Inhibition Effects of a Synthesized Novel 4-Aminoantipyrine Derivative on the Corrosion of Mild Steel in Hydrochloric Acid Solution together with Quantum Chemical Studies
Abstract
:1. Introduction
2. Results and Discussion
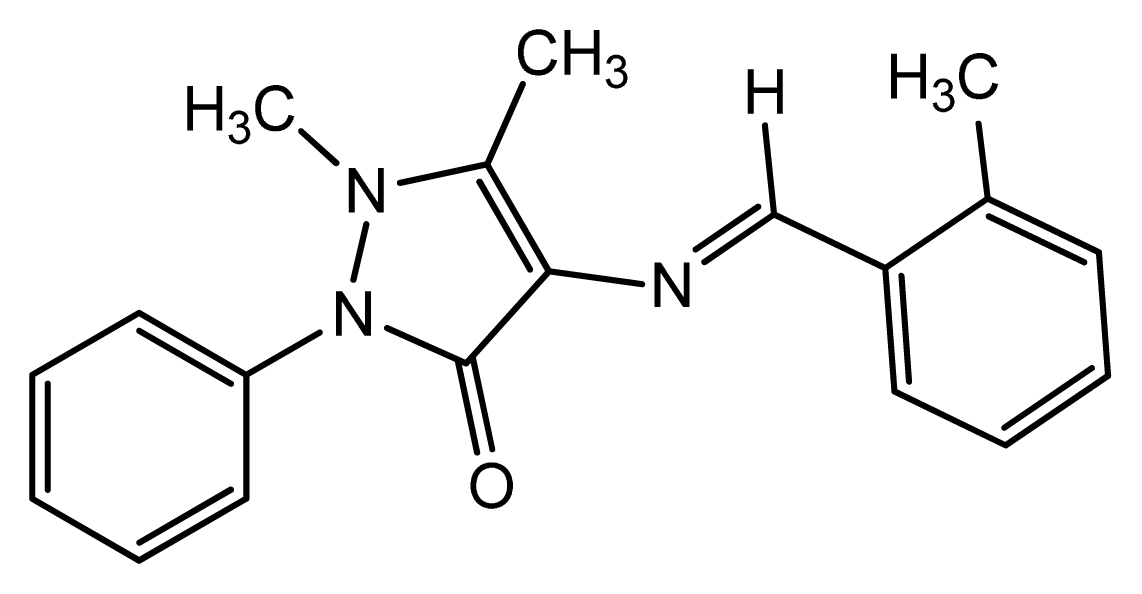
2.1. Chemistry
2.2. Electrochemical
Electrochemical Impedance Spectroscopy (EIS) Measurements
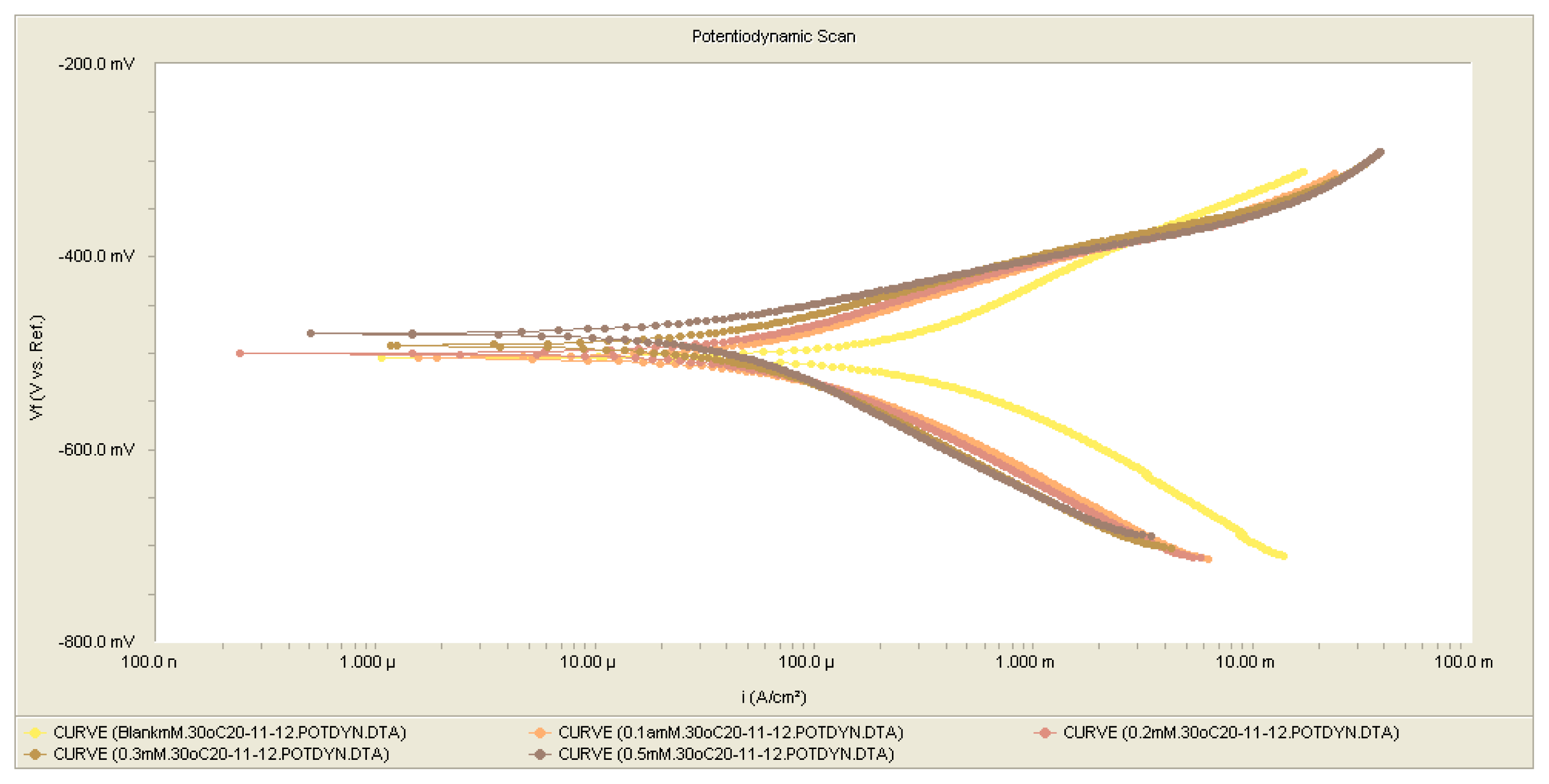
2.3. Polarization Measurements
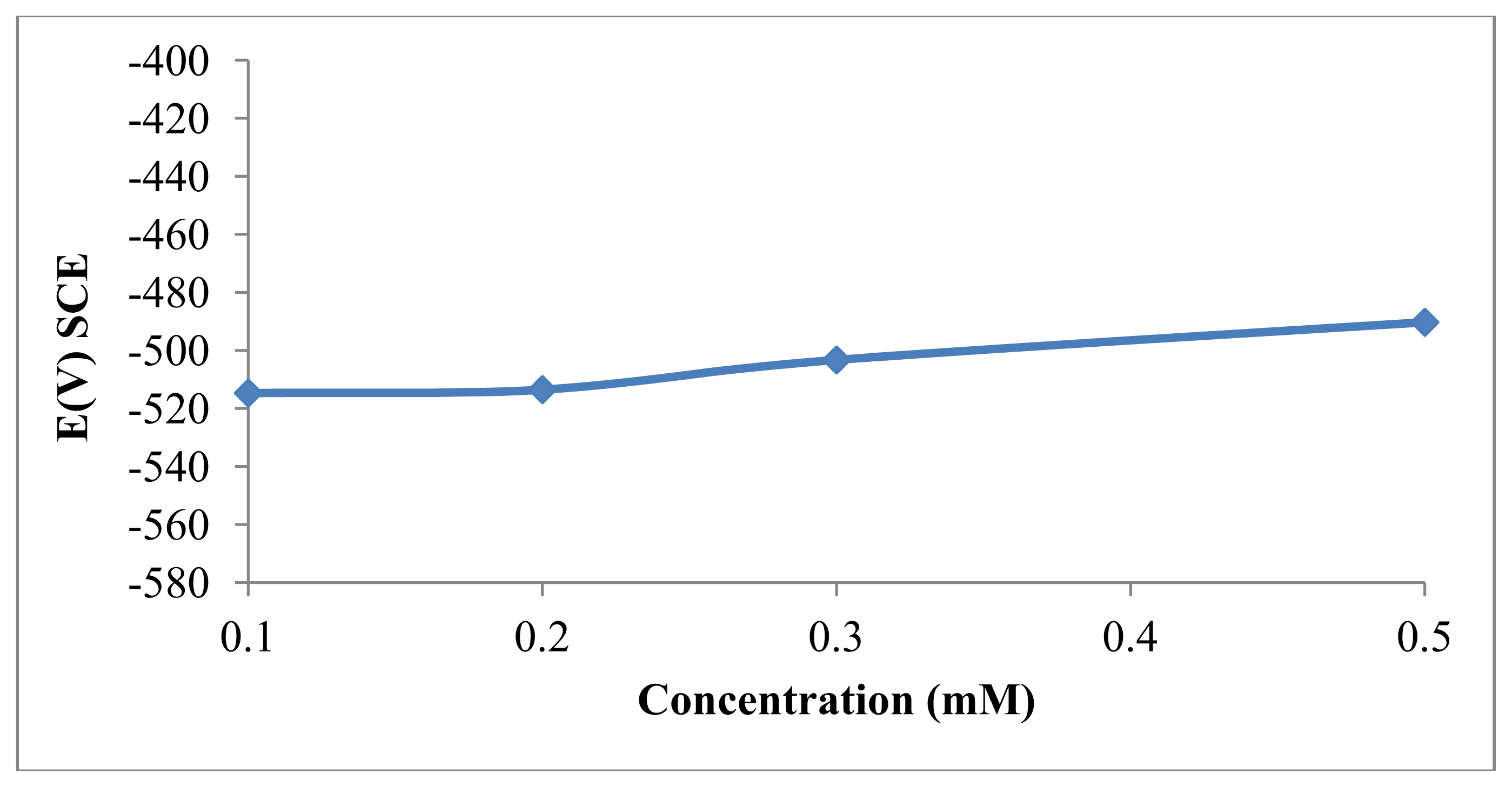
2.3.1. Open Circuit Potential (OCP) Measurements
2.3.2. Electrochemical Frequency Modulation Measurement
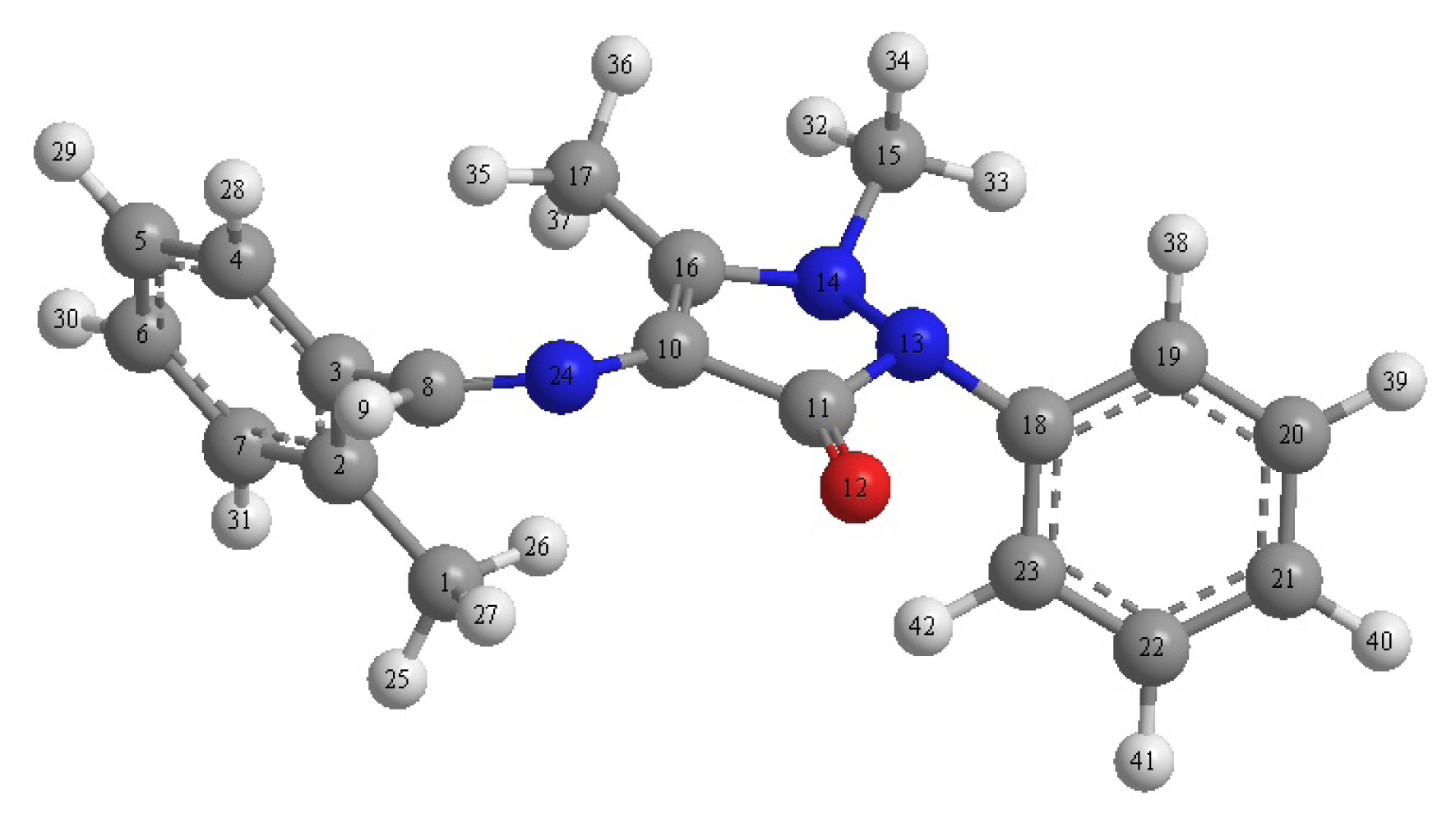
2.3.3. Quantum Chemical Calculations
3. Experimental Section
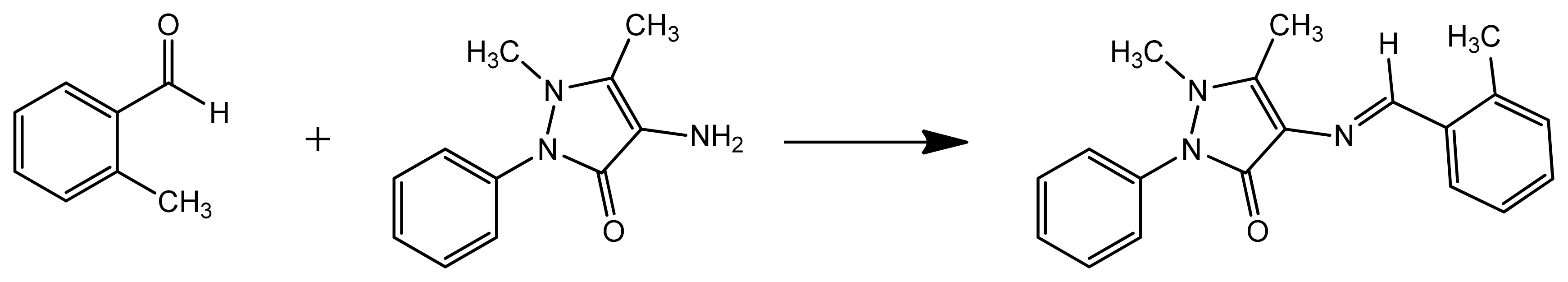
3.1. Synthesis of Corrosion Inhibitor DMPO
3.2. Electrochemical Measurements
3.3. Theory and Computational Detail
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Roberge, P.R. Handbook of Corrosion Engineering; McGraw-Hill Professional Publishing: New York, NY, USA, 1999; pp. 1–3. [Google Scholar]
- Sing, D.N.; Dey, A.K. Synergistic effects of inorganic and organic cations on inhibitive performance of propargyl alcohol on steel dissolution in boiling hydrochloric acid solution. Corrosion 1993, 49, 594–600. [Google Scholar]
- Banerjee, G.; Malhotra, S.N. Contribution to adsorption of aromatic amines on mild steel surface from HCl solutions by impedance, UV, and raman spectroscopy. Corrosion 1992, 48, 10–15. [Google Scholar]
- Arab, S.T.; Noor, E.A. Inhibition of acid corrosion of steel by some S- Alkylisothiouronium. Corrosion 1993, 49, 122–129. [Google Scholar]
- Khaled, F.H. Investigation of the inhibitive effect of ortho-substituted on corrosion of iron in 0.5 M H2SO4 solutions. Mater. Chem. Phys 2003, 82, 949–960. [Google Scholar]
- Lin, W. Inhibiting effect of 2-mercaptopyrimidine on the corrosion of a low carbon steel in phosphoric acid. Corros. Sci 2001, 43, 1637–1644. [Google Scholar]
- Shorky, H.; Yuasa, M.; Sekine, I.; Issa, R.M.; El-Baradie, H.Y.; Gomma, G.K. Corrosion inhibition of mild steel by schiff base compounds in various aqueous solutions. Corros. Sci 1998, 40, 2173–2186. [Google Scholar]
- Abdallah, M. Rhodanine azosulpha drugs as corrosion inhibitor. Corros. Sci 2002, 44, 717–728. [Google Scholar]
- Prabhu, R.A.; Venkatesha, T.V.; Shanbhag, A.V.; Kulkarni, G.M.; Kalkhambkar, R.G. Inhibition effects of some Schiff’s bases on the corrosion of mild steel in hydrochloric acid solution. Corros. Sci 2008, 50, 3356–3362. [Google Scholar]
- Prabhu, R.A.; Shanbhag, A.V.; Venkatesha, T.V. Influence of tramadol [2-[(dimethylamino)methyl]-1-(3-methoxyphenylcyclohexanolhydrate] on corrosion inhibition of mild steel in acidic media. J. Appl. Electrochem 2007, 37, 491–497. [Google Scholar]
- El-Naggar, M. Corrosion inhibition of mild steel in acidic medium by some sulfa drugs compounds. Corros. Sci 2007, 49, 2226–2236. [Google Scholar]
- Obot, I.B.; Obi-Egbedi, N.O.; Umoren, S.A. Drugs as corrosion inhibitors for aluminium in 0.1 M HCl. Corros. Sci 2009, 51, 1868–1875. [Google Scholar]
- Shukla, S.K.; Quraishi, M.A. 4-Substituted anilinomethylpropionate: New and efficient. Corrosion inhibitors for mild steel. Corros. Sci 2009, 51, 1007–1011. [Google Scholar]
- Shukla, S.K.; Singh, A.K.; Ahamad, I.; Quraishi, M.A. Drug as corrosion inhibitor for mild steel in hydrochloric acid solution. Mater. Lett 2009, 63, 819–822. [Google Scholar]
- Al-Amiery, A.A.; Al-Bayati, R.I.; Saed, F.M.; Ali, W.B.; Kadhum, A.H.; Mohamad, A.B. Novel pyranopyrazoles: Synthesis and theoretical studies. Molecules 2012, 17, 10377–10389. [Google Scholar]
- Kadhum, A.A.H.; Mohamad, A.; Al-Amiery, A.A. Antimicrobial and anti-oxidant activities of new metal complexes derived from 3-aminocoumarin. Molecules 2011, 16, 6969–6984. [Google Scholar]
- Kadhum, A.A.H.; Al-Amiery, A.A.; Shikara, M.; Mohamad, A.; Al-Bayati, R. Synthesis, structure elucidation and DFT studies of new thiadiazoles. Int. J. Phys. Sci 2012, 6, 6692–6697. [Google Scholar]
- Al-Amiery, A.A. Synthesis and antioxidant, antimicrobial evaluation, DFT studies of novel metal complexes derivate from Schiff base. Res. Chem. Intermediat 2012, 38, 745–759. [Google Scholar]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A. Antifungal activities of new coumarins. Molecules 2012, 17, 5713–5723. [Google Scholar]
- Junaedi, S.; Kadhum, A.A.H.; Al-Amiery, A.A.; Mohamad, A.; Takriff, M. Synthesis andcharacterization of novel corrosion inhibitor derived from oleic acid: 2-Amino 5-Oleyl-1,3,4Thiadiazol (AOT). Int. J. Electrochem. Sci 2012, 7, 3543–3554. [Google Scholar]
- Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A.B.; Daud, A.R.; Takriff, M.S.; Kamarudin, S.K. A comparative study of the corrosion inhibition of mild steel in sulphuric acid by 4,4-dimethyloxazolidine-2-thione. Corros. Sci 2009, 51, 2393–2399. [Google Scholar]
- Hleli, S.; Abdelghani, A.; Tlili, A. Impedance spectroscopy technique for DNA hybridization. Sensors 2003, 3, 472–479. [Google Scholar]
- Wang, Z. The inhibition effect of Bis-Benzimidazole compound for mild steel in 0.5 M HCl solution. Int. J. Electrochem. Sci 2012, 7, 11149–11160. [Google Scholar]
- Ramesh Saliyan, V.; Adhikari, A.V. Quinolin-5-ylmethylene-3-{[8-(trifluoromethyl)quinolin-4- yl]thio}propanohydrazide as an effective inhibitor of mild steel corrosion in HCl solution. Corros. Sci 2008, 50, 55–61. [Google Scholar]
- Liu, F.G.; Du, M.; Zhang, J.; Qiu, M. Electrochemical behavior of Q235 steel in saltwater saturated with carbon dioxide based on new imidazoline derivative inhibitor. Corros. Sci 2009, 51, 102–109. [Google Scholar]
- De Souza, F.S. Caffeic acid as a green corrosion inhibitor for mild steel. Corros. Sci 2009, 51, 642–649. [Google Scholar]
- Hermas, A.A.; Morad, M.S. A comparative study on the corrosion behaviour of 304 austenitic stainless steel in sulfamic and sulfuric acid solutions. Corros. Sci 2008, 50, 2710–2717. [Google Scholar]
- Arslan, T.; Kandemirli, F.; Ebens, E.; Love, I.; Alemu, H. Quantum chemical studies on the corrosion inhibition of some sulphonamides on mild steel in acidic medium. Corros. Sci 2009, 51, 35–47. [Google Scholar]
- Obi-Egbedi, N.O. Inhibitive properties, thermodynamic and quantum chemical studies of alloxazine on mild steel corrosion in H2SO4. Corros. Sci 2011, 53, 263–275. [Google Scholar]
- Al-Amiery, A.A.; Al-Bayati, R.; Saour, K.; Radi, M. Cytotoxicity, antioxidant and antimicrobial activities of novel 2-quinolone derivatives derived from coumarins. Res. Chem. Intermediat 2012, 38, 559–569. [Google Scholar]
- Al-Amiery, A.A.; Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A. The antioxidant activity of new coumarin derivatives. Int. J. Mol. Sci 2011, 12, 5757–5761. [Google Scholar]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A. Antifungal and antioxidant activities of pyrrolidonethiosemicarbazone complexes. Bioinorg. Chem. Appl 2012, 2012, 1–5. [Google Scholar]
- Al-Amiery, A.A.; Al-Majedy, Y.; Abdulreazak, H.; Abood, H. Synthesis, characterization, theoretical crystal structure, and antibacterial activities of some transition metal complexes of the thiosemicarbazone. Bioinorg. Chem. Appl 2011, 2011, 1–6. [Google Scholar]
- Al-Amiery, A.A. Antimicrobial and antioxidant activities of new metal complexes derived from (E)-3-((5-phenyl-1,3,4-oxadiazol-2-ylimino)methyl)naphthalen-2-ol. Med. Chem. Res 2012, 21, 3204–3213. [Google Scholar]
- Kandemirli, F.; Sagdinc, S. Theoretical study of corrosion inhibition of amides and thiosemicarbazones. Corros. Sci 2007, 49, 2118–2130. [Google Scholar]
- Al-Amiery, A.A.; Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A. The use of umbelliferone in the synthesis of new heterocyclic compounds. Molecules 2011, 16, 6833–6843. [Google Scholar]
- Costa, J.M.; Lluch, J.M. The use of quantum mechanics calculations for the study of corrosion inhibitors. Corros. Sci 1984, 24, 924–933. [Google Scholar]
- Khalil, N. Quantum chemical approach of corrosion inhibition. Electrochim. Acta 2003, 48, 2635–2640. [Google Scholar]
- Bereket, G.; Ogretir, C.; Yurt, A. Quantum mechanical calculations on some 4-methyl-5-substituted imidazole derivatives as acidic corrosion inhibitor for zinc. J. Mol. Struct. (Theochem.) 2001, 571, 139–145. [Google Scholar]
- Xia, S.; Qiu, M.; Yu, L.; Liu, F.; Zhao, H. Molecular dynamics and density functional theory study on relationship between structure of imidazoline derivatives and inhibition performance. Corros. Sci 2008, 50, 2021–2029. [Google Scholar]
- Musa, A.Y.; Kadhum, A.H.; Mohamad, A.B.; Rahoma, A.B.; Mesmari, H. Electrochemical and quantum chemical calculations on 4,4-dimethyloxazolidine-2-thione as inhibitor for mild steel corrosion in hydrochloric acid. J. Mol. Struct 2010, 969, 233–327. [Google Scholar]
- Obot, I.B.; Ebenso, E.E.; Akpan, I.A.; Gasem, Z.M.; Afolabi Ayo, S. 119Thermodynamic and density functional theory investigation of sulphathiazole as green corrosion inhibitor at mild steel/hydrochloric acid interface. Int. J. Electrochem. Sci 2012, 7, 1978–1996. [Google Scholar]
- Obot, I.B.; Obi-Egbedi, N.O. Anti-corrosive properties of xanthone on mild steel corrosion in sulphuric acid: Experimental and theoretical investigations. Curr. Appl. Phys 2011, 11, 382–392. [Google Scholar]
- Obot, I.B.; Obi-Egbedi, N.O.; Eseola, A.O. Anticorrosion potential of 2-Mesityl-1H-imidazo[4,5- f][1,10]phenanthroline on mild steel in sulfuric acid solution: Experimental and theoretical study. Ind. Eng. Chem. Res 2011, 50, 2098–2110. [Google Scholar]
- Obot, I.B.; Obi-Egbedi, N.O. 1171Theoretical study of benzimidazole and its derivatives and their potential activity as corrosion inhibitors. Corros. Sci 2010, 52, 657–660. [Google Scholar]
- ASTM (American Society for Testing and Materials). Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens; ASTM: West Conshohocken, PA, USA, 2003. Available online: http://www.cosasco.com/documents/ASTM_G1_Standard_Practice.pdf (accessed on 11 March 2013).
- Al-Amiery, A.A.; Abdul, A.H.K.; Abu, B.M.; Sutiana, J. A novel hydrazinecarbothioamide as a potential corrosion inhibitor for mild steel in HCl. Materials 2013, 6, 1420–1431. [Google Scholar]
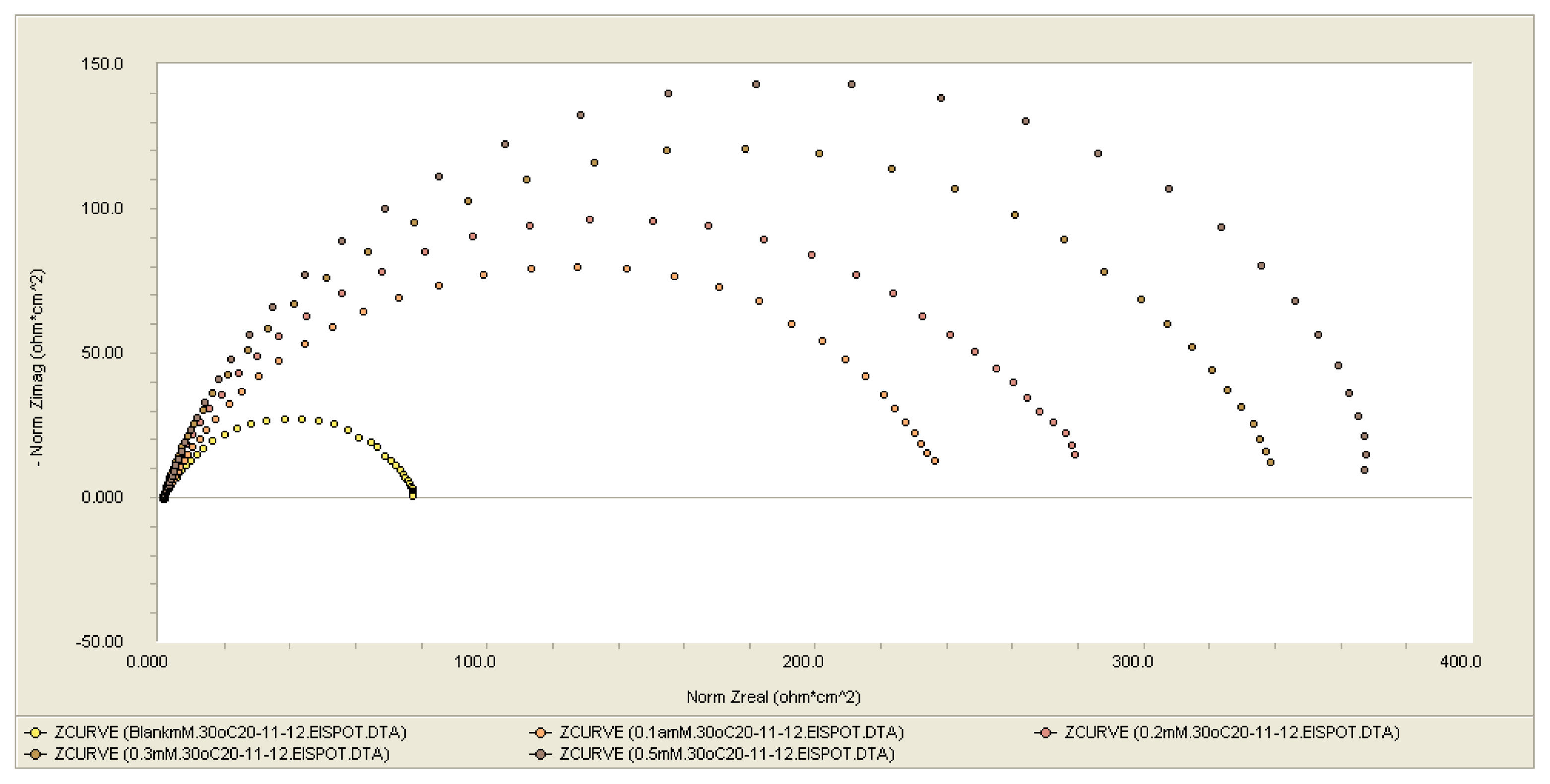


| Conc., 1 × 10−3M | Rs, ohm cm2 | Rct, ohm cm2 | CPEdl (Y0X10−5), ohm−1cm−2Sn | IE (%) |
|---|---|---|---|---|
| 0 | – | 77 | – | 0 |
| 0.1 | 1.55 | 239 | 39.4 | 71.08 |
| 0.2 | 1.56 | 259 | 22.2 | 70 |
| 0.3 | 1.63 | 328 | 17.7 | 77 |
| 0.5 | 1.73 | 376 | 23.3 | 80 |
| Conc., 1 × 10−3M | icorr (μA cm−2) | −Ecorr (mV vs. SCE) | βa (V dec−1) | βc (V dec−1) | IE% |
|---|---|---|---|---|---|
| 0 | 298 | 504 | 0.119 | 0.121 | 0 |
| 0.1 | 60 | 505 | 0.07 | 0.10 | 79.860 |
| 0.2 | 49 | 500 | 0.06 | 0.10 | 83.550 |
| 0.3 | 40.5 | 492 | 0.06 | 0.11 | 86.410 |
| 0.5 | 39.6 | 479 | 0.06 | 0.12 | 87.700 |
| Conc, mM | icorr, (μA·cm−2) | βa, (V·dec−1) | βc, (V·dec−1) | CR mmpy | IE (%) |
|---|---|---|---|---|---|
| 0 | 189.8 | 24.26e-3 | 27.00e-3 | 4.89 | 0 |
| 0.1 | 501.9 | 96.88e-3 | 152.5e-3 | 1.295 | 80 |
| 0.2 | 478.5 | 66.90e-3 | 173.2e-3 | 1.234 | 83 |
| 0.3 | 388.1 | 105.4e-3 | 152.4e-3 | 1.001 | 86 |
| 0.5 | 301.6 | 106.9e-3 | 120.8e-3 | 0.777 | 87 |
| Comp. | HOMO eV | LUMO eV | Band gap | Dipole moment | Total Energy | Conc. (mM) | IEExp% | IETheo% |
|---|---|---|---|---|---|---|---|---|
| DMPO | −8.051 | −1.593 | −6.458 | 1.4655 | 54.2342 Kcal/Mol | 0.1 | 80 | 67.30 |
| −8.051 | −1.593 | −6.458 | 1.4655 | 54.2342 Kcal/Mol | 0.5 | 87 | 84.26 |
| Atom | Charge | Atom | Charge | Atom | Charge | Atom | Charge |
|---|---|---|---|---|---|---|---|
| C(1) | −0.1893 | C(11) | 0.3262 | C(21) | −0.1205 | H(31) | 0.1352 |
| C(2) | −0.0376 | O(12) | −0.2802 | C(22) | −0.1268 | H(32) | 0.0969 |
| C(3) | −0.1087 | N(13) | −0.1996 | C(23) | −0.1147 | H(33) | 0.1058 |
| C(4) | −0.1179 | O(14) | −0.1130 | N(24) | −0.1022 | H(34) | 0.0774 |
| C(5) | −0.1377 | C(15) | −0.1202 | H(25) | 0.0865 | H(35) | 0.1126 |
| C(6) | −0.1181 | C(16) | 0.0037 | H(26) | 0.0922 | H(36) | 0.0946 |
| C(7) | −0.1366 | C(17) | −0.1875 | H(27) | 0.0972 | H(37) | 0.1073 |
| C(8) | −0.0139 | C(18) | 0.0272 | H(28) | 0.1370 | H(38) | 0.1420 |
| H(9) | 0.1550 | C(19) | −0.1048 | H(29) | 0.1351 | H(39) | 0.1344 |
| C(10) | −0.1499 | C(20) | −0.1332 | H(30) | 0.1344 | H(40) | 0.1341 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Junaedi, S.; Al-Amiery, A.A.; Kadihum, A.; Kadhum, A.A.H.; Mohamad, A.B. Inhibition Effects of a Synthesized Novel 4-Aminoantipyrine Derivative on the Corrosion of Mild Steel in Hydrochloric Acid Solution together with Quantum Chemical Studies. Int. J. Mol. Sci. 2013, 14, 11915-11928. https://doi.org/10.3390/ijms140611915
Junaedi S, Al-Amiery AA, Kadihum A, Kadhum AAH, Mohamad AB. Inhibition Effects of a Synthesized Novel 4-Aminoantipyrine Derivative on the Corrosion of Mild Steel in Hydrochloric Acid Solution together with Quantum Chemical Studies. International Journal of Molecular Sciences. 2013; 14(6):11915-11928. https://doi.org/10.3390/ijms140611915
Chicago/Turabian StyleJunaedi, Sutiana, Ahmed A. Al-Amiery, Abdulhadi Kadihum, Abdul Amir H. Kadhum, and Abu Bakar Mohamad. 2013. "Inhibition Effects of a Synthesized Novel 4-Aminoantipyrine Derivative on the Corrosion of Mild Steel in Hydrochloric Acid Solution together with Quantum Chemical Studies" International Journal of Molecular Sciences 14, no. 6: 11915-11928. https://doi.org/10.3390/ijms140611915
APA StyleJunaedi, S., Al-Amiery, A. A., Kadihum, A., Kadhum, A. A. H., & Mohamad, A. B. (2013). Inhibition Effects of a Synthesized Novel 4-Aminoantipyrine Derivative on the Corrosion of Mild Steel in Hydrochloric Acid Solution together with Quantum Chemical Studies. International Journal of Molecular Sciences, 14(6), 11915-11928. https://doi.org/10.3390/ijms140611915






