In Vitro Antimetastatic Effect of Phosphatidylinositol 3-Kinase Inhibitor ZSTK474 on Prostate Cancer PC3 Cells
Abstract
:1. Introduction
2. Results and Discussion
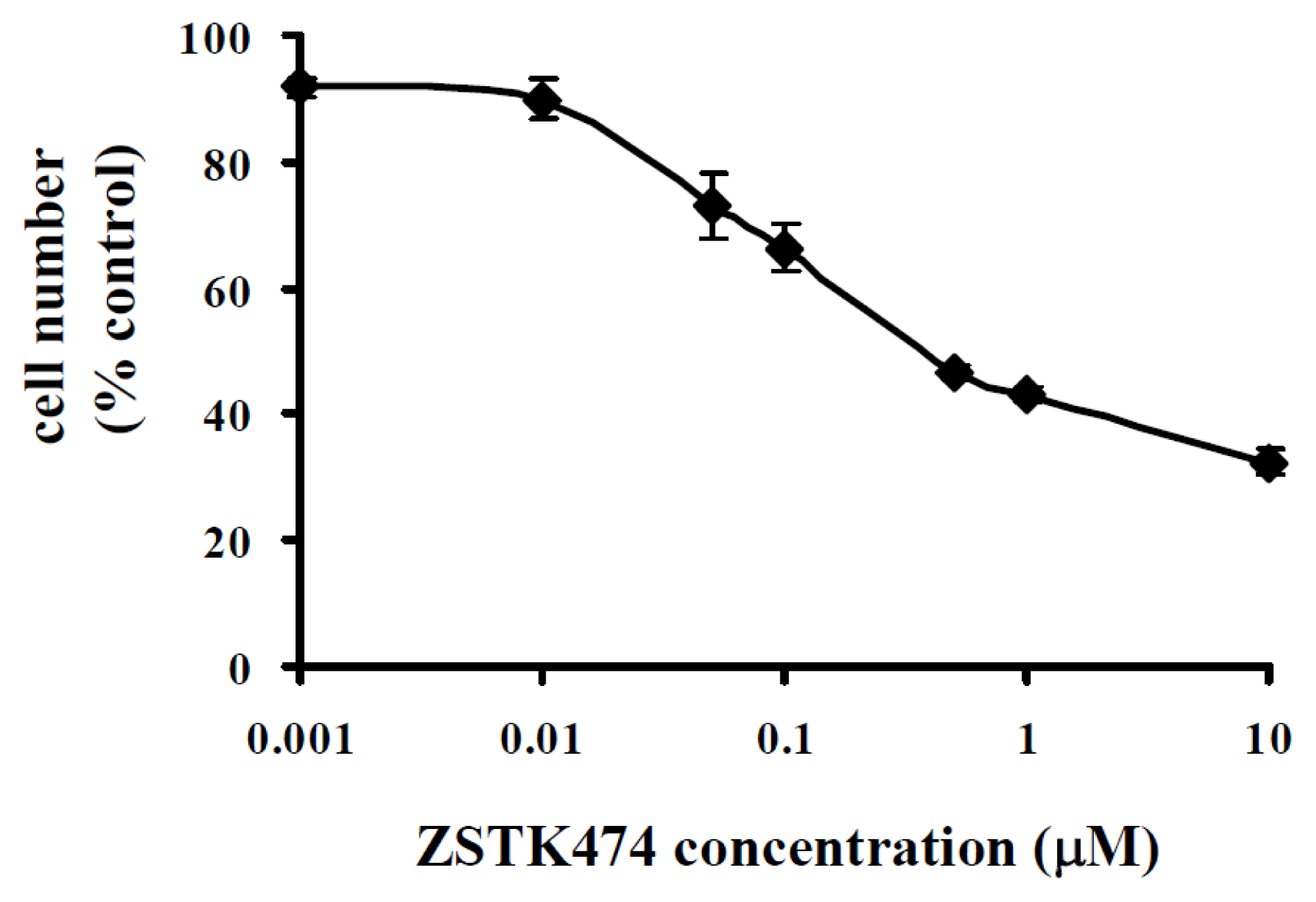
2.1. ZSTK474 Inhibited PC3 Proliferation
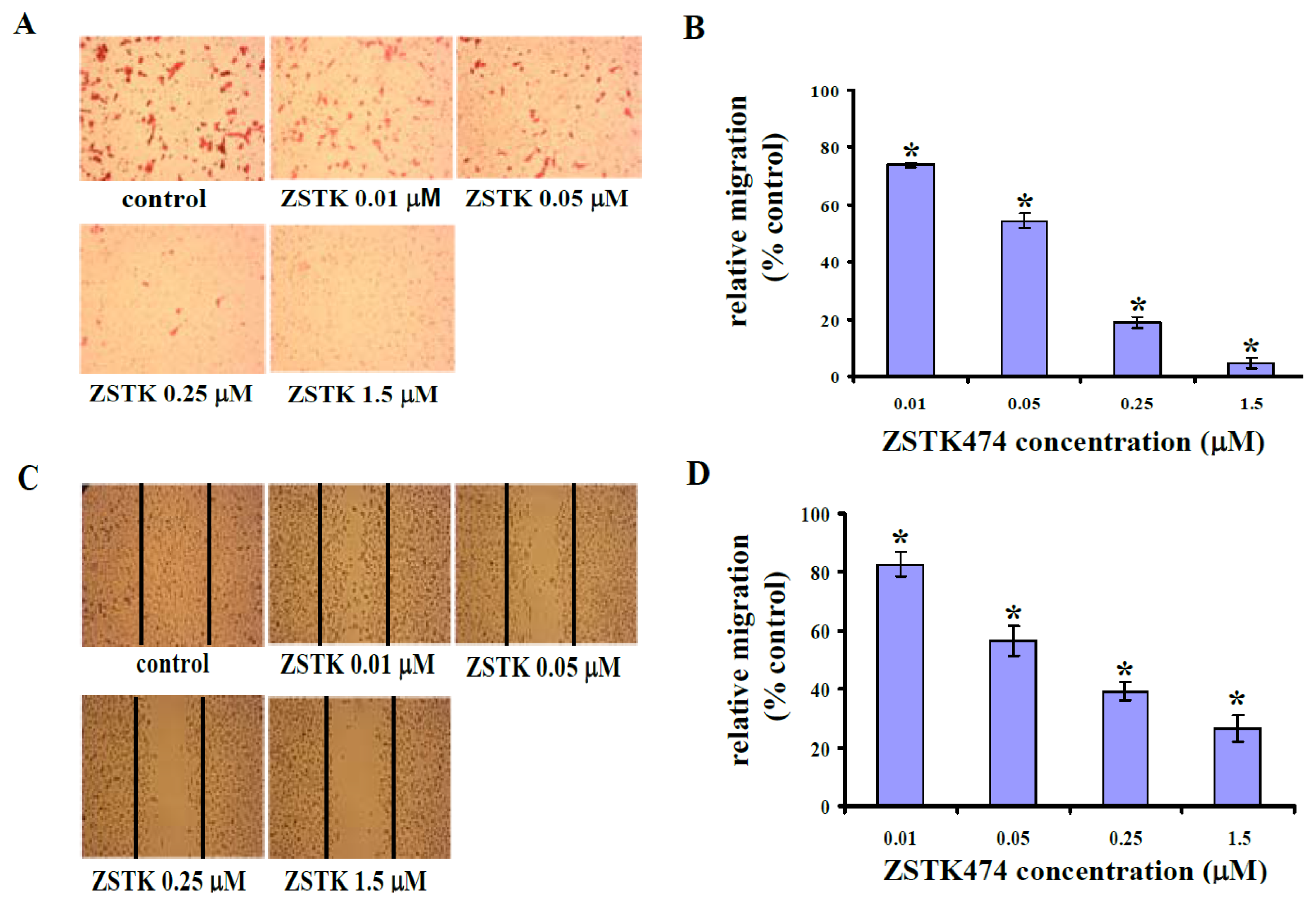
2.2. ZSTK474 Blocked PC3 Migration
2.3. ZSTK474 Reduced PC3 Invasion
2.4. ZSTK474 Inhibited PC3 Adhesion
2.5. ZSTK474 Inhibited Phosphorylation of Girdin in PC3 Cells
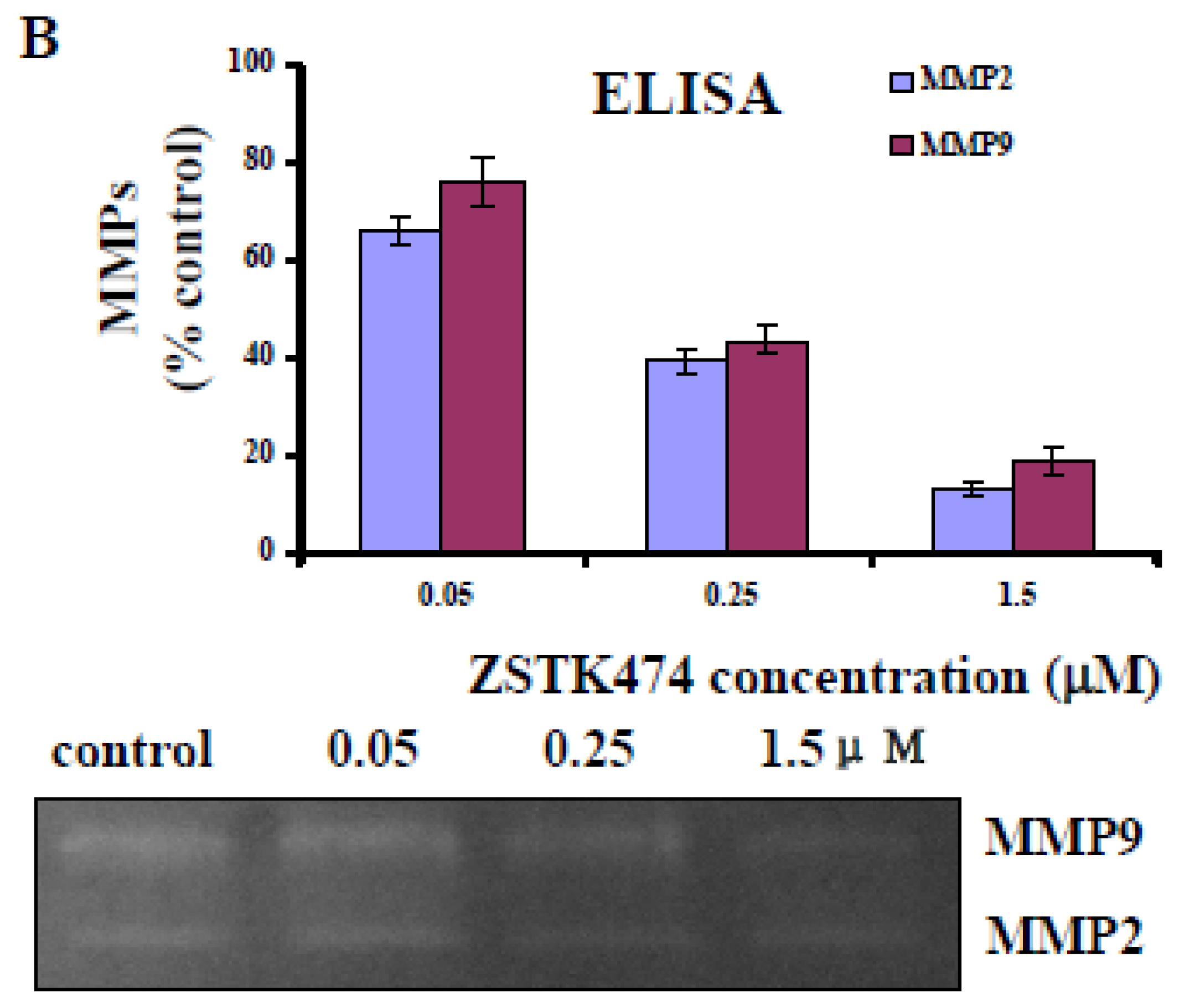
2.6. ZSTK474 Inhibited the Secretion of MMP2 and MMP9 by PC3 Cells
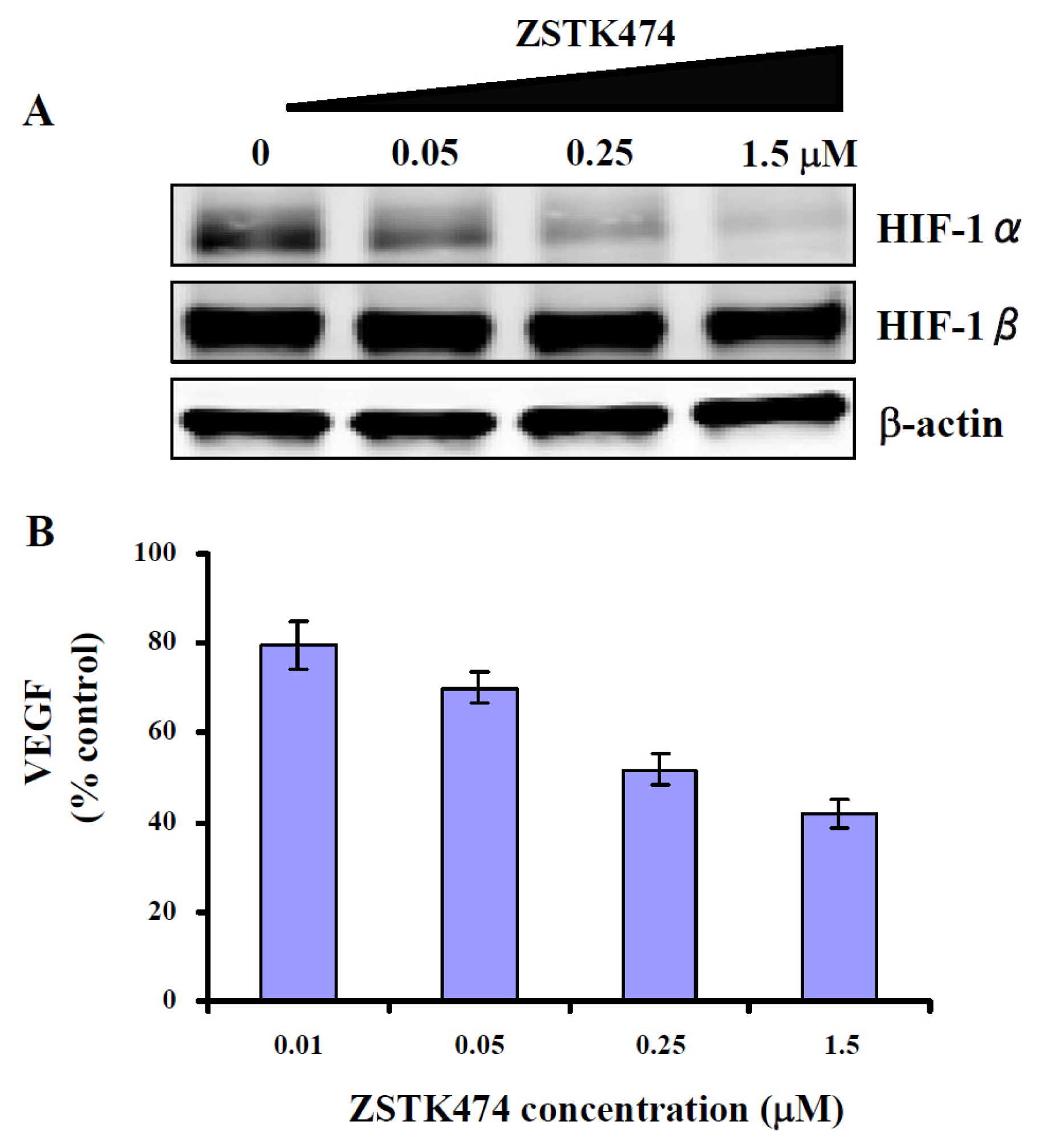
2.7. ZSTK474 Inhibited the Expression of HIF-1α and VEGF in PC3 Cancer Cells
2.8. ZSTK474-Reduced VEGF Inhibited Tube Formation by HUVEC Cells
3. Experimental Section
3.1. Reagents
3.2. Cell Culture
3.3. Cell Growth Inhibition Assay
3.4. Transwell Migration Assay
3.5. Wound Healing Assay
3.6. Transwell Invasion Assay
3.7. Cell Adhesion Assay
3.8. Western Blot Analysis
3.9. Enzyme-Linked Immunosorbent Assay (ELISA)
3.10. Gelatin Zymography Assay
3.11. Tube Formation Assay
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar]
- Toker, A.; Cantley, L.C. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 1997, 387, 673–676. [Google Scholar]
- Franke, T.F.; Kaplan, D.R.; Cantley, L.C. PI3K: downstream AKTion blocks apoptosis. Cell 1997, 88, 435–437. [Google Scholar]
- Kong, D.; Yamori, T. Advances in development of phosphatidylinositol 3-kinase inhibitors. Curr. Med. Chem 2009, 16, 2839–2854. [Google Scholar]
- Samuels, Y.; Wang, Z.; Bardelli, A.; Silliman, N.; Ptak, J.; Szabo, S.; Yan, H.; Gazdar, A.; Powell, S.M.; Riggins, G.J.; et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 2004, 304, 554. [Google Scholar]
- Kong, D.; Yamori, T. Phosphatidylinositol 3-kinase inhibitors: promising drug candidates for cancer therapy. Cancer Sci 2008, 99, 1734–1740. [Google Scholar]
- Kong, D.X.; Yamori, T. ZSTK474, a novel phosphatidylinositol 3-kinase inhibitor identified using the JFCR39 drug discovery system. Acta Pharmacol. Sin 2010, 31, 1189–1197. [Google Scholar]
- Yaguchi, S.; Fukui, Y.; Koshimizu, I.; Yoshimi, H.; Matsuno, T.; Gouda, H.; Hirono, S.; Yamazaki, K.; Yamori, T. Antitumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. J. Natl. Cancer Inst 2006, 98, 545–556. [Google Scholar]
- Kong, D.; Yamori, T. JFCR39, a panel of 39 human cancer cell lines, and its application in the discovery and development of anticancer drugs. Bioorg. Med. Chem 2012, 20, 1947–1951. [Google Scholar]
- Kong, D.; Yamori, T. ZSTK474 is an ATP-competitive inhibitor of class I phosphatidylinositol 3 kinase isoforms. Cancer Sci 2007, 98, 1638–1642. [Google Scholar]
- Kong, D.; Yaguchi, S.; Yamori, T. Effect of ZSTK474, a novel phosphatidylinositol 3-kinase inhibitor, on DNA-dependent protein kinase. Biol. Pharm. Bull 2009, 32, 297–300. [Google Scholar]
- Kong, D.; Dan, S.; Yamazaki, K.; Yamori, T. Inhibition profiles of phosphatidylinositol 3-kinase inhibitors against PI3K superfamily and human cancer cell line panel JFCR39. Eur. J. Cancer 2010, 46, 1111–1121. [Google Scholar]
- Kong, D.; Okamura, M.; Yoshimi, H.; Yamori, T. Antiangiogenic effect of ZSTK474, a novel phosphatidylinositol 3-kinase inhibitor. Eur. J. Cancer 2009, 45, 857–865. [Google Scholar]
- Toyama, S.; Tamura, N.; Haruta, K.; Karakida, T.; Mori, S.; Watanabe, T.; Yamori, T.; Takasaki, Y. Inhibitory effects of ZSTK474, a novel phosphoinositide 3-kinase inhibitor, on osteoclasts and collagen-induced arthritis in mice. Arthritis Res. Ther 2010, 12, R92. [Google Scholar]
- Serra, V.; Markman, B.; Scaltriti, M.; Eichhorn, P.J.; Valero, V.; Guzman, M.; Botero, M.L.; Llonch, E.; Atzori, F.; Di Cosimo, S.; et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res 2008, 68, 8022–8030. [Google Scholar]
- GraphPad Prism, version 4. Available online: http://www.graphpad.com/scientific-software/prism/ (on Accessed 26, June, 2013).
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar]
- Kitamura, T.; Asai, N.; Enomoto, A.; Maeda, K.; Kato, T.; Ishida, M.; Jiang, P.; Watanabe, T.; Usukura, J.; Kondo, T.; et al. Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nat. Cell Biol 2008, 10, 329–337. [Google Scholar]
- Jiang, P.; Enomoto, A.; Jijiwa, M.; Kato, T.; Hasegawa, T.; Ishida, M.; Sato, T.; Asai, N.; Murakumo, Y.; Takahashi, M. An actin-binding protein Girdin regulates the motility of breast cancer cells. Cancer Res 2008, 68, 1310–1318. [Google Scholar]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov 2007, 6, 273–286. [Google Scholar]
- Jiang, B.H.; Jiang, G.; Zheng, J.Z.; Lu, Z.; Hunter, T.; Vogt, P.K. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ 2001, 12, 363–369. [Google Scholar]
- Rini, B.I.; Small, E.J. Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. J. Clin. Oncol 2005, 23, 1028–1043. [Google Scholar]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar]
- Ferrara, N. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med 1999, 77, 527–543. [Google Scholar]
- Ferrara, N.; Gerber, H.P. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol 2001, 106, 148–156. [Google Scholar]
- Folkman, J. Angiogenesis. Annu. Rev. Med 2006, 57, 1–18. [Google Scholar]
- Kong, D.; Yamori, T.; Kobayashi, M.; Duan, H. Antiproliferative and antiangiogenic activities of smenospongine, a marine sponge sesquiterpene aminoquinone. Mar. Drugs 2011, 9, 154–161. [Google Scholar]
- Lee, H.S.; Seo, E.Y.; Kang, N.E.; Kim, W.K. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J. Nutr. Biochem. 2008, 19, 313–319. [Google Scholar]
- ImageJ, version 1.47. Available online: http://rsbweb.nih.gov/ij/ (on accessed 26 June 2013).
- Hsiech, C.Y.; Tsai, P.C.; Tseng, C.H.; Chen, Y.L.; Chang, L.S.; Lin, S.R. Inhibition of EGF/EGFR activation with naphtho[1,2-b]furan-4,5-dione blocks migration and invasion of MDA-MB-231 cells. Toxicol. In Vitro 2013, 27, 1–10. [Google Scholar]
- MetaMorph, version 6.3. Available online: http://www.moleculardevices.com/Products/Software/Meta-Imaging-Series/MetaMorph.html (on accessed 26 June 2013).



© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, W.; Guo, W.; Zhou, Q.; Ma, S.-N.; Wang, R.; Qiu, Y.; Jin, M.; Duan, H.-Q.; Kong, D. In Vitro Antimetastatic Effect of Phosphatidylinositol 3-Kinase Inhibitor ZSTK474 on Prostate Cancer PC3 Cells. Int. J. Mol. Sci. 2013, 14, 13577-13591. https://doi.org/10.3390/ijms140713577
Zhao W, Guo W, Zhou Q, Ma S-N, Wang R, Qiu Y, Jin M, Duan H-Q, Kong D. In Vitro Antimetastatic Effect of Phosphatidylinositol 3-Kinase Inhibitor ZSTK474 on Prostate Cancer PC3 Cells. International Journal of Molecular Sciences. 2013; 14(7):13577-13591. https://doi.org/10.3390/ijms140713577
Chicago/Turabian StyleZhao, Wennan, Wenzhi Guo, Qianxiang Zhou, Sheng-Nan Ma, Ran Wang, Yuling Qiu, Meihua Jin, Hong-Quan Duan, and Dexin Kong. 2013. "In Vitro Antimetastatic Effect of Phosphatidylinositol 3-Kinase Inhibitor ZSTK474 on Prostate Cancer PC3 Cells" International Journal of Molecular Sciences 14, no. 7: 13577-13591. https://doi.org/10.3390/ijms140713577



