The Functions and Applications of RGD in Tumor Therapy and Tissue Engineering
Abstract
:1. Introduction
2. The Functions of RGD
2.1. The Sequence and the Structure of RGD
2.2. RGD-Mediated Recognition and Adhesion with Cells
3. The Applications of RGD
3.1. Radiolabelled RGD Peptides for Tumor Imaging and Diagnostics
3.2. RGD Inhibiting Tumor
3.2.1. RGD Peptides Affecting Tumor Cell Adhesion and Migration
3.2.2. RGD Peptides Inducing Tumor Cell Apoptosis
3.2.3. RGD Peptides Inhibiting Tumor Angiogenesis
3.3. RGD-Modified Carriers
3.3.1. RGD-Modified Gene Carriers Treating Tumor
3.3.2. RGD-Modified Drugs for Target Therapy
3.4. RGD Used in Tissue Engineering
3.4.1. Bone Regeneration
3.4.2. Cornea Repair
3.4.3. Artificial Neovascularization
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Colombo, M.; Bianchi, A. Click chemistry for the synthesis of rgd-containing integrin ligands. Molecules 2010, 15, 178–197. [Google Scholar]
- Pierschbacher, M.D.; Ruoslahti, E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 1984, 309, 30–33. [Google Scholar]
- Kim, J.; Nam, H.Y.; Kim, T.-I.; Kim, P.-H.; Ryu, J.; Yun, C.-O.; Kim, S.W. Active targeting of RGD-conjugated bioreducible polymer for delivery of oncolytic adenovirus expressing shRNA against IL-8 mRNA. Biomaterials 2011, 32, 5158–5166. [Google Scholar]
- Hynes, R.O. Integrins: A family of cell surface receptors. Cell 1987, 48, 549–554. [Google Scholar]
- Yamada, K.M.; Geiger, B. Molecular interactions in cell adhesion complexes. Curr. Opin. Cell Biol 1997, 9, 76–85. [Google Scholar]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci 2006, 119, 3901–3903. [Google Scholar]
- Temming, K.; Schiffelers, R.M.; Molema, G.; Kok, R.J. RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resist. Update 2005, 8, 381–402. [Google Scholar]
- Verrier, S.; Pallu, S.; Bareille, R.; Jonczyk, A.; Meyer, J.; Dard, M.; Amedee, J. Function of linear and cyclic RGD-containing peptides in osteoprogenitor cells adhesion process. Biomaterials 2002, 23, 585–596. [Google Scholar]
- Frochot, C.; Stasio, B.D.; Vanderesse, R.; Belgy, M.-J.; Dodeller, M.; Guillemin, F.; Viriot, M.-L.; Barberi-Heyob, M. Interest of RGD-containing linear or cyclic peptide targeted tetraphenylchlorin as novel photosensitizers for selective photodynamic activity. Bioorg. Chem 2007, 35, 205–220. [Google Scholar]
- Enwerem, I.; Wang, J.; Leszczynski, J. In search of active RGD peptides: Theoretical study of hydrogen bonding in five-member ring Cyclic-RGD isomers. Comput. Theor. Chem 2012, 998, 141–147. [Google Scholar]
- Hilgenbrink, A.R.; Low, P.S. Folate receptor-mediated drug targeting: From therapeutics to diagnostics. J. Pharm. Sci 2005, 94, 2135–2146. [Google Scholar]
- Bellis, S.L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar]
- Hwang, D.S.; Sim, S.B.; Cha, H.J. Cell adhesion biomaterial based on mussel adhesive protein fused with RGD peptide. Biomaterials 2007, 28, 4039–4046. [Google Scholar]
- Zitzmann, S.; Ehemann, V.; Schwab, M. Arginine-glycine-aspartic acid (RGD)-peptide binds to both tumor and tumor-endothelial cells in vivo. Cancer Res 2002, 62, 5139–5143. [Google Scholar]
- Delouvrié, B.; Al-Kadhimi, K.; Arnould, J.-C.; Barry, S.T.; Cross, D.A.; Didelot, M.; Gavine, P.R.; Germain, H.; Harris, C.S.; Hughes, A.M. Structure activity relationship of a series of non peptidic RGD integrin antagonists targeting α5β1. Part 2. Bioorg. Med. Chem. Lett 2012, 22, 4117–4121. [Google Scholar]
- Bella, J.; Humphries, M.J. Cα–H···O=C hydrogen bonds contribute to the specificity of RGD cell-adhesion interactions. BMC Struct. Biol 2005, 5, 4, , doi:10.1186/1472-6807-5-4.. [Google Scholar]
- Plow, E.F.; Haas, T.A.; Zhang, L.; Loftus, J.; Smith, J.W. Ligand binding to integrins. J. Biol. Chem 2000, 275, 21785–21788. [Google Scholar]
- Delouvrié, B.; Al-Kadhimi, K.; Arnould, J.-C.; Barry, S.T.; Cross, D.A.; Didelot, M.; Gavine, P.R.; Germain, H.; Harris, C.S.; Hughes, A.M. Structure activity relationship of a series of non peptidic RGD integrin antagonists targeting α5β1. Part 1. Bioorg. Med. Chem. Lett 2012, 22, 4111–4116. [Google Scholar]
- Liu, S. Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3 targeted radiotracers for tumor imaging. Mol. Pharm 2006, 3, 472–487. [Google Scholar]
- Haubner, R.; Wester, H.J. Radiolabeled tracers for imaging of tumor angiogenesis and evaluation of anti-angiogenic therapies. Curr. Pharm. Design 2004, 10, 1439–1455. [Google Scholar]
- Choi, N.; Kim, S.-M.; Hong, K.S.; Cho, G.; Cho, J.-H.; Lee, C.; Ryu, E.K. The use of the fusion protein RGD-HSA-TIMP2 as a tumor targeting imaging probe for SPECT and PET. Biomaterials 2011, 32, 7151–7158. [Google Scholar]
- Zerda, A.D.L.; Liu, Z.; Bodapati, S.; Teed, R.; Vaithilingam, S.; Khuri-Yakub, B.T.; Chen, X.; Dai, H.; Gambhir, S.S. Ultrahigh sensitivity carbon nanotube agents for photoacoustic molecular imaging in living mice. Nano Lett 2010, 10, 2168–2172. [Google Scholar]
- Li, Z.; Huang, P.; Zhang, X.; Lin, J.; Yang, S.; Liu, B.; Gao, F.; Xi, P.; Ren, Q.; Cui, D. RGD-conjugated dendrimer-modified gold nanorods for in vivo tumor targeting and photothermal therapy. Mol. Pharm 2009, 7, 94–104. [Google Scholar]
- Park, J.; Lee, J.J.; Jung, J.C.; Yu, D.Y.; Oh, C.; Ha, S.; Kim, T.J.; Chang, Y. Gd-DOTA conjugate of RGD as a potential tumor-targeting mri contrast agent. Chem Bio Chem 2008, 9, 2811–2813. [Google Scholar]
- Wu, Y.; Cai, W.; Chen, X. Near-infrared fluorescence imaging of tumor integrin αvβ3 expression with Cy7-labeled RGD multimers. Mol. Imaging Biol 2006, 8, 226–236. [Google Scholar]
- Kang, I.C.; Kim, D.S.; Jang, Y.; Chung, K.H. Suppressive mechanism of salmosin, a novel disintegrin in B16 melanoma cell metastasis. Biochem. Bioph. Res. Comm 2000, 275, 169–173. [Google Scholar]
- Ritchie, C.K.; Giordano, A.; Khalili, K. Integrin involvement in glioblastoma multiforme: Possible regulation by NF-κB. J. Cell Physiol 2000, 184, 214–221. [Google Scholar]
- Mitjans, F.; Meyer, T.; Fittschen, C.; Goodman, S.; Jonczyk, A.; Marshall, J.F.; Reyes, G.; Piulats, J. In vivo therapy of malignant melanoma by means of antagonists of αv integrins. Int. J. Cancer 2000, 87, 716–723. [Google Scholar]
- Anuradha, C.; Kanno, S.; Hirano, S. RGD peptide-induced apoptosis in human leukemia HL-60 cells requires caspase-3 activation. Cell Biol. Toxicol 2000, 16, 275–283. [Google Scholar]
- Chen, Y.; Xu, X.; Hong, S.; Chen, J.; Liu, N.; Underhill, C.B.; Creswell, K.; Zhang, L. RGD-Tachyplesin inhibits tumor growth. Cancer Res 2001, 61, 2434–2438. [Google Scholar]
- Aguzzi, M.S.; Giampietri, C.; de Marchis, F.; Padula, F.; Gaeta, R.; Ragone, G.; Capogrossi, M.C.; Facchiano, A. RGDS peptide induces caspase 8 and caspase 9 activation in human endothelial cells. Blood 2004, 103, 4180–4187. [Google Scholar]
- Chen, X.; Wang, J.; Fu, B.; Yu, L. RGD-containing peptides trigger apoptosis in glomerular mesangial cells of adult human kidneys. Biochem. Bioph. Res. Comm 1997, 234, 594–599. [Google Scholar]
- Eliceiri, B.P.; Cheresh, D.A. The role of alphav integrins during angiogenesis: Insights into potential mechanisms of action and clinical development. J. Clin. Invest 1999, 103, 1227–1230. [Google Scholar]
- Chavakis, E.; Riecke, B.; Lin, J.; Linn, T.; Bretzel, R.; Preissner, K.; Brownlee, M.; Hammes, H.P. Kinetics of integrin expression in the mouse model of proliferative retinopathy and success of secondary intervention with cyclic RGD peptides. Diabetologia 2002, 45, 262–267. [Google Scholar]
- Vachutinsky, Y.; Oba, M.; Miyata, K.; Hiki, S.; Kano, M.R.; Nishiyama, N.; Koyama, H.; Miyazono, K.; Kataoka, K. Antiangiogenic gene therapy of experimental pancreatic tumor by sFlt-1 plasmid DNA carried by RGD-modified crosslinked polyplex micelles. J. Control. Release 2011, 149, 51–57. [Google Scholar]
- Yonenaga, N.; Kenjo, E.; Asai, T.; Tsuruta, A.; Shimizu, K.; Dewa, T.; Nango, M.; Oku, N. RGD-based active targeting of novel polycation liposomes bearing siRNA for cancer treatment. J. Control. Release 2012, 160, 177–181. [Google Scholar]
- Orsi, S.; Guarnieri, D.; de Capua, A.; Netti, P.A. Gene-activated and cell-migration guiding PEG matrices based on three dimensional patterning of RGD peptides and DNA complexes. Acta Biomater 2012, 8, 3228–3240. [Google Scholar]
- Zhan, C.; Meng, Q.; Li, Q.; Feng, L.; Zhu, J.; Lu, W. Cyclic RGD-polyethylene glycol-polyethylenimine for intracranial glioblastoma-targeted gene delivery. Chem. Asian J 2012, 7, 91–96. [Google Scholar]
- Sakae, M.; Ito, T.; Yoshihara, C.; Iida-Tanaka, N.; Yanagie, H.; Eriguchi, M.; Koyama, Y. Highly efficient in vivo gene transfection by plasmid/PEI complexes coated by anionic PEG derivatives bearing carboxyl groups and RGD peptide. Biomed. Pharmacother 2008, 62, 448–453. [Google Scholar]
- Hood, J.D.; Bednarski, M.; Frausto, R.; Guccione, S.; Reisfeld, R.A.; Xiang, R.; Cheresh, D.A. Tumor regression by targeted gene delivery to the neovasculature. Science 2002, 296, 2404–2407. [Google Scholar]
- Jiang, J.; Yang, S.; Wang, J.; Yang, L.; Xu, Z.; Yang, T.; Liu, X.; Zhang, Q. Sequential treatment of drug-resistant tumors with RGD-modified liposomes containing siRNA or doxorubicin. Eur. J. Pharm. Biopharm 2010, 76, 170–178. [Google Scholar]
- Kibria, G.; Hatakeyama, H.; Ohga, N.; Hida, K.; Harashima, H. Dual-ligand modification of PEGylated liposomes shows better cell selectivity and efficient gene delivery. J. Control. Release 2011, 153, 141–148. [Google Scholar]
- Samanta, S.; Sistla, R.; Chaudhuri, A. The use of RGDGWK-lipopeptide to selectively deliver genes to mouse tumor vasculature and its complexation with p53 to inhibit tumor growth. Biomaterials 2010, 31, 1787–1797. [Google Scholar]
- Leng, Q.; Mixson, A.J. Modified branched peptides with a histidine-rich tail enhance in vitro gene transfection. Nucleic Acids Res. 2005, 33. [Google Scholar] [CrossRef]
- Przystal, J.M.; Umukoro, E.; Stoneham, C.A.; Yata, T.; O’Neill, K.; Syed, N.; Hajitou, A. Proteasome inhibition in cancer is associated with enhanced tumor targeting by the adeno-associated virus/phage. Mol. Oncol 2013, 7, 55–66. [Google Scholar]
- Katayama, K.; Furuki, R.; Yokoyama, H.; Kaneko, M.; Tachibana, M.; Yoshida, I.; Nagase, H.; Tanaka, K.; Sakurai, F.; Mizuguchi, H. Enhanced in vivo gene transfer into the placenta using RGD fiber-mutant adenovirus vector. Biomaterials 2011, 32, 4185–4193. [Google Scholar]
- Meyer, A.; Auernheimer, J.; Modlinger, A.; Kessler, H. Targeting RGD recognizing integrins: Drug development, biomaterial research, tumor imaging and targeting. Curr. Pharm. Design 2006, 12, 2723–2747. [Google Scholar]
- Zhu, S.; Qian, L.; Hong, M.; Zhang, L.; Pei, Y.; Jiang, Y. RGD-modified PEG-PAMAM-DOX conjugate: In vitro and in vivo targeting to both tumor neovascular endothelial cells and tumor cells. Adv. Mater 2011, 23, H84–H89. [Google Scholar]
- Wang, Z.; Lee, T.Y.; Ho, P.C. A novel dextran-oleate-cRGDfK conjugate for self-assembly of nanodrug. Nanomed. Nanotechnol 2012, 8, 194–203. [Google Scholar]
- Xu, Q.; Liu, Y.; Su, S.; Li, W.; Chen, C.; Wu, Y. Anti-tumor activity of paclitaxel through dual-targeting carrier of cyclic RGD and transferrin conjugated hyperbranched copolymer nanoparticles. Biomaterials 2012, 33, 1627–1639. [Google Scholar]
- Zhang, L.; Zhu, S.; Qian, L.; Pei, Y.; Qiu, Y.; Jiang, Y. RGD-modified PEG-PAMAM-DOX conjugates: In vitro and in vivo studies for glioma. Eur. J. Pharm. Biopharm 2011, 79, 232–240. [Google Scholar]
- Zhang, Y.; Wang, J.; Bian, D.; Zhang, X.; Zhang, Q. Targeted delivery of RGD-modified liposomes encapsulating both combretastatin A-4 and doxorubicin for tumor therapy: In vitro and in vivo studies. Eur. J. Pharm. Biopharm 2010, 74, 467–473. [Google Scholar]
- Naik, S.; Patel, D.; Chuttani, K.; Mishra, A.K.; Misra, A. In vitro mechanistic study of cell death and in vivo performance evaluation of RGD grafted PEGylated docetaxel liposomes in breast cancer. Nanomed. Nanotechnol 2012, 8, 951–962. [Google Scholar]
- Yue, W.; Sun, Q.; Landreneau, R.; Wu, C.; Siegfried, J.M.; Yu, J.; Zhang, L. Fibulin-5 suppresses lung cancer invasion by inhibiting matrix metalloproteinase-7 expression. Cancer Res 2009, 69, 6339–6346. [Google Scholar]
- Yan, J.H.; Yang, G.W.; Wang, J.P.; Wu, N.; Zhuang, G.H. Gene expression and activity analysis of a novel fusion protein (RGD) 3/tTF. Chin. J. Biotechnol 2007, 23, 409–413. [Google Scholar]
- Tugulu, S.; Silacci, P.; Stergiopulos, N.; Klok, H.A. RGD—Functionalized polymer brushes as substrates for the integrin specific adhesion of human umbilical vein endothelial cells. Biomaterials 2007, 28, 2536–2546. [Google Scholar]
- Tanabe, N.; Wheal, B.D.; Kwon, J.; Chen, H.H.; Shugg, R.; Sims, S.M.; Goldberg, H.A.; Dixon, S.J. Osteopontin signals through calcium and nuclear factor of activated T cells (NFAT) in osteoclasts: A novel RGD-dependent pathway promoting cell survival. J. Biol. Chem 2011, 286, 39871–39881. [Google Scholar]
- García, A.J. Get a grip: Integrins in cell-biomaterial interactions. Biomaterials 2005, 26, 7525–7529. [Google Scholar]
- Shachar, M.; Tsur-Gang, O.; Dvir, T.; Leor, J.; Cohen, S. The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering. Acta Biomater 2011, 7, 152–162. [Google Scholar]
- Zheng, W.; Wang, Z.; Song, L.; Zhao, Q.; Zhang, J.; Li, D.; Wang, S.; Han, J.; Zheng, X.-L.; Yang, Z. Endothelialization and patency of RGD-functionalized vascular grafts in a rabbit carotid artery model. Biomaterials 2012, 33, 2880–2891. [Google Scholar]
- Zhang, H.; Lin, C.Y.; Hollister, S.J. The interaction between bone marrow stromal cells and RGD-modified three-dimensional porous polycaprolactone scaffolds. Biomaterials 2009, 30, 4063–4069. [Google Scholar]
- Tocce, E.; Liliensiek, S.; Broderick, A.; Jiang, Y.; Murphy, K.; Murphy, C.; Lynn, D.; Nealey, P. The influence of biomimetic topographic features and the extracellular matrix peptide RGD on human corneal epithelial contact guidance. Acta Biomater 2013, 9, 5040–5051. [Google Scholar]
- Hennessy, K.M.; Clem, W.C.; Phipps, M.C.; Sawyer, A.A.; Shaikh, F.M.; Bellis, S.L. The effect of RGD peptides on osseointegration of hydroxyapatite biomaterials. Biomaterials 2008, 29, 3075–3083. [Google Scholar]
- Chen, J.; Bly, R.; Saad, M.; AlKhodary, M.; El-Backly, R.; Cohen, D.; Kattamis, N.; Fatta, M.; Moore, W.; Arnold, C. In vivo study of adhesion and bone growth around implanted laser groove/RGD-functionalized Ti-6Al-4V pins in rabbit femurs. Mat. Sci. Eng. C 2011, 31, 826–832. [Google Scholar]
- Wohlrab, S.; Müller, S.; Schmidt, A.; Neubauer, S.; Kessler, H.; Leal-Egaña, A.; Scheibel, T. Cell adhesion and proliferation on RGD-modified recombinant spider silk proteins. Biomaterials 2012, 33, 6650–6659. [Google Scholar]
- Patra, C.; Ricciardi, F.; Engel, F.B. The functional properties of nephronectin: An adhesion molecule for cardiac tissue engineering. Biomaterials 2012, 33, 4327–4335. [Google Scholar]
- Jo, D.H.; Lee, T.G.; Kim, J.H. Nanotechnology and nanotoxicology in retinopathy. Int. J. Mol. Sci 2011, 12, 8288–8301. [Google Scholar]
- Gil, E.S.; Mandal, B.B.; Park, S.-H.; Marchant, J.K.; Omenetto, F.G.; Kaplan, D.L. Helicoidal multi-lamellar features of RGD-functionalized silk biomaterials for corneal tissue engineering. Biomaterials 2010, 31, 8953–8963. [Google Scholar]




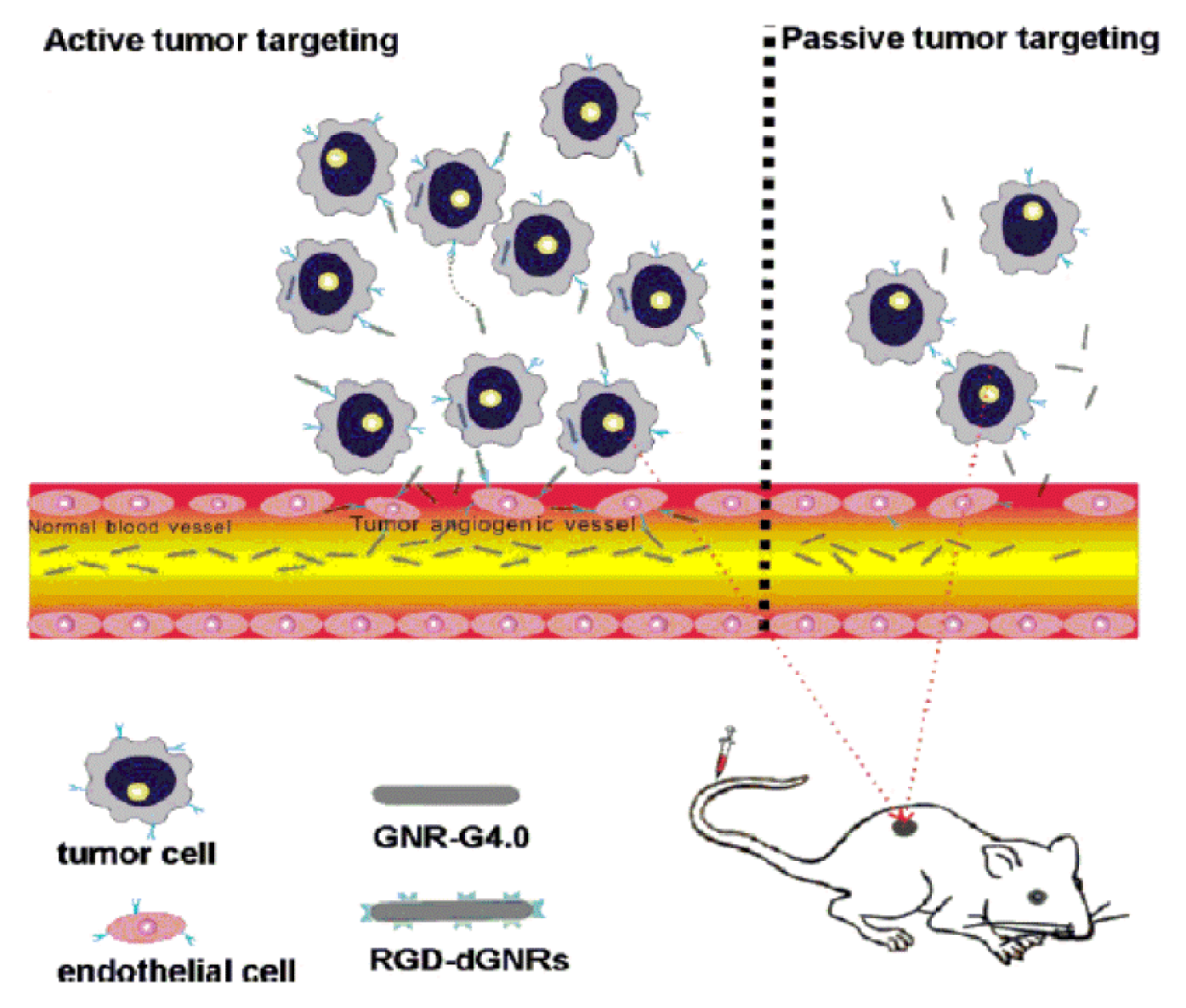
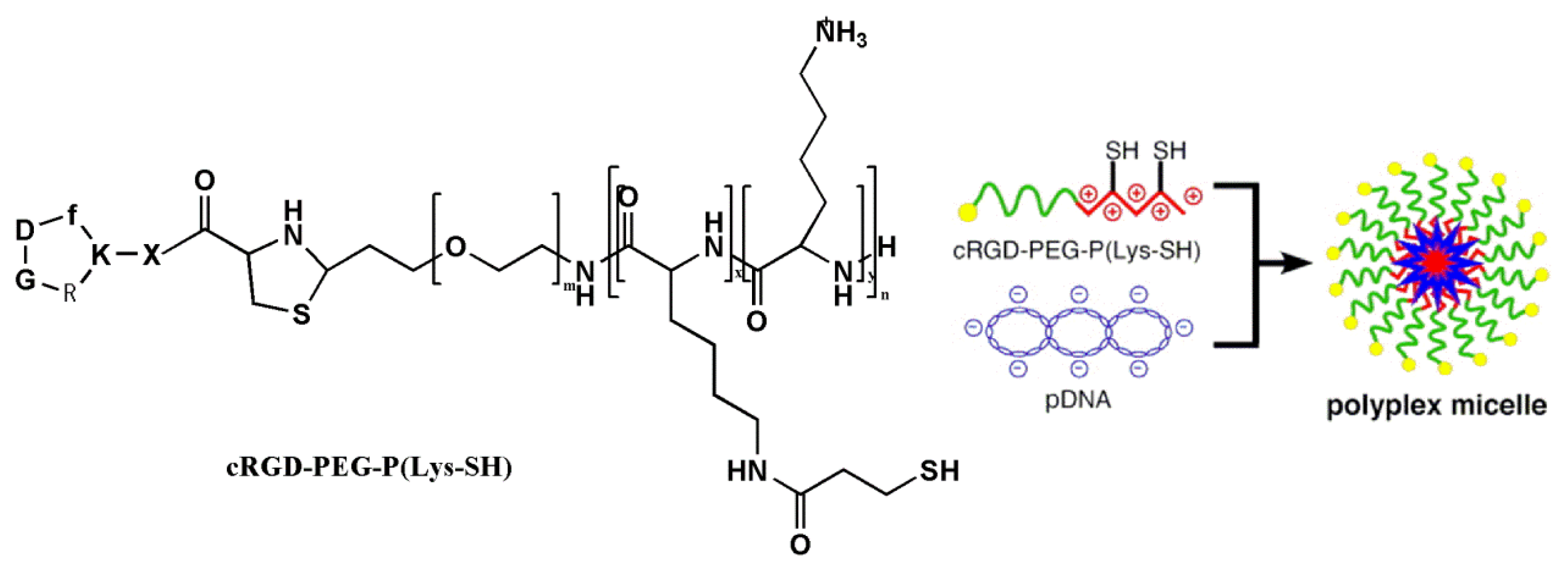
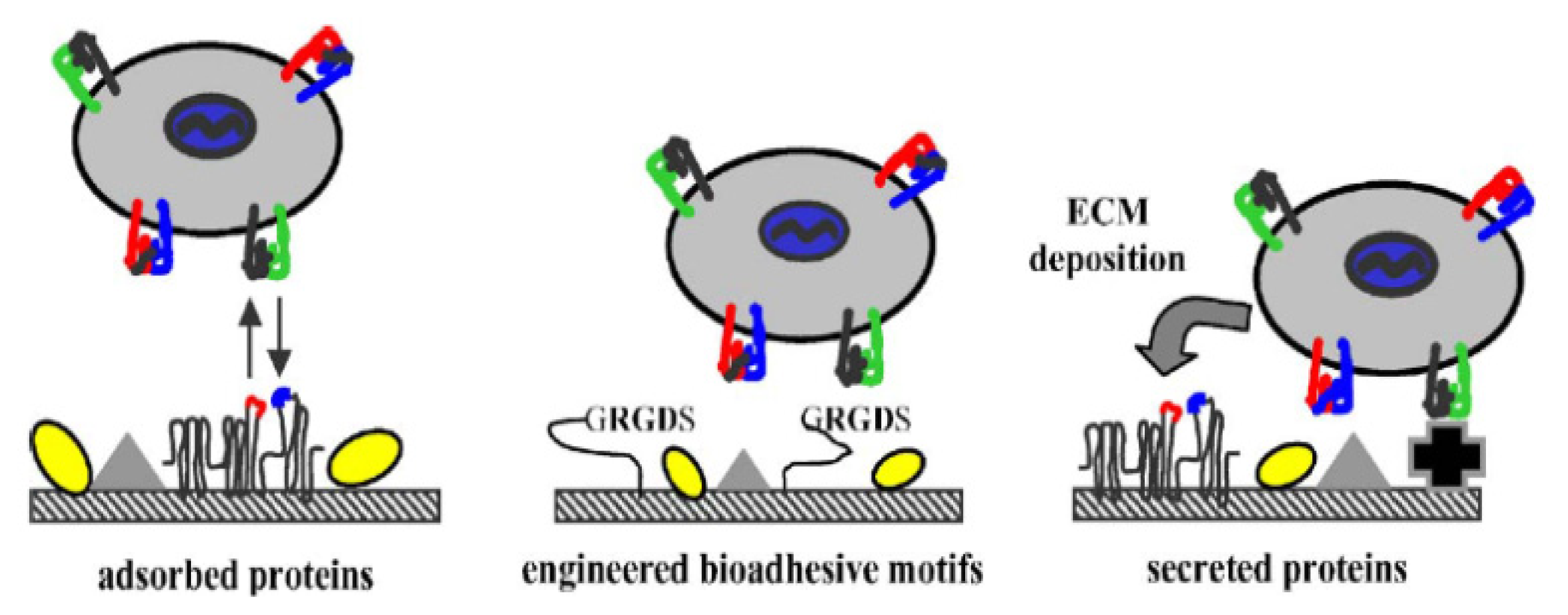
| Carriers | Production | Gene | Experimental model |
|---|---|---|---|
| Polymer | RGD-PEG-PCL | siRNA | B16F10-luc2 lung metastatic [36] |
| RGD-PEG | PEG-PEI/DNA complexes | NIH3T3 cells [37] | |
| RGD-PEG-PEI | Plasmid DNA | Intracranial glioblastoma [38] | |
| RGD-PEG-Suc | Plasmid DNA coding luciferase | Melanoma cell [39] | |
| Lipid | Polymerized lipid nanoparticle | ATP-Raf | M21-L/CT26 colon carcinoma [40] |
| Liposomes | RGD-SSL-DOX liposomes | RGD-Lipo-siRNA(MDR1) | Breast cancer MCF7/A cells [41] |
| RGD-PEGylated liposomes | siRNA | Pigment epithelial cells [42] | |
| Peptides | RGDGWK-lipopeptde | Anti-cancer p53 gene | B16F10 tumor [43] |
| RGD-HK-branched peptides | siLacZ, siLuciferase | MDA-MB-435c, MCF7 [44] | |
| Production | Gene | Experimental model |
|---|---|---|
| RGD4C/AAV/phage hybrid | GFP or Luc reporter genes | Human M21 Melanoma Human U87 glioblastoma [45] |
| Ad/CD-PEG500-RGD | shRNA | Various cancer cells [3] |
| (Adenovirus) Ad-RGD | Firefly luciferase gene | Mouse TS cells [46] |
| Category | Drug | Production | Experimental model |
|---|---|---|---|
| small molecule drugs | Paclitaxel | PTX-RGD/Tf-NPs (nanoparticles) | HeLa cells [50] |
| Doxorubicin | RGD-PEG-PAMAM-DOX | C6 glioma cells [51] | |
| Combretastatin A-4 (CA-4) and doxorubicin (Dox) | RGD-CA-4 and Dox liposomes | B16 and B16F10 melanoma cells [52] | |
| Docetaxel | RGD-PEG-LP-DC (Liposomes) | BT-20 and MDA-MB-231 cells [53] | |
| therapeutic proteins and peptides | Fibulin-5 | RGD-fibulin-5 | A549, H1299 and H460 cells [54] |
| tTf | (RGD)3/tTF | H460 lung cancer cells [55] | |
| The angiogenic factor Del1 | RGD-Mediated angiogenic factor | Human umbilical vein endothelial cells [56] | |
| Osteopontin | RGD-containing Osteopontin | Avian osteoclast-like cells [57] |
| Category | Biomaterials | Compound | Experimental model |
|---|---|---|---|
| Polymer | Alginate scaffolds | RGD-immobilized alginate scaffolds | Cardiac cell [59] |
| PCL (polycaprolactone) | RGD-PCL | Vascular grafts rabbit carotid artery [60] | |
| PCL | RGD-modified 3D-PCL | Bone marrow stromal cells [61] | |
| Poly (ethylene imine)-poly(2-vinyl-4,4-dimethylazlactone) | RGD-PEI/PVDMA | Human corneal epithelial cell [62] | |
| Inorganic materials | Hydroxyapatite (HA) | RGD-coated HA | Rat tibiae [63] |
| Ti6-Al-4V pins | RGD-coated Ti6-Al-4V pins | Rabbit femurs [64] | |
| Proteins | Spider silk | RGD-modified spider silk | BALB/3T3 mouse fibroblasts [65] |
| Nephronectin | RGD nephronectin | Cardiomyocytes [66] |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, F.; Li, Y.; Shen, Y.; Wang, A.; Wang, S.; Xie, T. The Functions and Applications of RGD in Tumor Therapy and Tissue Engineering. Int. J. Mol. Sci. 2013, 14, 13447-13462. https://doi.org/10.3390/ijms140713447
Wang F, Li Y, Shen Y, Wang A, Wang S, Xie T. The Functions and Applications of RGD in Tumor Therapy and Tissue Engineering. International Journal of Molecular Sciences. 2013; 14(7):13447-13462. https://doi.org/10.3390/ijms140713447
Chicago/Turabian StyleWang, Fen, Yuanyuan Li, Yingqiang Shen, Anming Wang, Shuling Wang, and Tian Xie. 2013. "The Functions and Applications of RGD in Tumor Therapy and Tissue Engineering" International Journal of Molecular Sciences 14, no. 7: 13447-13462. https://doi.org/10.3390/ijms140713447
APA StyleWang, F., Li, Y., Shen, Y., Wang, A., Wang, S., & Xie, T. (2013). The Functions and Applications of RGD in Tumor Therapy and Tissue Engineering. International Journal of Molecular Sciences, 14(7), 13447-13462. https://doi.org/10.3390/ijms140713447





