Ibuprofen Rescues Abnormalities in Periodontal Tissues in Conditional Presenilin 1 and Presenilin 2 Double Knockout Mice
Abstract
:1. Introduction
2. Results and Discussion
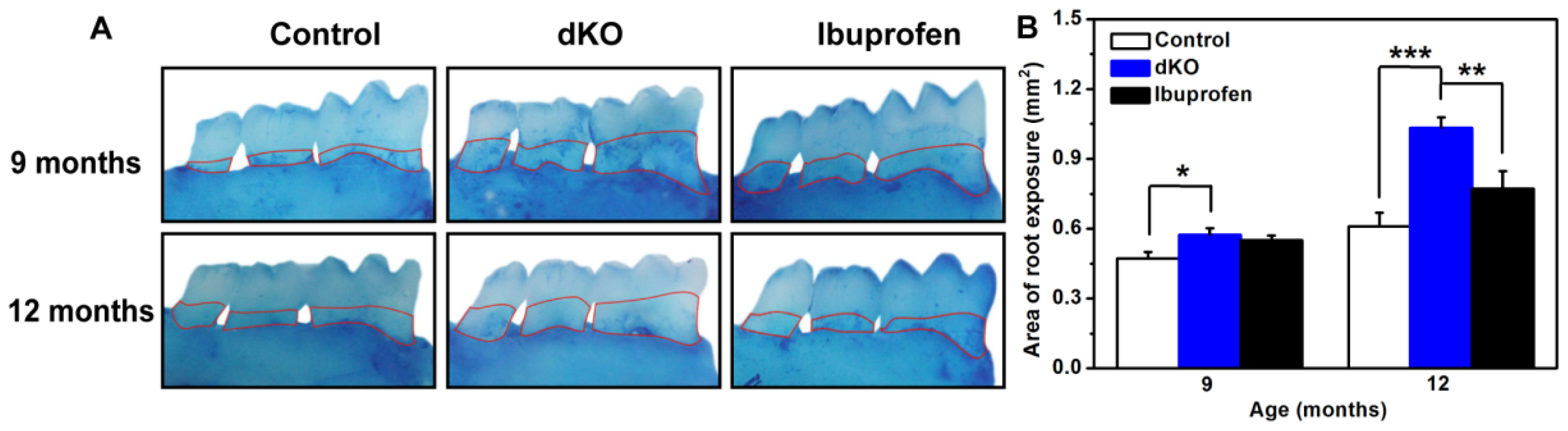
2.1. Ibuprofen Decreased Alveolar Bone Loss of the Mandibular Molar Region in PS-dKO Mice
2.2. The Number of Osteoclasts in the Periodontal Ligament Was Significantly Less in Ibuprofen-Treated Mice
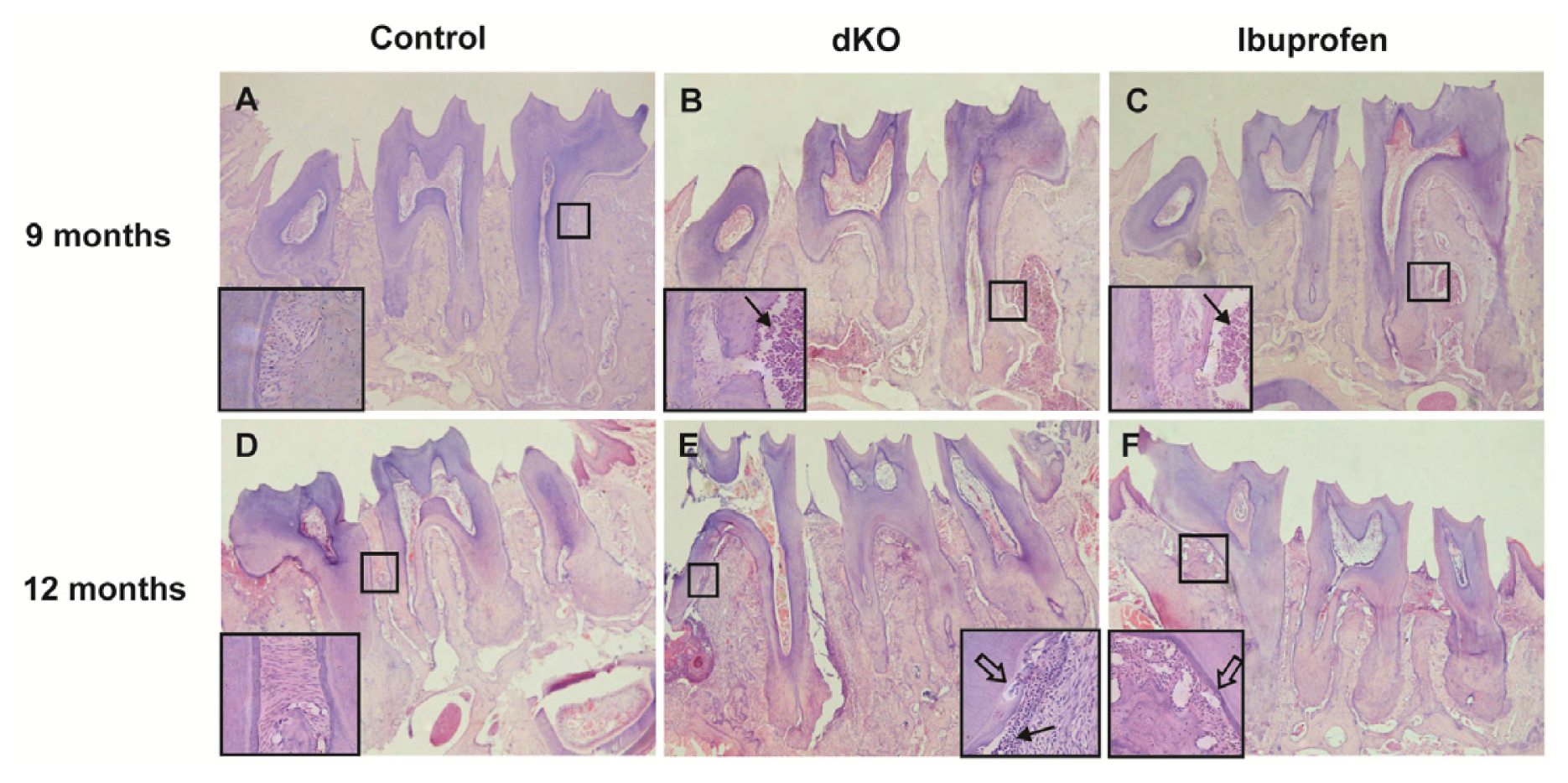
2.3. Ibuprofen Alleviated Histomorphological Abnormalities in the Periodontal Tissue of dKO Mice
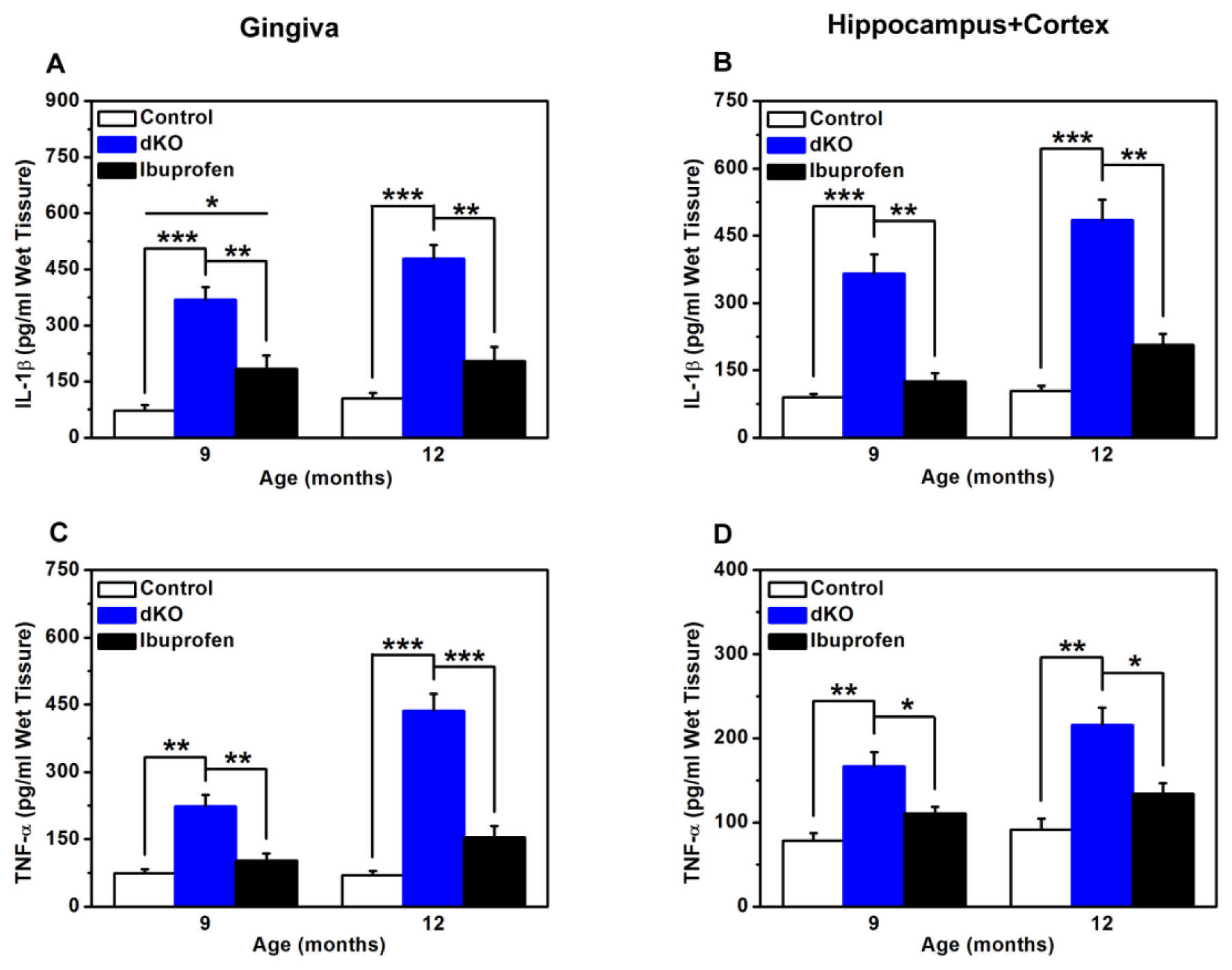
2.4. Inflammatory Mediators in the Brain and Gingiva Were Reduced with Ibuprofen Treatment
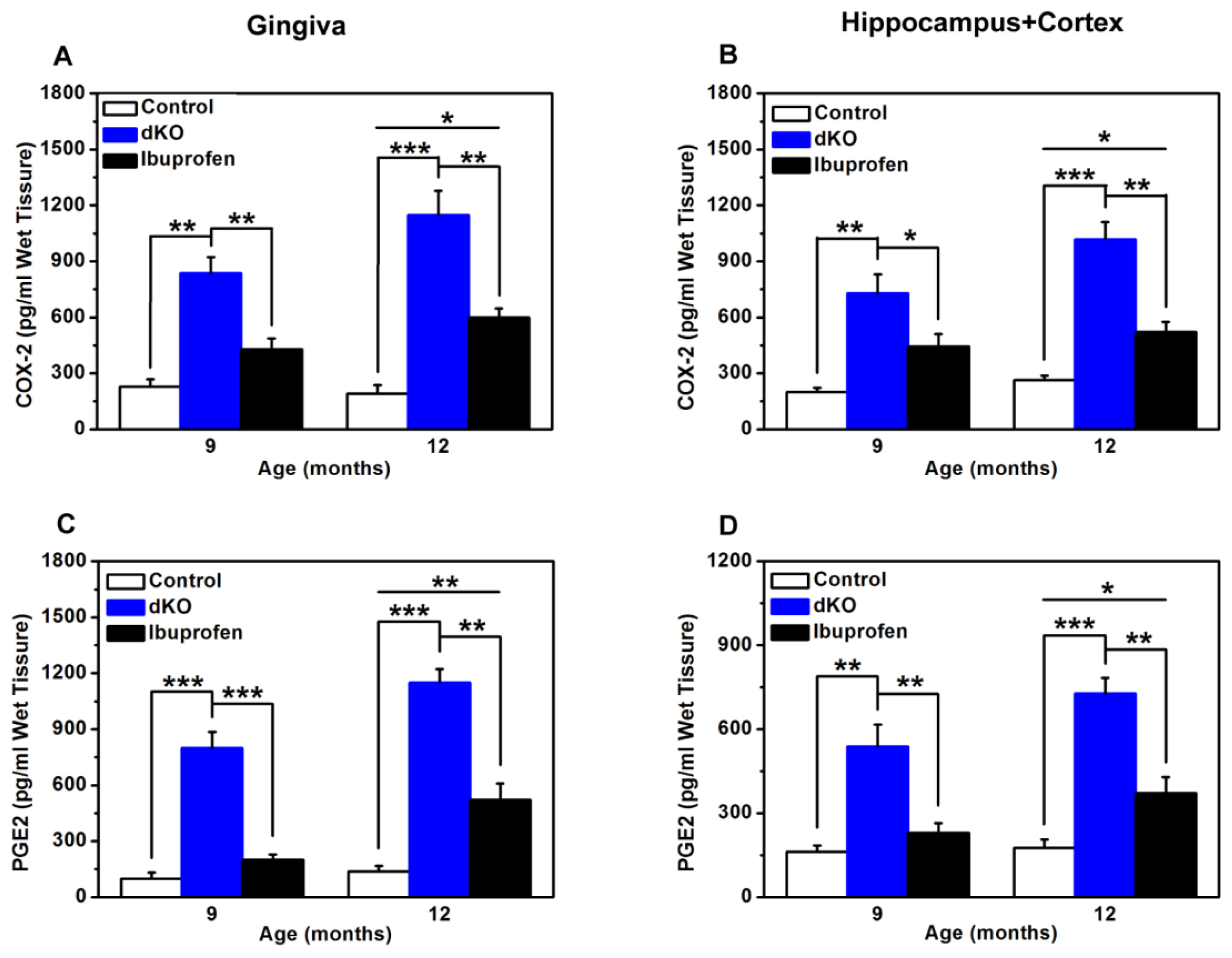
2.5. COX-2 and PGE2 Levels Were Significantly Lower after Ibuprofen Treatment
3. Experimental Section
3.1. Mice Breeding and Genotyping
3.2. Immunohistochemical Analysis of the Calcitonin Receptor in the Periodontal Ligament
3.3. Histological Observation of Periodontal Tissues
3.4. Morphometric Bone Observation
3.5. Enzyme-Linked Immunosorbent Assay (ELISA) for Inflammatory Mediators
3.6. Statistical Analysis
4. Conclusions
Acknowledgements
Conflicts of Interest
References
- Hutton, M.; Hardy, J. The presenilins and Alzheimer’s disease. Hum. Mol. Genet 1997, 6, 1639–1646. [Google Scholar]
- Feng, R.; Rampon, C.; Tang, Y.P.; Shrom, D.; Jin, J.; Kyin, M.; Sopher, B.; Miller, M.W.; Ware, C.B.; Martin, G.M.; et al. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron 2001, 32, 911–926. [Google Scholar]
- Donoviel, D.B.; Hadjantonakis, A.K.; Ikeda, M.; Zheng, H.; Hyslop, P.S.; Bernstein, A. Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev 1999, 13, 2801–2810. [Google Scholar]
- Shen, J.; Kelleher, R.J., 3rd. The presenilin hypothesis of Alzheimer’s disease: Evidence for a loss-of-function pathogenic mechanism. Proc. Natl. Acad. Sci. USA 2007, 104, 403–409. [Google Scholar]
- Saura, C.A.; Choi, S.Y.; Beglopoulos, V.; Malkani, S.; Zhang, D.; Shankaranarayana Rao, B.S.; Chattarji, S.; Kelleher, R.J., 3rd; Kandel, E.R.; Duff, K.; et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron 2004, 42, 23–36. [Google Scholar]
- Feng, R.; Wang, H.; Wang, J.; Shrom, D.; Zeng, X.; Tsien, J.Z. Forebrain degeneration and ventricle enlargement caused by double knockout of Alzheimer’s presenilin-1 and presenilin-2. Proc. Natl. Acad. Sci. USA 2004, 101, 8162–8167. [Google Scholar]
- Jiang, X.; Zhang, D.; Shi, J.; Chen, Y.; Zhang, P.; Mei, B. Increased inflammatory response both in brain and in periphery in presenilin 1 and presenilin 2 conditional double knock-out mice. J. Alzheimer’s Dis 2009, 18, 515–523. [Google Scholar]
- Beglopoulos, V.; Sun, X.; Saura, C.A.; Lemere, C.A.; Kim, R.D.; Shen, J. Reduced beta-amyloid production and increased inflammatory responses in presenilin conditional knock-out mice. J. Biol.Chem 2004, 279, 46907–46914. [Google Scholar]
- Wang, D.; Yang, L.; Su, J.; Niu, Y.; Lei, X.; Xiong, J.; Cao, X.; Hu, Y.; Mei, B.; Hu, J.F. Attenuation of neurodegenerative phenotypes in Alzheimer-like presenilin 1/presenilin 2 conditional double knockout mice by EUK1001, a promising derivative of xanomeline. Biochem. Biophys. Res. Comm 2011, 410, 229–234. [Google Scholar]
- Armitage, G.C. Periodontal diagnoses and classification of periodontal diseases. Periodontology 2000 2004, 34, 9–21. [Google Scholar]
- Ebersole, J.L.; Cappelli, D.; Mathys, E.C.; Steffen, M.J.; Singer, R.E.; Montgomery, M.; Mott, G.E.; Novak, M.J. Periodontitis in humans and non-human primates: Oral-systemic linkage inducing acute phase proteins. Ann. Periodontol 2002, 7, 102–111. [Google Scholar]
- Kamer, A.R.; Morse, D.E.; Holm-Pedersen, P.; Mortensen, E.L.; Avlund, K. Periodontal inflammation in relation to cognitive function in an older adult Danish population. J. Alzheimer’s Dis 2012, 28, 613–624. [Google Scholar]
- Stewart, R.; Sabbah, W.; Tsakos, G.; D’Aiuto, F.; Watt, R.G. Oral health and cognitive function in the Third National Health and Nutrition Examination Survey (NHANES III). Psychosom. Med 2008, 70, 936–941. [Google Scholar]
- Grabe, H.J.; Schwahn, C.; Volzke, H.; Spitzer, C.; Freyberger, H.J.; John, U.; Mundt, T.; Biffar, R.; Kocher, T. Tooth loss and cognitive impairment. J. Clin. Periodontol 2009, 36, 550–557. [Google Scholar]
- Kaye, E.K.; Valencia, A.; Baba, N.; Spiro, A., 3rd; Dietrich, T.; Garcia, R.I. Tooth loss and periodontal disease predict poor cognitive function in older men. J. Am. Geriatr. Soc 2010, 58, 713–718. [Google Scholar]
- Ellefsen, B.; Holm-Pedersen, P.; Morse, D.E.; Schroll, M.; Andersen, B.B.; Waldemar, G. Assessing caries increments in elderly patients with and without dementia: A one-year follow-up study. J. Am. Dent. Assoc 2009, 140, 1392–1400. [Google Scholar]
- Avlund, K.; Holm-Pedersen, P.; Morse, D.E.; Viitanen, M.; Winblad, B. Tooth loss and caries prevalence in very old Swedish people: The relationship to cognitive function and functional ability. Gerodontology 2004, 21, 17–26. [Google Scholar]
- Ellefsen, B.; Holm-Pedersen, P.; Morse, D.E.; Schroll, M.; Andersen, B.B.; Waldemar, G. Caries prevalence in older persons with and without dementia. J. Am. Geriatr. Soc 2008, 56, 59–67. [Google Scholar]
- Han, W.; Ji, T.; Wang, L.; Yan, L.; Wang, H.; Luo, Z.; Mei, B.; Su, J. Abnormalities in periodontal and salivary tissues in conditional presenilin 1 and presenilin 2 double knockout mice. Mol. Cell. Biochem 2011, 347, 13–20. [Google Scholar]
- McGeer, P.L.; Schulzer, M.; McGeer, E.G. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: A review of 17 epidemiologic studies. Neurology 1996, 47, 425–432. [Google Scholar]
- Stewart, W.F.; Kawas, C.; Corrada, M.; Metter, E.J. Risk of Alzheimer’s disease and duration of NSAID use. Neurology 1997, 48, 626–632. [Google Scholar]
- Vlad, S.C.; Miller, D.R.; Kowall, N.W.; Felson, D.T. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology 2008, 70, 1672–1677. [Google Scholar]
- Howell, T.H.; Williams, R.C. Nonsteroidal antiinflammatory drugs as inhibitors of periodontal disease progression. Crit. Rev. Oral Biol. Med 1993, 4, 177–196. [Google Scholar]
- Lim, G.P.; Yang, F.; Chu, T.; Chen, P.; Beech, W.; Teter, B.; Tran, T.; Ubeda, O.; Ashe, K.H.; Frautschy, S.A.; et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J. Neurosci 2000, 20, 5709–5714. [Google Scholar]
- Lim, G.P.; Yang, F.; Chu, T.; Gahtan, E.; Ubeda, O.; Beech, W.; Overmier, J.B.; Hsiao-Ashec, K.; Frautschy, S.A.; Cole, G.M. Ibuprofen effects on Alzheimer pathology and open field activity in APPsw transgenic mice. Neurobiol. Aging 2001, 22, 983–991. [Google Scholar]
- Cochran, D.L. Inflammation and bone loss in periodontal disease. J. Periodontol 2008, 79, 1569–1576. [Google Scholar]
- Yamamoto, T.; Domon, T.; Takahashi, S.; Islam, N.; Suzuki, R.; Wakita, M. The structure and function of periodontal ligament cells in acellular cementum in rat molars. Ann. Anat 1998, 180, 519–522. [Google Scholar]
- Noble, J.M.; Scarmeas, N. (Cognitive Impairment) Improving Oral Health for the Elderly, 1st ed; Springer Science & Business Media: New York, NY, USA, 2008; pp. 99–126. [Google Scholar]
- Mei, B. East China Normal University: Shanghai, China, Unpublished observation 2013.
- Stalder, A.K.; Carson, M.J.; Pagenstecher, A.; Asensio, V.C.; Kincaid, C.; Benedict, M.; Powell, H.C.; Masliah, E.; Campbell, I.L. Late-onset chronic inflammatory encephalopathy in immune-competent and severe combined immune-deficient (SCID) mice with astrocyte-targeted expression of tumor necrosis factor. Am. J. Pathol 1998, 153, 767–783. [Google Scholar]
- Yang, Y.; Quitschke, W.W.; Brewer, G.J. Upregulation of amyloid precursor protein gene promoter in rat primary hippocampal neurons by phorbol ester, IL-1 and retinoic acid, but not by reactive oxygen species. Brain Res 1998, 60, 40–49. [Google Scholar]
- Li, Y.; Liu, L.; Barger, S.W.; Griffin, W.S. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J. Neurosci 2003, 23, 1605–1611. [Google Scholar]
- In’t Veld, B.A.; Ruitenberg, A.; Hofman, A.; Launer, L.J.; van Duijn, C.M.; Stijnen, T.; Breteler, M.M.; Stricker, B.H. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N. Eng. J. Med 2001, 345, 1515–1521. [Google Scholar]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev 2004, 56, 387–437. [Google Scholar]
- McGeer, P.L. Cyclo-oxygenase-2 inhibitors: Rationale and therapeutic potential for Alzheimer’s disease. Drugs Aging 2000, 17, 1–11. [Google Scholar]
- Heneka, M.T.; Sastre, M.; Dumitrescu-Ozimek, L.; Hanke, A.; Dewachter, I.; Kuiperi, C.; O’Banion, K.; Klockgether, T.; van Leuven, F.; Landreth, G.E. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1–42 levels in APPV717I transgenic mice. Brain 2005, 128, 1442–1453. [Google Scholar]
- Hoozemans, J.J.; Veerhuis, R.; Rozemuller, A.J.; Eikelenboom, P. Non-steroidal anti-inflammatory drugs and cyclooxygenase in Alzheimer’s disease. Curr. Drug Targets 2003, 4, 461–468. [Google Scholar]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar]
- Dong, S.; Li, C.; Wu, P.; Tsien, J.Z.; Hu, Y. Environment enrichment rescues the neurodegenerative phenotypes in presenilins-deficient mice. Eur. J. Neurosci 2007, 26, 101–112. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Su, J.; Gu, J.; Dong, Z.; Mei, B. Ibuprofen Rescues Abnormalities in Periodontal Tissues in Conditional Presenilin 1 and Presenilin 2 Double Knockout Mice. Int. J. Mol. Sci. 2013, 14, 18457-18469. https://doi.org/10.3390/ijms140918457
Su J, Gu J, Dong Z, Mei B. Ibuprofen Rescues Abnormalities in Periodontal Tissues in Conditional Presenilin 1 and Presenilin 2 Double Knockout Mice. International Journal of Molecular Sciences. 2013; 14(9):18457-18469. https://doi.org/10.3390/ijms140918457
Chicago/Turabian StyleSu, Jiansheng, Jiamei Gu, Zhuo Dong, and Bing Mei. 2013. "Ibuprofen Rescues Abnormalities in Periodontal Tissues in Conditional Presenilin 1 and Presenilin 2 Double Knockout Mice" International Journal of Molecular Sciences 14, no. 9: 18457-18469. https://doi.org/10.3390/ijms140918457



