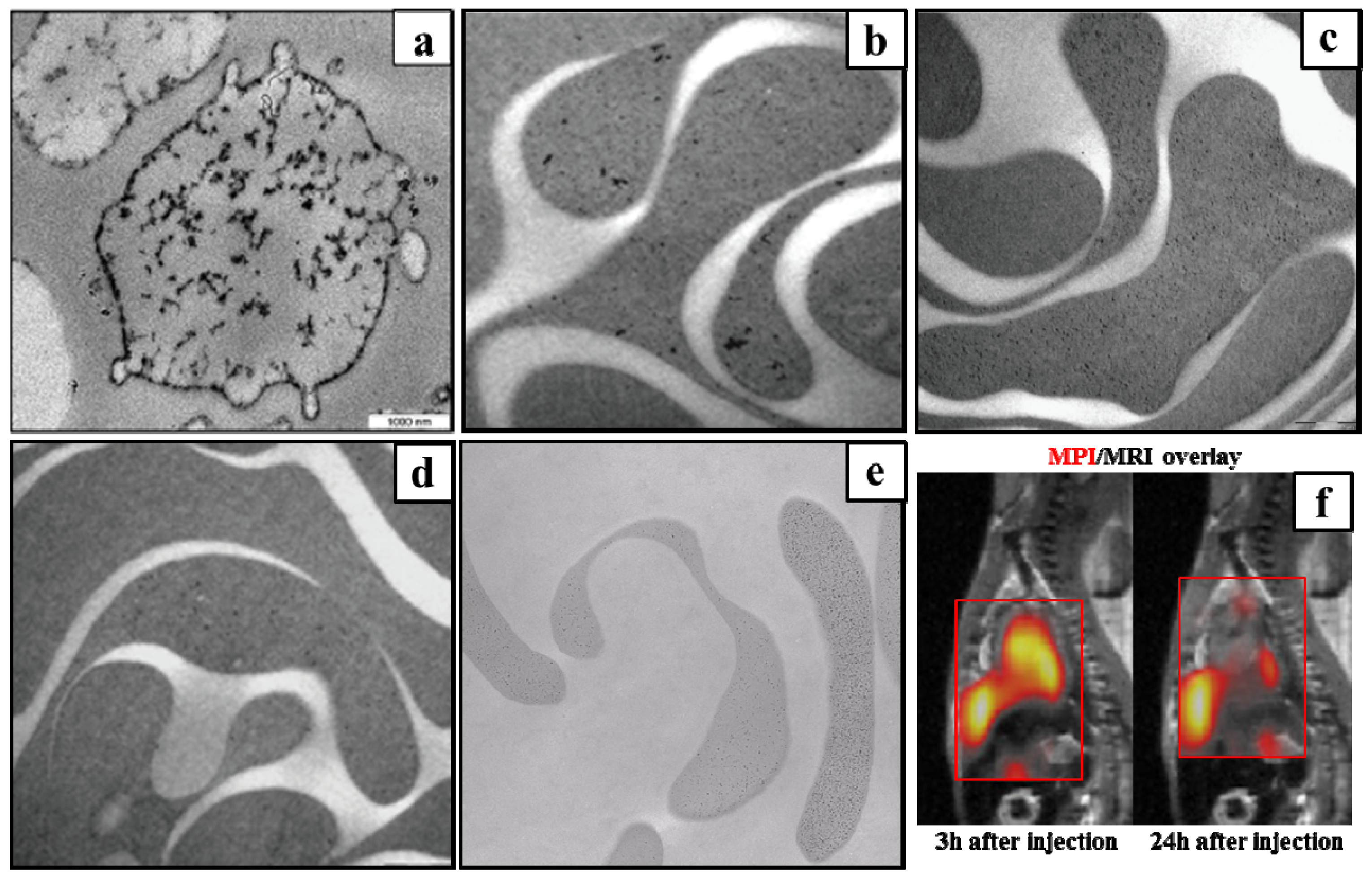
4.1.1. Safe Angiography
4.1.2. Red Blood Cell Labeling
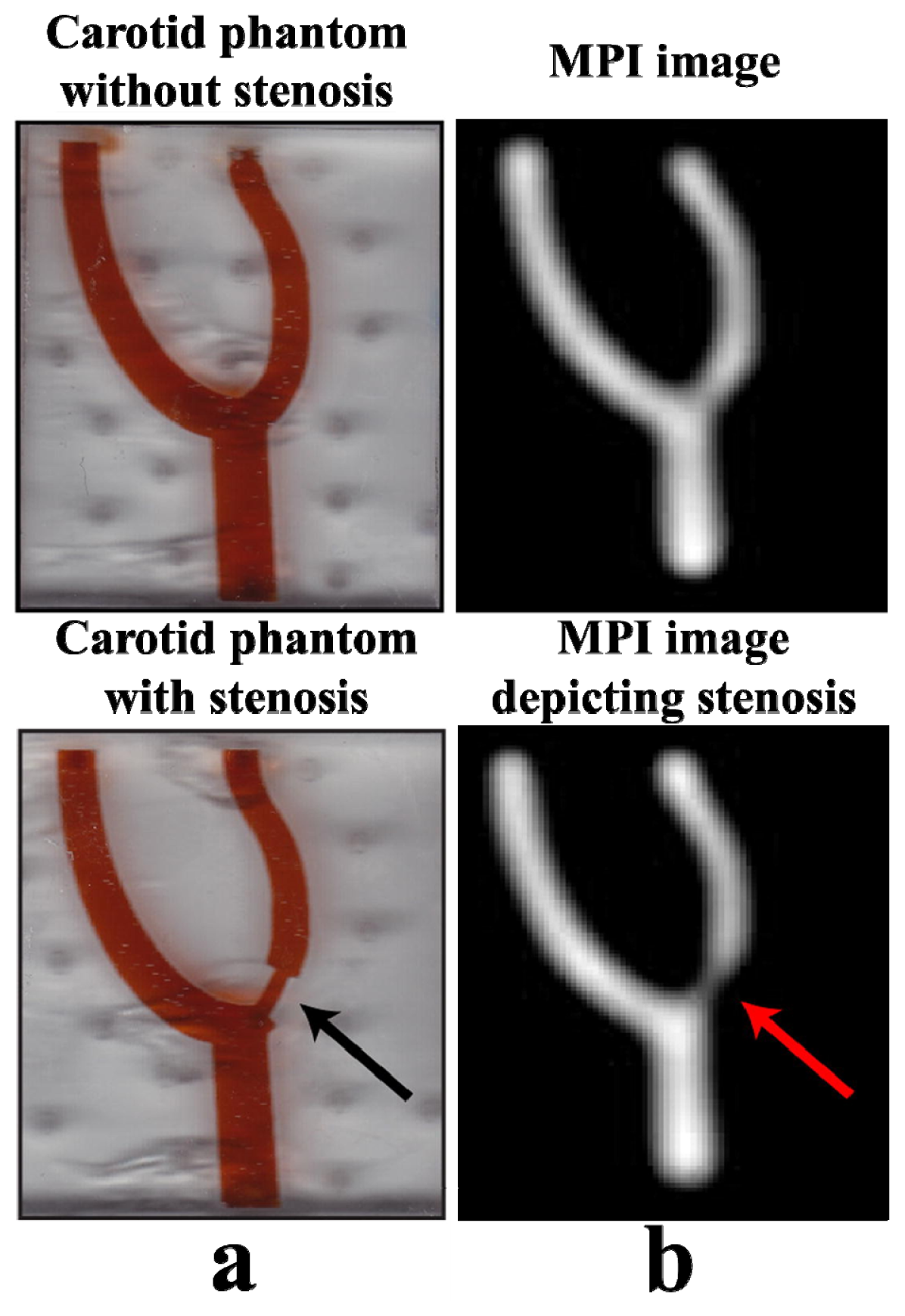
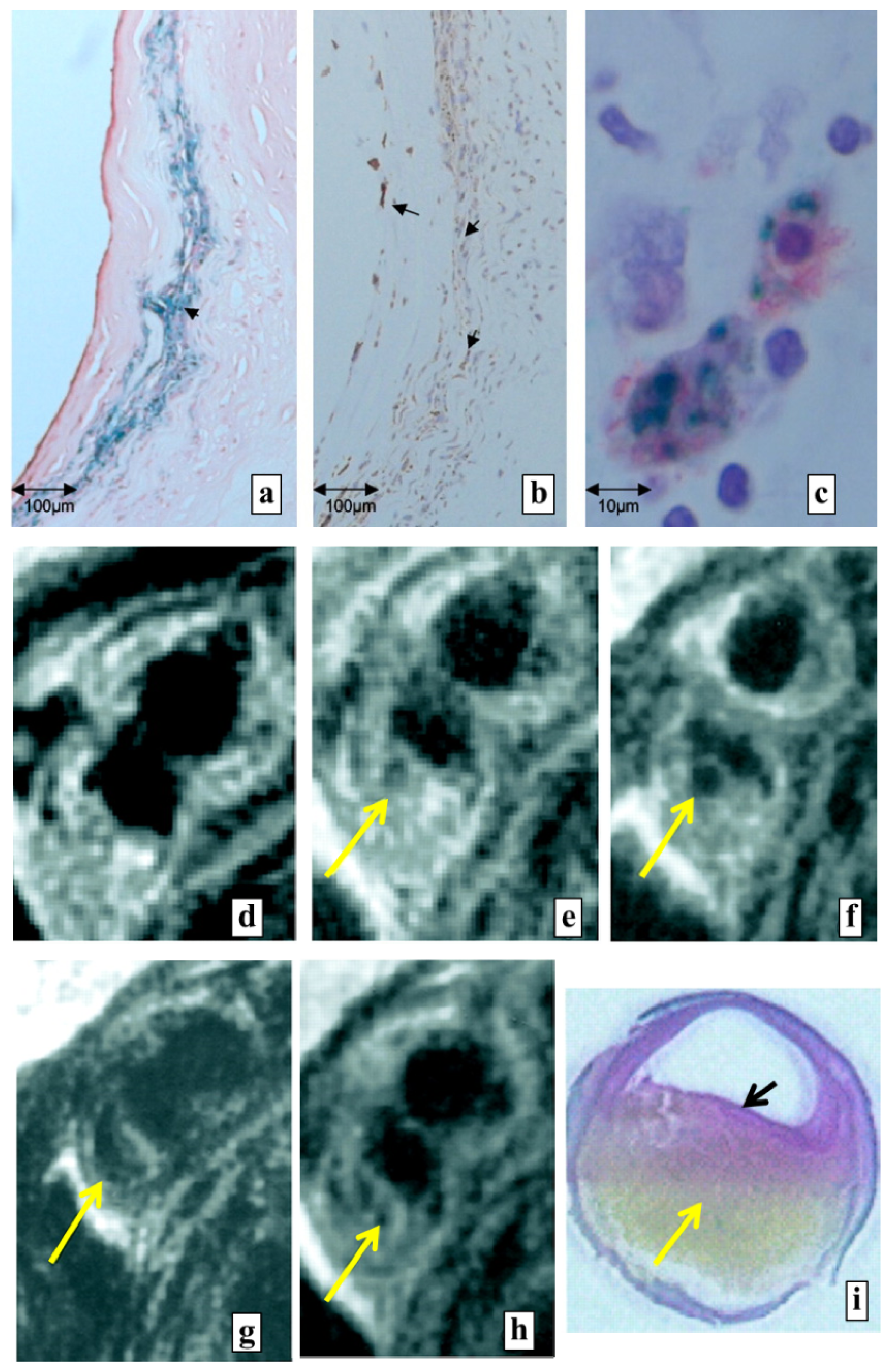
4.1.3. Atherosclerotic Plaque
4.2. Oncology
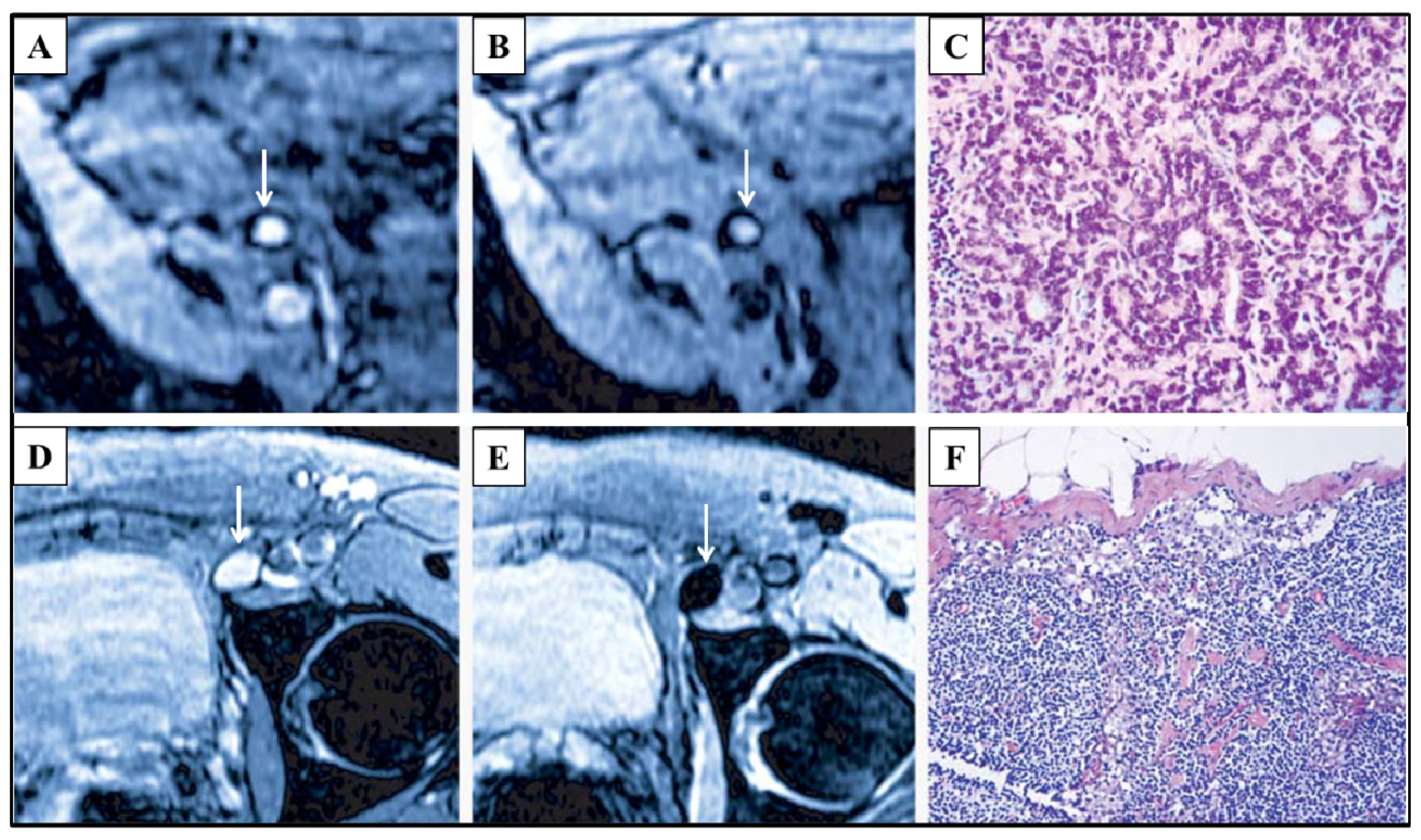
4.2.1. Diagnostic Imaging
4.2.2. Therapeutic Imaging
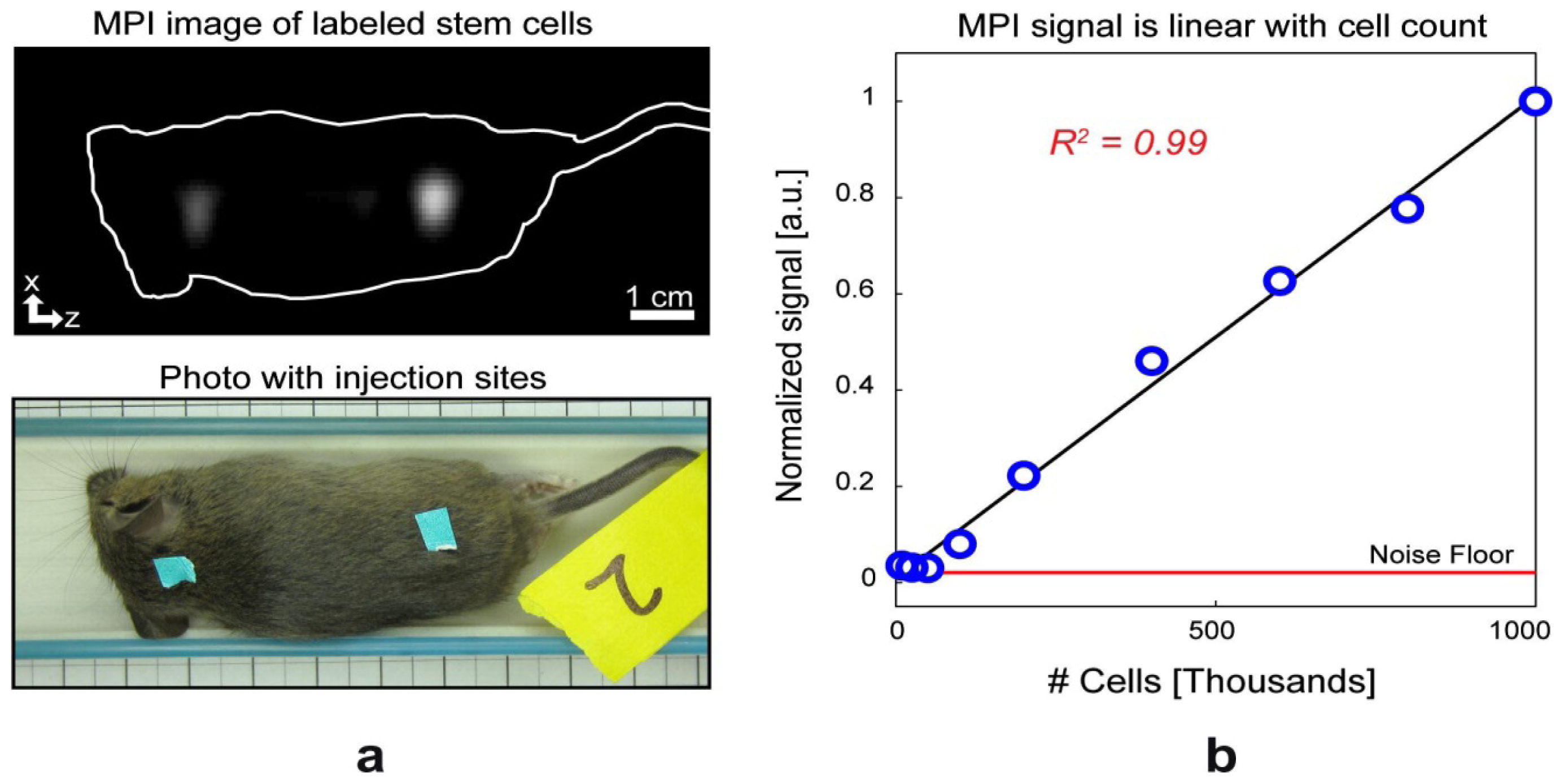
4.3. Stem Cell Tracking
4.4. Immune Related Imaging
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gleich, B.; Weizenecker, J. Tomographic imaging using the nonlinear response of magnetic particles. Nature 2005, 435, 1214–1217. [Google Scholar]
- Knopp, T.; Buzug, T.M. Introduction. In Magnetic Particle Imaging: An Introduction to Imaging Principles and Scanner Instrumentation; Springer Berlin Heidelberg: Berlin, Germany, 2012; pp. 1–9. [Google Scholar]
- Ferguson, R.M.; Minard, K.R.; Krishnan, K.M. Optimization of nanoparticle core size for magnetic particle imaging. J. Magn. Magn. Mater 2009, 321, 1548–1551. [Google Scholar]
- Krishnan, K.M.; Pakhomov, A.B.; Bao, Y.; Blomqvist, P.; Chun, Y.; Gonzales, M.; Griffin, K.; Ji, X.; Roberts, B.K. Nanomagnetism and spin electronics: Materials, microstructure and novel properties. J. Mater. Sci 2006, 41, 793–815. [Google Scholar]
- Bedanta, S.; Kleemann, W. Supermagnetism. J. Phys. D: Appl. Phys 2009, 42, 013001, :1– 013001:28.. [Google Scholar]
- Herrera, A.P.; Barrera, C.; Zayas, Y.; Rinaldi, C. Monitoring colloidal stability of polymer-coated magnetic nanoparticles using AC susceptibility measurements. J. Colloid. Interf. Sci 2010, 342, 540–549. [Google Scholar]
- Capadona, J.R.; Shanmuganathan, K.; Tyler, D.J.; Rowan, S.J.; Weder, C. Stimuli-responsive polymer nanocomposites inspired by the sea cucumber dermis. Science 2008, 319, 1370–1374. [Google Scholar]
- Zhang, J.; Misra, R.D.K. Magnetic drug-targeting carrier encapsulated with thermosensitive smart polymer: Core–shell nanoparticle carrier and drug release response. Acta Biomater 2007, 3, 838–850. [Google Scholar]
- Zhang, J.L.; Srivastava, R.S.; Misra, R.D.K. Core–shell magnetite nanoparticles surface encapsulated with smart stimuli-responsive polymer: Synthesis, characterization, and LCST of viable drug-targeting delivery system. Langmuir 2007, 23, 6342–6351. [Google Scholar]
- Bronstein, L.M.; Huang, X.; Retrum, J.; Schmucker, A.; Pink, M.; Stein, B.D.; Dragnea, B. Influence of iron oleate complex structure on iron oxide nanoparticle formation. Chem. Mater 2007, 19, 3624–3632. [Google Scholar]
- Briley-Saebo, K.; Bjørnerud, A.; Grant, D.; Ahlstrom, H.; Berg, T.; Kindberg, G.M. Hepatic cellular distribution and degradation of iron oxide nanoparticles following single intravenous injection in rats: Implications for magnetic resonance imaging. Cell Tissue Res 2004, 316, 315–323. [Google Scholar]
- Clement, J.H.; Schwalbe, M.; Buske, N.; Wagner, K.; Schnabelrauch, M.; Gornert, P.; Kliche, K.O.; Pachmann, K.; Weitschies, W.; Hoffken, K. Differential interaction of magnetic nanoparticles with tumor cells and peripheral blood cells. J. Cancer. Res. Clin 2006, 132, 287–292. [Google Scholar]
- Khandhar, A.P.; Ferguson, R.M.; Simon, J.A.; Krishnan, K.M. Tailored magnetic nanoparticles for optimizing magnetic fluid hyperthermia. J. Biomed. Mater. Res. A 2012, 100A, 728–737. [Google Scholar]
- Alexiou, C.; Jurgons, R.; Seliger, C.; Brunke, O.; Iro, H.; Odenbach, S. Delivery of superparamagnetic nanoparticles for local chemotherapy after intraarterial infusion and magnetic drug targeting. Anticancer Res 2007, 27, 2019–2022. [Google Scholar]
- Reimer, P.; Tombach, B. Hepatic MRI with SPIO: Detection and characterization of focal liver lesions. Eur. Radiol 1998, 8, 1198–1204. [Google Scholar]
- Grubnic, S.; Padhani, A.R.; Revell, P.B.; Husband, J.E. Comparative efficacy of and sequence choice for two oral contrast agents used during MR imaging. Am. J. Roentgenol 1999, 173, 173–178. [Google Scholar]
- Mitchell, D.G. MR imaging contrast agents—What’s in a name? J. Magn. Reson. Imaging 1997, 7, 1–4. [Google Scholar]
- Saritas, E.U.; Goodwill, P.W.; Croft, L.R.; Konkle, J.J.; Lu, K.; Zheng, B.; Conolly, S.M. Magnetic particle imaging (MPI) for NMR and MRI researchers. J. Magn. Reson 2013, 229, 116–126. [Google Scholar]
- Borgert, J.; Schmidt, J.D.; Schmale, I.; Rahmer, J.; Bontus, C.; Gleich, B.; David, B.; Eckart, R.; Woywode, O.; Weizenecker, J.; et al. Fundamentals and applications of magnetic particle imaging. J. Cardiovasc. Comput. Tomogr 2012, 6, 149–153. [Google Scholar]
- Weizenecker, J.; Borgert, J.; Gleich, B. A simulation study on the resolution and sensitivity of magnetic particle imaging. Phys. Med. Biol 2007, 52, 6363–6374. [Google Scholar]
- Murase, K.; Konishi, T.; Takeuchi, Y.; Takata, H.; Saito, S. Experimental and simulation studies on the behavior of signal harmonics in magnetic particle imaging. Radiol. Phys. Technol. 2013, 1–16. [Google Scholar]
- Rahmer, J.; Weizenecker, J.; Gleich, B.; Borgert, J. Signal encoding in magnetic particle imaging: Properties of the system function. BMC Med. Imaging 2009, 9, 4, :1–4:21.. [Google Scholar]
- Goodwill, P.W.; Saritas, E.U.; Croft, L.R.; Kim, T.N.; Krishnan, K.M.; Schaffer, D.V.; Conolly, S.M. X-space MPI: Magnetic nanoparticles for safe medical imaging. Adv. Mater 2012, 24, 3870–3877. [Google Scholar]
- Khandhar, A.P.; Ferguson, R.M.; Arami, H.; Krishnan, K.M. Monodisperse magnetite nanoparticle tracers for in vivo magnetic particle imaging. Biomaterials 2013, 34, 3837–3845. [Google Scholar]
- Knopp, T.; Buzug, T.M. How Magnetic Particle Imaging Works. In Magnetic Particle Imaging: An Introduction to Imaging Principles and Scanner Instrumentation; Springer Berlin Heidelberg: Berlin, Germany, 2012; pp. 11–70. [Google Scholar]
- Krishnan, K.M. Biomedical nanomagnetics: A spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans. Magn 2010, 46, 2523–2558. [Google Scholar]
- Knopp, T.; Biederer, S.; Sattel, T.F.; Erbe, M.; Buzug, T.M. Prediction of the spatial resolution of magnetic particle imaging using the modulation transfer function of the imaging process. IEEE Trans. Med. Imaging 2011, 30, 1284–1292. [Google Scholar]
- Goodwill, P.W.; Tamrazian, A.; Croft, L.R.; Lu, C.D.; Johnson, E.M.; Pidaparthi, R.; Ferguson, R.M.; Khandhar, A.P.; Krishnan, K.M.; Conolly, S.M. Ferrohydrodynamic relaxometry for magnetic particle imaging. Appl. Phys. Lett 2011, 98, 262502, :1–262502:3.. [Google Scholar]
- Ferguson, R.M.; Khandhar, A.P.; Krishnan, K.M. Tracer design for magnetic particle imaging (invited). J. Appl. Phys 2012, 111, 07, B318:1–07B318:5.. [Google Scholar]
- Ferguson, R.M.; Minard, K.R.; Khandhar, A.P.; Krishnan, K.M. Optimizing magnetite nanoparticles for mass sensitivity in magnetic particle imaging. Med. Phys 2011, 38, 1619–1626. [Google Scholar]
- Herrera, A.P.; Polo-Corrales, L.; Chavez, E.; Cabarcas-Bolivar, J.; Uwakweh, O.N.; Rinaldi, C. Influence of aging time of oleate precursor on the magnetic relaxation of cobalt ferrite nanoparticles synthesized by the thermal decomposition method. J. Magn. Magn. Mater 2013, 328, 41–52. [Google Scholar]
- Shliomis, M.I. Magnetic fluids. Sov. Phys. Uspekhi 1974, 17, 153–169. [Google Scholar]
- Yokoyama, M.; Ohta, E.; Sato, T. Magnetic properties of ultrafine particles and bulk material of cadmium ferrite. J. Magn. Magn. Mater 1998, 183, 173–180. [Google Scholar]
- Chantrell, R.W.; Popplewell, J.; Charles, S.W. Measurements of particle size distribution parameters in ferrofluids. IEEE Trans. Magn 1978, 14, 975–977. [Google Scholar]
- Kiss, L.B.; Söderlund, J.; Niklasson, G.A.; Granqvist, C.G. New approach to the origin of lognormal size distributions of nanoparticles. Nanotechnology 1999, 10, 25–28. [Google Scholar]
- Goodwill, P.W.; Conolly, S.M. The X-space formulation of the magnetic particle imaging process: 1-D signal, resolution, bandwidth, SNR, SAR, and magnetostimulation. IEEE Trans. Med. Imaging 2010, 29, 1851–1859. [Google Scholar]
- Fannin, P.C. Investigating magnetic fluids by means of complex susceptibility measurements. J. Magn. Magn. Mater. 2003, 258–259, 446–451. [Google Scholar]
- Starmans, L.W.E.; Burdinski, D.; Haex, N.P.M.; Moonen, R.P.M.; Strijkers, G.J.; Nicolay, K.; Grüll, H. Iron oxide nanoparticle-micelles (ION-micelles) for sensitive (molecular) magnetic particle imaging and magnetic resonance imaging. PLoS One 2013, 8, e57335, :1–e57335:9.. [Google Scholar]
- Weizenecker, J.; Gleich, B.; Rahmer, J.; Dahnke, H.; Borgert, J. Three-dimensional real-time in vivo magnetic particle imaging. Phys. Med. Biol 2009, 54, L1–L10. [Google Scholar]
- McCullough, P.A. Contrast-induced acute kidney injury. J. Am. Coll. Cardiol 2008, 51, 1419–1428. [Google Scholar]
- Kuo, P.H.; Kanal, E.; Abu-Alfa, A.K.; Cowper, S.E. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology 2007, 242, 647–649. [Google Scholar]
- Katzberg, R.W.; Haller, C. Contrast-induced nephrotoxicity: clinical landscape. Kidney Int 2006, 69, S3–S7. [Google Scholar]
- Ix, J.H.; Mercado, N.; Shlipak, M.G.; Lemos, P.A.; Boersma, E.; Lindeboom, W.; O’Neill, W.W.; Wijns, W.; Serruys, P.W. Association of chronic kidney disease with clinical outcomes after coronary revascularization: The arterial revascularization therapies study (ARTS). Am. Heart J 2005, 149, 512–519. [Google Scholar]
- Bettmann, M.A. Frequently asked questions: Iodinated contrast agents. Radiographics 2004, 24, S3–S10. [Google Scholar]
- Gleich, B.; Weizenecker, J.; Borgert, J. Experimental results on fast 2D-encoded magnetic particle imaging. Phys. Med. Biol 2008, 53, N81–N84. [Google Scholar]
- Laconte, L.; Nitin, N.; Bao, G. Magnetic nanoparticle probes. Mater. Today 2005, 8, 32–38. [Google Scholar]
- Neuwelt, E.A.; Hamilton, B.E.; Varallyay, C.G.; Rooney, W.R.; Edelman, R.D.; Jacobs, P.M.; Watnick, S.G. Ultrasmall superparamagnetic iron oxides (USPIOs): A future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int 2009, 75, 465–474. [Google Scholar]
- Weissleder, R.; Stark, D.D.; Engelstad, B.L.; Bacon, B.R.; Compton, C.C.; White, D.L.; Jacobs, P.; Lewis, J. Superparamagnetic iron oxide: Pharmacokinetics and toxicity. Am. J. Roentgenol 1989, 152, 167–173. [Google Scholar]
- Reimer, P.; Balzer, T. Ferucarbotran (resovist): A new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: Properties, clinical development, and applications. Eur. Radiol 2003, 13, 1266–1276. [Google Scholar]
- Lu, M.; Cohen, M.H.; Rieves, D.; Pazdur, R. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am. J. Hematol 2010, 85, 315–319. [Google Scholar]
- Maddux, J.; Chen, S.; Groves, B.; Messenger, J.; Wink, O.; Carroll, J. Rotational angiography and 3D coronary modeling: Revolutions in the cardiac cath lab. MedicaMundi 2003, 47, 8–14. [Google Scholar]
- Almeida, J.P.M.; Chen, A.L.; Foster, A.; Drezek, R. In vivo biodistribution of nanoparticles. Nanomedicine 2011, 6, 815–835. [Google Scholar]
- Antonelli, A.; Sfara, C.; Manuali, E.; Bruce, I.J.; Magnani, M. Encapsulation of superparamagnetic nanoparticles into red blood cells as new carriers of MRI contrast agents. Nanomedicine 2011, 6, 211–223. [Google Scholar]
- Berry, C.C.; Curtis, A.S.G. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys 2003, 36, R198–R206. [Google Scholar]
- Fujita, T.; Nishikawa, M.; Ohtsubo, Y.; Ohno, J.; Takakura, Y.; Sezaki, H.; Hashida, M. Control of in vivo fate of albumin derivatives utilizing combined chemical modification. J. Drug Target 1994, 2, 157–165. [Google Scholar]
- Papisov, M.; Bogdanov, A., Jr; Schaffer, B.; Nossiff, N.; Shen, T.; Weissleder, R.; Brady, T. Colloidal magnetic resonance contrast agents: Effect of particle surface on biodistribution. J. Magn. Magn. Mater. 1993, 122, 383–386. [Google Scholar]
- Chouly, C.; Pouliquen, D.; Lucet, I.; Jeune, J.; Jallet, P. Development of superparamagnetic nanoparticles for MRI: Effect of particle size, charge and surface nature on biodistribution. J. Microencapsul 1996, 13, 245–255. [Google Scholar]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug. Deliver. Rev 2008, 60, 1252–1265. [Google Scholar]
- Decuzzi, P.; Ferrari, M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials 2006, 27, 5307–5314. [Google Scholar]
- Storm, G.; Belliot, S.O.; Daemen, T.; Lasic, D.D. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv. Drug. Deliver. Rev 1995, 17, 31–48. [Google Scholar]
- Chen, L.T.; Weiss, L. The role of the sinus wall in the passage of erythrocytes through the spleen. Blood 1973, 41, 529–537. [Google Scholar]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol 2007, 25, 1165–1170. [Google Scholar]
- Sarin, H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J. Angiogenes. Res 2010, 2, 14, :1–14:19.. [Google Scholar]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng 2012, 14, 1–16. [Google Scholar]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm 2008, 5, 505–515. [Google Scholar]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar]
- Rahmer, J.; Antonelli, A.; Sfara, C.; Tiemann, B.; Gleich, B.; Magnani, M.; Weizenecker, J.; Borgert, J. Nanoparticle encapsulation in red blood cells enables blood-pool magnetic particle imaging hours after injection. Phys. Med. Biol 2013, 58, 3965–3977. [Google Scholar]
- Markov, D.E.; Boeve, H.; Gleich, B.; Borgert, J.; Antonelli, A.; Sfara, C.; Magnani, M. Human erythrocytes as nanoparticle carriers for magnetic particle imaging. Phys. Med. Biol 2010, 55, 6461–6473. [Google Scholar]
- Magnani, M.; Rossi, L.; D’Ascenzo, M.; Panzani, I.; Bigi, L.; Zanella, A. Erythrocyte engineering for drug delivery and targeting. Biotechnol. Appl. Bioc 1998, 28, 1–6. [Google Scholar]
- Antonelli, A.; Sfara, C.; Magnani, M.; Rahmer, J.; Gleich, B.; Borgert, J.; Weizenecker, J. Red Blood Cells as Magnetic Carriers for MPI Applications. In Magnetic Particle Imaging; Buzug, T.M., Borgert, J., Eds.; Springer: London, UK, 2012; Volume 140, pp. 175–179. [Google Scholar]
- Zink, S.I.; Ohki, S.K.; Stein, B.; Zambuto, D.A.; Rosenberg, R.J.; Choi, J.J.; Tubbs, D.S. Noninvasive evaluation of active lower gastrointestinal bleeding: Comparison between contrast-enhanced MDCT and 99mTc-labeled RBC scintigraphy. Am. J. Roentgenol 2008, 191, 1107–1114. [Google Scholar]
- Howarth, D.M. The role of nuclear medicine in the detection of acute gastrointestinal bleeding. Semin. Nucl. Med 2006, 36, 133–146. [Google Scholar]
- Zheng, J.G.; Yao, Z.M.; Shu, C.Y.; Zhang, Y.; Zhang, X. Role of SPECT/CT in diagnosis of hepatic hemangiomas. World J. Gastroentero 2005, 11, 5336–5341. [Google Scholar]
- Brähler, M.; Georgieva, R.; Buske, N.; Müller, A.; Müller, S.; Pinkernelle, J.; Teichgräber, U.; Voigt, A.; Bäumler, H. Magnetite-loaded carrier erythrocytes as contrast agents for magnetic resonance imaging. Nano Lett 2006, 6, 2505–2509. [Google Scholar]
- Antonelli, A.; Sfara, C.; Manuali, E.; Salamida, S.; Louin, G.; Magnani, M. SPIE Medical Imaging. In Magnetic Red Blood Cells as New Contrast Agents for MRI Applications; International Society for Optics and Photonics: Washington, USA, 2013; pp. 86721D:1–86721D:8. [Google Scholar]
- Fayad, Z.A.; Fuster, V. Clinical imaging of the high-risk or vulnerable atherosclerotic plaque. Circ. Res 2001, 89, 305–316. [Google Scholar]
- Yang, J.; Yang, H.; Cao, L.; Li, S. MR and targeted molecular MRI of vulnerable plaques. Intervent. Neurol 2012, 1, 124–131. [Google Scholar]
- Klink, A.; Lancelot, E.; Ballet, S.; Vucic, E.; Fabre, J.E.; Gonzalez, W.; Medina, C.; Corot, C.; Mulder, W.J.M.; Mallat, Z.; et al. Magnetic resonance molecular imaging of thrombosis in an arachidonic acid mouse model using an activated platelet targeted probe. Arterioscl. Throm. Vas 2010, 30, 403–410. [Google Scholar]
- Lancelot, E.; Amirbekian, V.; Brigger, I.; Raynaud, J.S.; Ballet, S.; David, C.; Rousseaux, O.; Le Greneur, S.; Port, M.; Lijnen, H.R.; et al. Evaluation of matrix metalloproteinases in atherosclerosis using a novel noninvasive imaging approach. Arterioscl. Throm. Vas 2008, 28, 425–432. [Google Scholar]
- Saam, T.; Cai, J.; Ma, L.; Cai, Y.Q.; Ferguson, M.S.; Polissar, N.L.; Hatsukami, T.S.; Yuan, C. Comparison of symptomatic and asymptomatic atherosclerotic carotid plaque features with in vivo MR imaging. Radiology 2006, 240, 464–472. [Google Scholar]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol 2011, 12, 204–212. [Google Scholar]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol 2006, 6, 508–519. [Google Scholar]
- Epstein, F.H.; Ross, R. Atherosclerosis—An Inflammatory disease. N. Engl. J. Med 1999, 340, 115–126. [Google Scholar]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part II. Circulation 2003, 108, 1772–1778. [Google Scholar]
- Faxon, D.P.; Fuster, V.; Libby, P.; Beckman, J.A.; Hiatt, W.R.; Thompson, R.W.; Topper, J.N.; Annex, B.H.; Rundback, J.H.; Fabunmi, R.P.; et al. Atherosclerotic vascular disease conference: Writing group III: Pathophysiology. Circulation 2004, 109, 2617–2625. [Google Scholar]
- Nighoghossian, N.; Derex, L.; Douek, P. The vulnerable carotid artery plaque: Current imaging methods and new perspectives. Stroke 2005, 36, 2764–2772. [Google Scholar]
- Tang, T.Y.; Howarth, S.P.S.; Miller, S.R.; Graves, M.J.; Jean-Marie, U.K.I.; Trivedi, R.A.; Li, Z.Y.; Walsh, S.R.; Brown, A.P.; Kirkpatrick, P.J.; et al. Comparison of the inflammatory burden of truly asymptomatic carotid atheroma with atherosclerotic plaques contralateral to symptomatic carotid stenosis: An ultra small superparamagnetic iron oxide enhanced magnetic resonance study. J. Neurol. Neurosur. Ps 2007, 78, 1337–1343. [Google Scholar]
- Trivedi, R.A.; Mallawarachi, C.; Jean-Marie, U.K.I.; Graves, M.J.; Horsley, J.; Goddard, M.J.; Brown, A.; Wang, L.; Kirkpatrick, P.J.; Brown, J.; et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscl. Throm. Vas 2006, 26, 1601–1606. [Google Scholar]
- Trivedi, R.A.; Jean-Marie, U.K.I.; Graves, M.J.; Cross, J.J.; Horsley, J.; Goddard, M.J.; Skepper, J.N.; Quartey, G.; Warburton, E.; Joubert, I.; et al. In vivo detection of macrophages in human carotid atheroma: Temporal dependence of ultrasmall superparamagnetic particles of iron oxide-enhanced MRI. Stroke 2004, 35, 1631–1635. [Google Scholar]
- Rogers, W.J.; Basu, P. Factors regulating macrophage endocytosis of nanoparticles: Implications for targeted magnetic resonance plaque imaging. Atherosclerosis 2005, 178, 67–73. [Google Scholar]
- Litovsky, S.; Madjid, M.; Zarrabi, A.; Casscells, S.W.; Willerson, J.T.; Naghavi, M. Superparamagnetic iron oxide-based method for quantifying recruitment of monocytes to mouse atherosclerotic lesions in vivo: Enhancement by tissue necrosis factor-alpha, interleukin-1beta, and interferon-gamma. Circulation 2003, 107, 1545–1549. [Google Scholar]
- Ruehm, S.G.; Corot, C.; Vogt, P.; Kolb, S.; Debatin, J.F. Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation 2001, 103, 415–422. [Google Scholar]
- Kooi, M.E.; Cappendijk, V.C.; Cleutjens, K.B.J.M.; Kessels, A.G.H.; Kitslaar, P.J.E.H.M.; Borgers, M.; Frederik, P.M.; Daemen, M.J.A.P.; van Engelshoven, J.M.A. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation 2003, 107, 2453–2458. [Google Scholar]
- Metz, S.; Beer, A.J.; Settles, M.; Pelisek, J.; Botnar, R.M.; Rummeny, E.J.; Heider, P. Characterization of carotid artery plaques with USPIO-enhanced MRI: Assessment of inflammation and vascularity as in vivo imaging biomarkers for plaque vulnerability. Int. J. Cardiovasc. Imaging 2011, 27, 901–912. [Google Scholar]
- Jaffer, F.A.; Libby, P.; Weissleder, R. Molecular and cellular imaging of atherosclerosis: emerging applications. J. Am. Coll. Cardiol 2006, 47, 1328–1338. [Google Scholar]
- Nagaoka, K.; Takahara, K.; Tanaka, K.; Yoshida, H.; Steinman, R.M.; Saitoh, S.I.; Akashi-Takamura, S.; Miyake, K.; Kang, Y.S.; Park, C.G.; et al. Association of SIGNR1 with TLR4–MD-2 enhances signal transduction by recognition of LPS in Gram-negative Bacteria. Int. Immunol 2005, 17, 827–836. [Google Scholar]
- Iida, H.; Eberl, S. Quantitative assessment of regional myocardial blood flow with thallium-201 and SPECT. J. Nucl. Cardiol 1998, 5, 313–331. [Google Scholar]
- Di Carli, M.F.; Dorbala, S.; Meserve, J.; El Fakhri, G.; Sitek, A.; Moore, S.C. Clinical myocardial perfusion PET/CT. J. Nucl. Med 2007, 48, 783–793. [Google Scholar]
- Rauwerdink, A.M.; Giustini, A.J.; Weaver, J.B. Simultaneous quantification of multiple magnetic nanoparticles. Nanotechnology 2010, 21, 455101, :1–455101:5.. [Google Scholar]
- Wojtczyk, H.; Haegele, J.; Grüttner, M.; Tenner, W.; Bringout, G.; Graeser, M.; Vogt, F.M.; Barkhausen, J.; Buzug, T.M. Visualization of Instruments in interventional Magnetic Particle Imaging (iMPI): A Simulation Study on SPIO Labelings. In Magnetic Particle Imaging; Buzug, T.M., Borgert, J., Eds.; Springer: London, UK, 2012; Volume 140, pp. 167–172. [Google Scholar]
- Haegele, J.; Rahmer, J.; Gleich, B.; Bontus, C.; Borgert, J.; Wojtczyk, H.; Buzug, T.M.; Barkhausen, J.; Vogt, F.M. Visualization of Instruments for Cardiovascular Intervention Using MPI. In Magnetic Particle Imaging; Buzug, T.M., Borgert, J., Eds.; Springer: London, UK, 2012; Volume 140, pp. 211–215. [Google Scholar]
- Haegele, J.; Biederer, S.; Wojtczyk, H.; Gräser, M.; Knopp, T.; Buzug, T.M.; Barkhausen, J.; Vogt, F.M. Toward cardiovascular interventions guided by magnetic particle imaging: First instrument characterization. Magnet. Reson. Med 2013, 69, 1761–1767. [Google Scholar]
- Saxena, V.; Gonzalez-Gomez, I.; Laug, W.E. A noninvasive multimodal technique to monitor brain tumor vascularization. Phys. Med. Biol 2007, 52, 5295–5308. [Google Scholar]
- Mazaheri, Y.; Shukla-Dave, A.; Muellner, A.; Hricak, H. MRI of the prostate: Clinical relevance and emerging applications. J. Magn. Reson. Imaging 2011, 33, 258–274. [Google Scholar]
- Kobayashi, H.; Kawamoto, S.; Bernardo, M.; Brechbiel, M.W.; Knopp, M.V.; Choyke, P.L. Delivery of gadolinium-labeled nanoparticles to the sentinel lymph node: Comparison of the sentinel node visualization and estimations of intra-nodal gadolinium concentration by the magnetic resonance imaging. J. Control. Release 2006, 111, 343–351. [Google Scholar]
- Dowlatshahi, K.; Fan, M.; Snider, H.C.; Habib, F.A. Lymph node micrometastases from breast carcinoma. Cancer 1997, 80, 1188–1197. [Google Scholar]
- Fisher, B.; Bauer, M.; Wickerham, D.L.; Redmond, C.K.; Fisher, E.R.; Cruz, A.B.; Foster, R.; Gardner, B.; Lerner, H.; Margolese, R.; et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer: An NSABP update. Cancer 1983, 52, 1551–1557. [Google Scholar]
- Goldhirsch, A.; Glick, J.H.; Gelber, R.D.; Coates, A.S.; Senn, H.J. Meeting highlights: International consensus panel on the treatment of primary breast cancer. J. Clin. Oncol 2001, 19, 3817–3827. [Google Scholar]
- Luciani, A.; Itti, E.; Rahmouni, A.; Meignan, M.; Clement, O. Lymph node imaging: Basic principles. Eur. J. Radiol 2006, 58, 338–344. [Google Scholar]
- Martínez-Palones, J.M.; Gil-Moreno, A.; Pérez-Benavente, M.A.; Roca, I.; Xercavins, J. Intraoperative sentinel node identification in early stage cervical cancer using a combination of radiolabeled albumin injection and isosulfan blue dye injection. Gynecol. Oncol 2004, 92, 845–850. [Google Scholar]
- Cabanas, R.M. Anatomy and biopsy of sentinel lymph nodes. Urol. Clin. North Am 1992, 19, 267–276. [Google Scholar]
- Jain, R.; Dandekar, P.; Patravale, V. Diagnostic nanocarriers for sentinel lymph node imaging. J. Control. Release 2009, 138, 90–102. [Google Scholar]
- Corot, C.; Robert, P.; Idee, J.M.; Port, M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev 2006, 58, 1471–1504. [Google Scholar]
- Harisinghani, M.G.; Barentsz, J.; Hahn, P.F.; Deserno, W.M.; Tabatabaei, S.; van de Kaa, C.H.; de la Rosette, J.; Weissleder, R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N. Engl.J. Med 2003, 348, 2491–2499. [Google Scholar]
- Ross, R.W.; Zietman, A.L.; Xie, W.; Coen, J.J.; Dahl, D.M.; Shipley, W.U.; Kaufman, D.S.; Islam, T.; Guimaraes, A.R.; Weissleder, R.; et al. Lymphotropic nanoparticle-enhanced magnetic resonance imaging (LNMRI) identifies occult lymph node metastases in prostate cancer patients prior to salvage radiation therapy. Clin. Imaging 2009, 33, 301–305. [Google Scholar]
- Guimaraes, A.R.; Tabatabei, S.; Dahl, D.; McDougal, W.S.; Weissleder, R.; Harisinghani, M.G. Pilot study evaluating use of lymphotrophic nanoparticle-enhanced magnetic resonance imaging for assessing lymph nodes in renal cell cancer. Urology 2008, 71, 708–712. [Google Scholar]
- Heesakkers, R.A.M.; Jager, G.J.; Hövels, A.M.; de Hoop, B.; van den Bosch, H.C.M.; Raat, F.; Witjes, J.A.; Mulders, P.F.A.; van der Kaa, C.H.; Barentsz, J.O. Prostate cancer: Detection of lymph node metastases outside the routine surgical area with ferumoxtran-10–enhanced MR imaging. Radiology 2009, 251, 408–414. [Google Scholar]
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys 2009, 42, 224001, :1–224001:15.. [Google Scholar]
- Lee, J.H.; Jang, J.T.; Choi, J.S.; Moon, S.H.; Noh, S.H.; Kim, J.W.; Kim, J.G.; Kim, I.S.; Park, K.I.; Cheon, J. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotechnol 2011, 6, 418–422. [Google Scholar]
- Carrey, J.; Mehdaoui, B.; Respaud, M. Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: Application to magnetic hyperthermia optimization. J. Appl. Phys 2011, 109, 083921, :1–083921:17.. [Google Scholar]
- Balivada, S.; Rachakatla, R.S.; Wang, H.; Samarakoon, T.N.; Dani, R.K.; Pyle, M.; Kroh, F.O.; Walker, B.; Leaym, X.; Koper, O.B.; et al. A/C magnetic hyperthermia of melanoma mediated by iron(0)/iron oxide core/shell magnetic nanoparticles: A mouse study. BMC Cancer 2010, 10, 119, :1–119:9.. [Google Scholar]
- Johannsen, M.; Gneveckow, U.; Thiesen, B.; Taymoorian, K.; Cho, C.H.; Waldofner, N.; Scholz, R.; Jordan, A.; Loening, S.A.; Wust, P. Thermotherapy of prostate cancer using magnetic nanoparticles: feasibility, imaging, and three-dimensional temperature distribution. Eur. Urol 2007, 52, 1653–1662. [Google Scholar]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hemat 2002, 43, 33–56. [Google Scholar]
- Rauwerdink, A.M.; Hansen, E.W.; Weaver, J.B. Nanoparticle temperature estimation in combined AC and DC magnetic fields. Phys. Med. Biol 2009, 54, L51–L55. [Google Scholar]
- Gonzales-Weimuller, M.; Zeisberger, M.; Krishnan, K.M. Size-dependant heating rates of iron oxide nanoparticles for magnetic fluid hyperthermia. J. Magn. Magn. Mater 2009, 321, 1947–1950. [Google Scholar]
- Khandhar, A.P.; Ferguson, R.M.; Krishnan, K.M. Monodispersed magnetite nanoparticles optimized for magnetic fluid hyperthermia: Implications in biological systems. J. Appl. Phys 2011, 109, 07, B310:1–07B310:3.. [Google Scholar]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater 2002, 252, 370–374. [Google Scholar]
- Kim, S.U.; de Vellis, J. Stem cell-based cell therapy in neurological diseases: A review. J. Neurosci. Res 2009, 87, 2183–2200. [Google Scholar]
- Hess, D.; Li, L.; Martin, M.; Sakano, S.; Hill, D.; Strutt, B.; Thyssen, S.; Gray, D.A.; Bhatia, M. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat. Biotechnol 2003, 21, 763–770. [Google Scholar]
- Jackson, K.A.; Majka, S.M.; Wang, H.; Pocius, J.; Hartley, C.J.; Majesky, M.W.; Entman, M.L.; Michael, L.H.; Hirschi, K.K.; Goodell, M.A. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J. Clin. Investig 2001, 107, 1395–1402. [Google Scholar]
- Kim, J.H.; Auerbach, J.M.; Rodríguez-Gómez, J.A.; Velasco, I.; Gavin, D.; Lumelsky, N.; Lee, S.H.; Nguyen, J.; Sánchez-Pernaute, R.; Bankiewicz, K.; et al. Dopamine neurons derived from embryonic stem cells function in an animal model of parkinson’s disease. Nature 2002, 418, 50–56. [Google Scholar]
- Elhami, E.; Goertzen, A.L.; Xiang, B.; Deng, J.; Stillwell, C.; Mzengeza, S.; Arora, R.C.; Freed, D.; Tian, G. Viability and proliferation potential of adipose-derived stem cells following labeling with a positron-emitting radiotracer. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1323–1334. [Google Scholar]
- Bindslev, L.; Haack-Sørensen, M.; Bisgaard, K.; Kragh, L.; Mortensen, S.; Hesse, B.; Kjær, A.; Kastrup, J. Labelling of human mesenchymal stem cells with indium-111 for SPECT imaging: Effect on cell proliferation and differentiation. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1171–1177. [Google Scholar]
- Gildehaus, F.J.; Haasters, F.; Drosse, I.; Wagner, E.; Zach, C.; Mutschler, W.; Cumming, P.; Bartenstein, P.; Schieker, M. Impact of indium-111 oxine labelling on viability of human mesenchymal stem cells in vitro, and 3D cell-tracking using SPECT/CT in vivo. Mol. Imaging Biol 2011, 13, 1204–1214. [Google Scholar]
- Hu, S.L.; Lu, P.G.; Zhang, L.J.; Li, F.; Chen, Z.; Wu, N.; Meng, H.; Lin, J.K.; Feng, H. In vivo magnetic resonance imaging tracking of SPIO-labeled human umbilical cord mesenchymal stem cells. J. Cell. Biochem 2012, 113, 1005–1012. [Google Scholar]
- Arbab, A.S.; Yocum, G.T.; Kalish, H.; Jordan, E.K.; Anderson, S.A.; Khakoo, A.Y.; Read, E.J.; Frank, J.A. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood 2004, 104, 1217–1223. [Google Scholar]
- Bulte, J.; Walczak, P.; Bernard, S.; Gleich, B.; Weizenecker, J.; Borgert, J.; Aerts, H.; Boeve, H. Magnetic Nanoparticles: Particle Science, Imaging Technology, and Clinical Applications: Proceedings of the First International Workshop on Magnetic Particle Imaging. In Developing Cellular MPI: Initial Experience; World Scientific Publishing Company: Singapore, Singapore, 2010; pp. 201–204. [Google Scholar]
- Neoh, K.G.; Kang, E.T. Surface modification of magnetic nanoparticles for stem cell labeling. Soft Matter 2012, 8, 2057–2069. [Google Scholar]
- Babic, M.; Horák, D.; Trchová, M.; Jendelová, P.; Glogarová, K.; Lesný, P.; Herynek, V.; Hájek, M.; Syková, E. Poly (l-lysine)-modified iron oxide nanoparticles for stem cell labeling. Bioconjugate Chem 2008, 19, 740–750. [Google Scholar]
- Thorek, D.L.J.; Tsourkas, A. Size, charge and concentration dependent uptake of iron oxide particles by non-phagocytic cells. Biomaterials 2008, 29, 3583–3590. [Google Scholar]
- Jo, J.I.; Aoki, I.; Tabata, Y. Design of iron oxide nanoparticles with different sizes and surface charges for simple and efficient labeling of mesenchymal stem cells. J. Control. Release 2010, 142, 465–473. [Google Scholar]
- Jiang, X.; Dausend, J.; Hafner, M.; Musyanovych, A.; Röcker, C.; Landfester, K.; Mailänder, V.; Nienhaus, G.U. Specific effects of surface amines on polystyrene nanoparticles in their interactions with mesenchymal stem cells. Biomacromolecules 2010, 11, 748–753. [Google Scholar]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Shuter, B.; Wang, S.C.; Poh, C.; Wang, W. (Carboxymethyl)chitosan-modified superparamagnetic iron oxide nanoparticles for magnetic resonance imaging of stem cells. ACS Appl. Mater. Interfaces 2009, 1, 328–335. [Google Scholar]
- Jander, S.; Schroeter, M.; Saleh, A. Imaging inflammation in acute brain ischemia. Stroke 2007, 38, 642–645. [Google Scholar]
- Rudd, J.H.F.; Hyafil, F.; Fayad, Z.A. Inflammation imaging in atherosclerosis. Arterioscl. Throm. Vas 2009, 29, 1009–1016. [Google Scholar]
- Smirnov, P.; Poirier-Quinot, M.; Wilhelm, C.; Lavergne, E.; Ginefri, J.C.; Combadière, B.; Clément, O.; Darrasse, L.; Gazeau, F. In vivo single cell detection of tumor-infiltrating lymphocytes with a clinical 1.5 tesla MRI system. Magnet. Reson. Med 2008, 60, 1292–1297. [Google Scholar]
- Hu, D.E.; Kettunen, M.I.; Brindle, K.M. Monitoring T-lymphocyte trafficking in tumors undergoing immune rejection. Magnet. Reson. Med 2005, 54, 1473–1479. [Google Scholar]
- Wuerfel, E.; Smyth, M.; Millward, J.M.; Schellenberger, E.; Glumm, J.; Prozorovski, T.; Aktas, O.; Schulze-Topphoff, U.; Schnorr, J.; Wagner, S.; et al. Electrostatically stabilized magnetic nanoparticles—An optimized protocol to label murine T Cells for in vivo MRI. Front. Neurol 2011, 2, 72, :1–72:9.. [Google Scholar]
- Rauwerdink, A.M.; Weaver, J.B. Viscous effects on nanoparticle magnetization harmonics. J. Magn. Magn. Mater 2010, 322, 609–613. [Google Scholar]
- Rauwerdink, A.M.; Weaver, J.B. Measurement of molecular binding using the brownian motion of magnetic nanoparticle probes. Appl. Phys. Lett 2010, 96, 033702, :1–033702:3.. [Google Scholar]
- Gleich, B.; Weizenecker, J.; Borgert, J. Theory, simulation and experimental results of the acoustic detection of magnetization changes in superparamagnetic iron oxide. BMC Med. Imaging 2011, 11, 16, :1–16:6.. [Google Scholar]
| Imaging technology | Principle | Spatial resolution | Acquisition time | Sensitivity | Quantifiability | Harmfulness |
|---|---|---|---|---|---|---|
| X-ray computed tomography (CT) | Scanning of radial projection of X-rays attenuated by the anatomical structures of the human body | ~0.5 mm | ~1 s | Millimolar level (contrast agent) | Yes | Ionizing radiation (X-ray) |
| Magnetic resonance imaging (MRI) | Registration of nuclear (water protons in human tissues) magnetic resonance | ~1 mm | 1 s–1 h | Millimolar level (contrast agent) | Yes (but require complex mathematical methodology) | Bio-effects caused by magnetic field |
| Positron emission tomography (PET) | Coincidence detection of two γ-quanta generated from the positron emission of radioactive tracers | ~4 mm | 1 min | Picomolar level (radioactive tracer) | Yes | Ionizing radiation (β/γ radiation) |
| Single photon emission computed tomography (SPECT) | Detection of the density of γ-quanta emitted by the radioactive tracers | ~10 mm | 1 min | Picomolar level (radioactive tracer) | Yes | Ionizing radiation (γ radiation) |
| Magnetic particle imaging (MPI) | Detection of the nonlinear response of superparamagnetic nanoparticles to the oscillating magnetic field | <1 mm | <0.1 s | Micromolar level (magnetic tracer) | Yes | Bio-effects caused by magnetic field |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Du, Y.; Lai, P.T.; Leung, C.H.; Pong, P.W.T. Design of Superparamagnetic Nanoparticles for Magnetic Particle Imaging (MPI). Int. J. Mol. Sci. 2013, 14, 18682-18710. https://doi.org/10.3390/ijms140918682
Du Y, Lai PT, Leung CH, Pong PWT. Design of Superparamagnetic Nanoparticles for Magnetic Particle Imaging (MPI). International Journal of Molecular Sciences. 2013; 14(9):18682-18710. https://doi.org/10.3390/ijms140918682
Chicago/Turabian StyleDu, Yimeng, Pui To Lai, Cheung Hoi Leung, and Philip W. T. Pong. 2013. "Design of Superparamagnetic Nanoparticles for Magnetic Particle Imaging (MPI)" International Journal of Molecular Sciences 14, no. 9: 18682-18710. https://doi.org/10.3390/ijms140918682