Chicken Cytochrome P450 1A5 Is the Key Enzyme for Metabolizing T-2 Toxin to 3'OH-T-2
Abstract
:1. Introduction
2. Results and Discussion
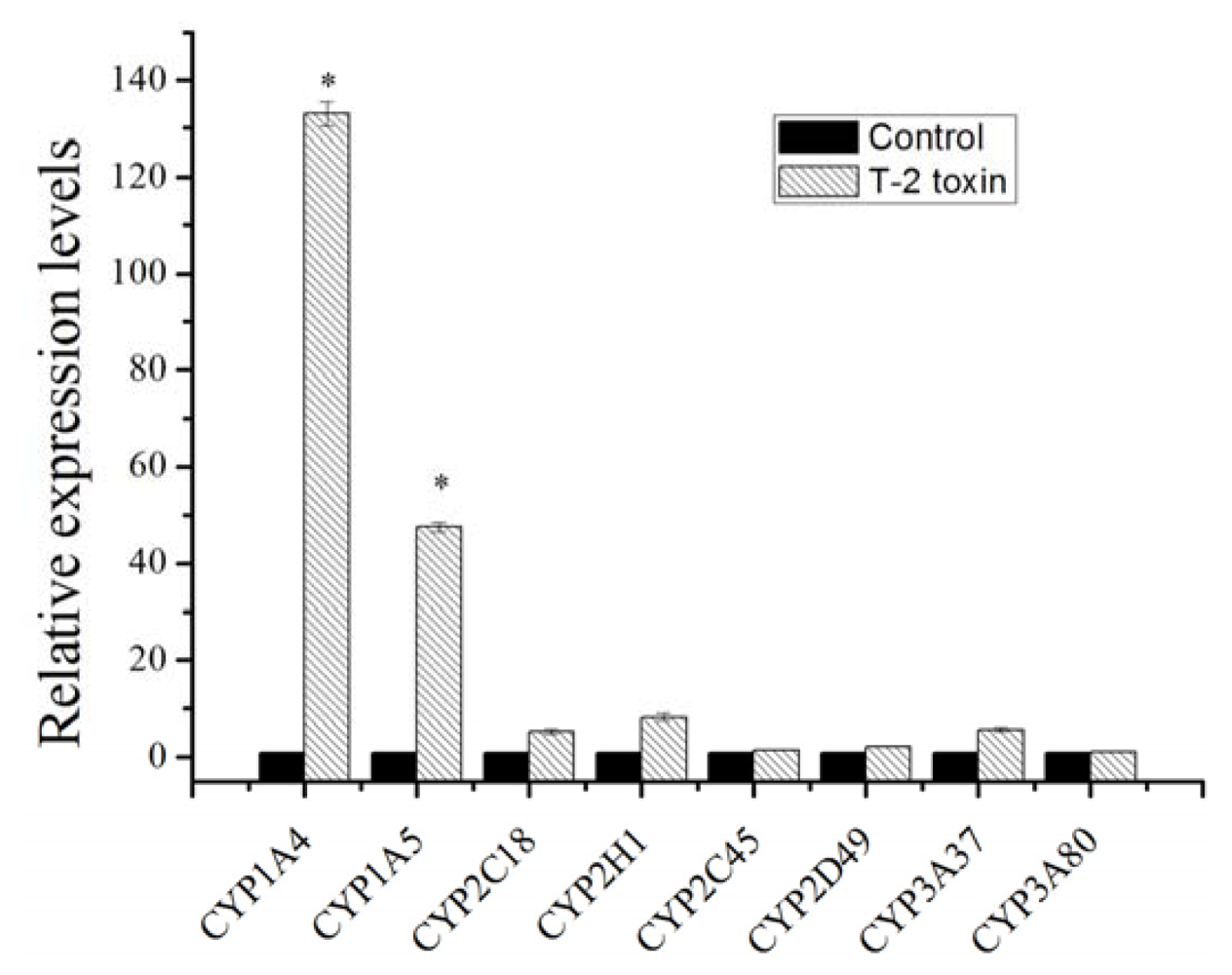
2.1. Expression Changes of Major Cytochrome P450 in Response to T-2 Exposure
2.2. The Catalytic Activity of S9 Fractions from HeLa-CYP1A4 and HeLa-CYP1A5
2.3. The Catalytic Activity and Secondary Structure of Recombinant CYP1A5
3. Experimental Section
3.1. Chicken Embryonic Hepatocytes Cell Isolation, Culture and Exposure to T-2
3.2. RNA Isolation and Quantitative Real-Time PCR Analysis
3.3. Vector Construction, Cell Culture, and Transfection and S9 Preparation
3.4. Enzyme Assay
3.5. Recombinant Protein Expression and Purification in Prokaryotes and Circular Dichroism (CD) Spectroscopy Detection
4. Conclusions
Supplementary Information
ijms-14-10809-s001.pdfAcknowledgments
Conflict of Interest
References
- Eriksen, G.S.; Alexander, J. Fusarium Toxins in Cereals: A Risk Assessment; Nordic Council of Ministers: Copenhagen, Denmark, 1998; Volume 502, pp. 7–44. [Google Scholar]
- Chi, M.; Robison, T.; Mirocha, C.; Behrens, J.; Shimoda, W. Transmission of radioactivity into eggs from laying hens (Gallus domesticus) administered tritium labeled T-2 toxin. Poult. Sci 1978, 57, 1234–1238. [Google Scholar]
- Chi, M.; Robison, T.; Mirocha, C.; Swanson, S.; Shimoda, W. Excretion and tissue distribution of radioactivity from tritium-labeled T-2 toxin in chicks. Toxicol. Appl. Pharmacol 1978, 45, 391–402. [Google Scholar]
- Robinson, T.; Mirocha, C.; Kurtz, H.; Behrens, J.; Chi, M.; Weaver, G.; Nystrom, S. Transmission of T-2 toxin into bovine and porcine milk. J. Dairy Sci 1979, 62, 637–641. [Google Scholar]
- Ueno, Y. Trichothecenes: Overview Address. In Mycotoxins in Human and Animal Health; Pathotox Publishers, Inc.: Park Forest South, IL, USA, 1977; pp. 189–207. [Google Scholar]
- Yoshizawa, T.; Sakamoto, T.; Ayano, Y.; Mirocha, C. 3′-Hydroxy T-2 and 3′-hydroxy HT-2 toxins: New metabolites of T-2 toxin, a trichothecene mycotoxin, in animals [lactating cow]. Agric. Biol. Chem 1982, 46, 2613–2615. [Google Scholar]
- Visconti, A.; Mirocha, C.J. Identification of various T-2 toxin metabolites in chicken excreta and tissues. Appl. Environ. Microbiol 1985, 49, 1246–1250. [Google Scholar]
- Yoshizawa, T.; Sakamoto, T.; Okamoto, K. In vitro formation of 3′-hydroxy T-2 and 3′-hydroxy HT-2 toxins from T-2 toxin by liver homogenates from mice and monkeys. Appl. Environ. Microbiol 1984, 47, 130–134. [Google Scholar]
- Wei, R.D.; Chu, F.S. Modification of in vitro metabolism of T-2 toxin by esterase inhibitors. Appl. Environ. Microbiol 1985, 50, 115–119. [Google Scholar]
- Ge, X.; Wang, J.; Liu, J.; Jiang, J.; Lin, H.; Wu, J.; Ouyang, M.; Tang, X.; Zheng, M.; Liao, M. The catalytic activity of cytochrome P450 3A22 is critical for the metabolism of T-2 toxin in porcine reservoirs. Catal. Commun 2010, 12, 71–75. [Google Scholar]
- Wang, J.; Jiang, J.; Zhang, H.; Cai, H.; Li, C.; Li, K.; Liu, J.; Guo, X.; Zou, G.; Wang, D.; et al. Integrated transcriptional and proteomic analysis with in vitro biochemical assay reveal the important role of CYP3A46 in T-2 toxin hydroxylation in porcine primary hepatocytes. Mol. Cell. Proteomics 2011, 10. [Google Scholar] [CrossRef]
- Bertz, R.J.; Granneman, G.R. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin. Pharmacokinet 1997, 32, 210–258. [Google Scholar]
- Mahajan, S.; Rifkind, A. Transcriptional activation of avian CYP1A4 and CYP1A5 by 2,3,7,8-tetrachlorodibenzo-p-dioxin: Differences in gene expression and regulation compared to mammalian CYP1A1 and CYP1A2. Toxicol. Appl. Pharmacol 1999, 155, 96–106. [Google Scholar]
- Gannon, M.; Gilday, D.; Rifkind, A.B. TCDD induces CYP1A4 and CYP1A5 in chick liver and kidney and only CYP1A4, an enzyme lacking arachidonic acid epoxygenase activity, in myocardium and vascular endothelium. Toxicol. Appl. Pharmacol 2000, 164, 24–37. [Google Scholar]
- Head, J.A.; Kennedy, S.W. Differential expression, induction, and stability of CYP1A4 and CYP1A5 mRNA in chicken and herring gull embryo hepatocytes. Comp. Biochem. Physiol. Toxicol. Pharmacol 2007, 145, 617–624. [Google Scholar]
- Wrighton, S.A.; VandenBranden, M.; Ring, B.J. The human drug metabolizing cytochromes P450. J. Pharmacokinet. Biopharm 1996, 24, 461–473. [Google Scholar]
- Kubota, A.; Iwata, H.; Goldstone, H.M.; Kim, E.Y.; Stegeman, J.J.; Tanabe, S. Cytochrome P450 1A4 and 1A5 in common cormorant (Phalacrocorax carbo): Evolutionary relationships and functional implications associated with dioxin and related compounds. Toxicol. Sci 2006, 92, 394–408. [Google Scholar]
- Orengo, C.A.; Michie, A.; Jones, S.; Jones, D.T.; Swindells, M.; Thornton, J.M. CATH—A hierarchic classification of protein domain structures. Structure 1997, 5, 1093–1109. [Google Scholar]
- Wang, J.F.; Zhang, C.C.; Chou, K.C.; Wei, D.Q. Structure of cytochrome p450s and personalized drug. Curr. Med. Chem 2009, 16, 232–244. [Google Scholar]
- Sansen, S.; Yano, J.K.; Reynald, R.L.; Schoch, G.A.; Griffin, K.J.; Stout, C.D.; Johnson, E.F. Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P450 1A2. J. Biol. Chem 2007, 282, 14348–14355. [Google Scholar]
- Scott, E.E.; Halpert, J.R. Structures of cytochrome P450 3A4. Trends Biochem. Sci. 2005, 30, 5–7. [Google Scholar]
- Williams, P.A.; Cosme, J.; Ward, A.; Angove, H.C.; Vinković, D.M.; Jhoti, H. Crystal structure of human cytochrome P450 2C9 with bound warfarin. Nature 2003, 424, 464–468. [Google Scholar]
- Sokolović, M.; Garaj-Vrhovac, V.; Šimpraga, B. T-2 toxin: incidence and toxicity in poultry. Arhiv. Za Higijenu Rada I Toksikologiju 2008, 59, 43–52. [Google Scholar]
- Gareis, M.; Bauer, J.; Enders, C.; Gedek, B. Contamination of Cereals and Feed with Fusarium mycotoxins in European Countries. In Fusarium Mycotoxins, Taxonomy and Pathogenicity; Chelkowski, J., Ed.; Elsevier Sciense Publishers BV: Amsterdam, The Netherlands, 1989; Volume 2, pp. 441–472. [Google Scholar]
- Langseth, W.; Rundberget, T. The occurrence of HT-2 toxin and other trichothecenes in Norwegian cereals. Mycopathologia 1999, 147, 157–165. [Google Scholar]
- Schollenberger, M.; Müller, H.-M.; Rüfle, M.; Suchy, S.; Plank, S.; Drochner, W. Natural occurrence of 16 Fusarium toxins in grains and feedstuffs of plant origin from Germany. Mycopathologia 2006, 161, 43–52. [Google Scholar]
- Cai, H.; Jiang, J.; Yang, Q.; Chen, Q.; Deng, Y. Functional characterization of a first avian cytochrome P450 of the CYP2D subfamily (CYP2D49). PLoS One 2012, 7, e38395. [Google Scholar]
- Burke, M.D.; Thompson, S.; Weaver, R.J.; Wolf, C.R.; Mayers, R.T. Cytochrome P450 specificities of alkoxyresorufin O-dealkylation in human and rat liver. Biochem. Pharmacol 1994, 48, 923–936. [Google Scholar]
- Muchmore, D.C.; McIntosh, L.P.; Russell, C.B.; Anderson, D.E.; Dahlquist, F.W. Expression and nitrogen-15 labeling of proteins for proton and nitrogen-15 nuclear magnetic resonance. Methods Enzymol 1989, 177, 44–73. [Google Scholar]
- Shang, S.; Tan, S.; Li, K.; Wu, J.; Lin, H.; Liu, S.; Deng, Y. Glu659Leu substitution of recombinant HIV fusion inhibitor C52L induces soluble expression in Escherichia coli with equivalent anti-HIV potency. Protein Eng. Des. Sel 2011, 24, 545–551. [Google Scholar]




© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shang, S.; Jiang, J.; Deng, Y. Chicken Cytochrome P450 1A5 Is the Key Enzyme for Metabolizing T-2 Toxin to 3'OH-T-2. Int. J. Mol. Sci. 2013, 14, 10809-10818. https://doi.org/10.3390/ijms140610809
Shang S, Jiang J, Deng Y. Chicken Cytochrome P450 1A5 Is the Key Enzyme for Metabolizing T-2 Toxin to 3'OH-T-2. International Journal of Molecular Sciences. 2013; 14(6):10809-10818. https://doi.org/10.3390/ijms140610809
Chicago/Turabian StyleShang, Shufeng, Jun Jiang, and Yiqun Deng. 2013. "Chicken Cytochrome P450 1A5 Is the Key Enzyme for Metabolizing T-2 Toxin to 3'OH-T-2" International Journal of Molecular Sciences 14, no. 6: 10809-10818. https://doi.org/10.3390/ijms140610809



