Characterization and Expression of the Gene Encoding En-MAPK1, an Intestinal Cell Kinase (ICK)-like Kinase Activated by the Autocrine Pheromone-Signaling Loop in the Polar Ciliate, Euplotes nobilii
Abstract
:1. Introduction
2. Results and Discussion
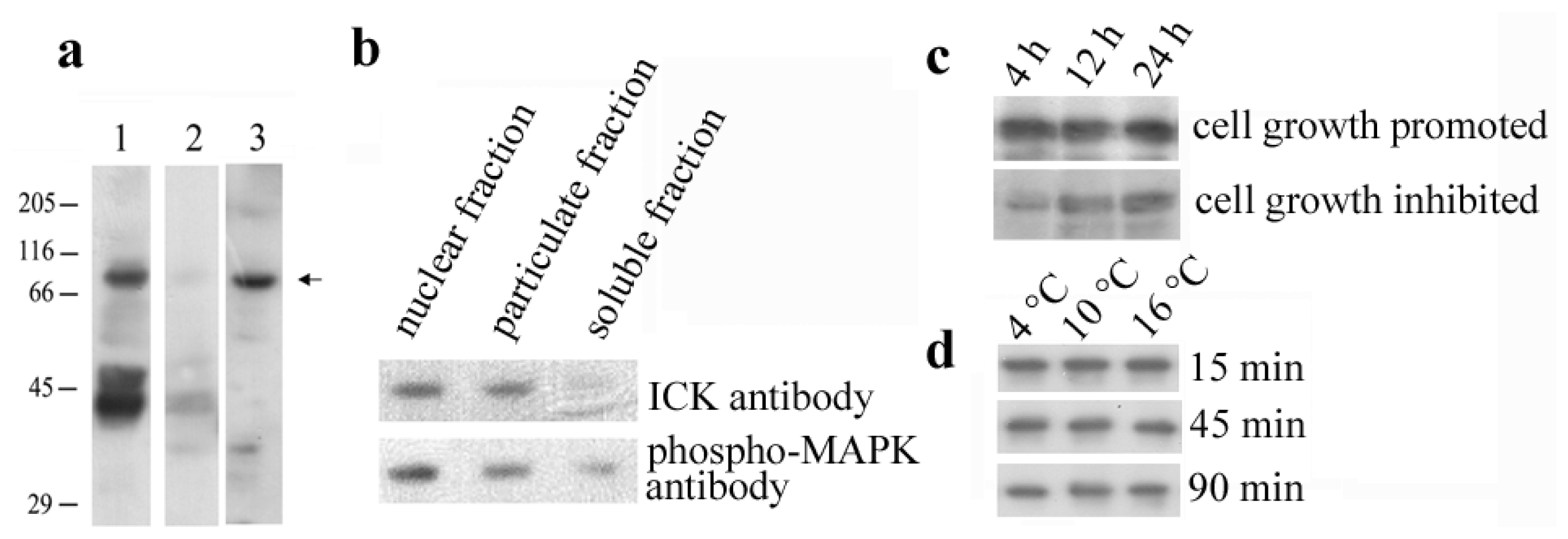
2.1. Identification of the En-MAPK1 Protein
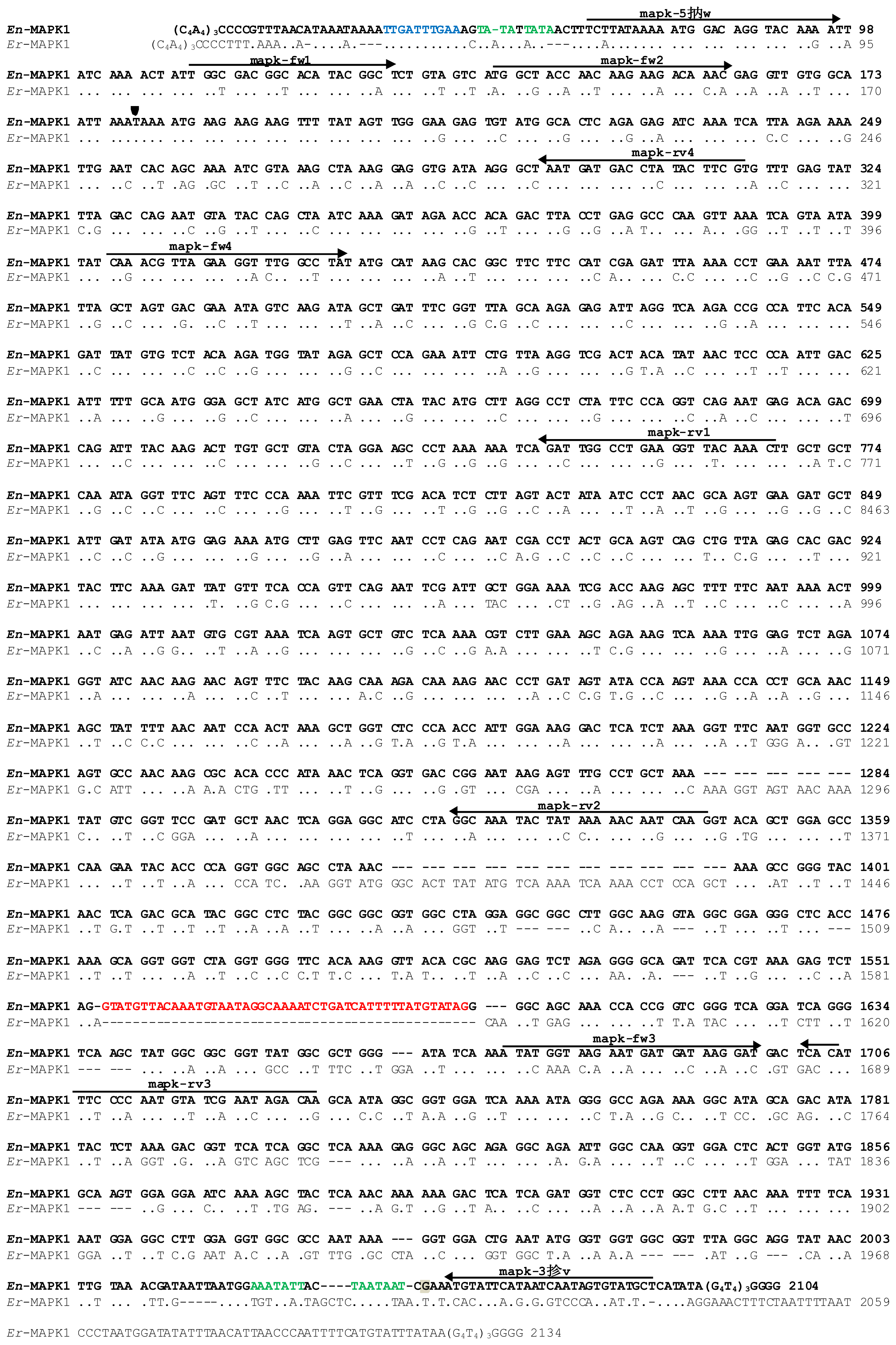
2.2. Molecular Cloning of the En-MAPK1 Coding Gene
2.3. En-MAPK1 Gene Structure and Expression
2.4. En-MAPK1 Protein Structure
3. Experimental Section
3.1. Cells
3.2. Western Blot Analysis
3.3. DNA and RNA Purification, and cDNA Synthesis
3.4. Polymerase Chain Reactions (PCR) and Molecular Cloning
4. Conclusions
Acknowledgments
References
- Togawa, K.; Yan, Y.X.; Inomoto, T.; Slaugenhaupt, S.; Rustgi, A.K. Intestinal cell kinase (ICK) localizes to the crypt region and requires a dual phosphorylation site found in map kinases. J. Cell Physiol 2000, 183, 129–139. [Google Scholar]
- Matsushime, H.; Jinno, A.; Takagi, N.; Shibuya, M. A novel mammalian protein kinase gene (mak) is highly expressed in testicular germ cells at and after meiosis. Mol. Cell. Biol 1990, 10, 2261–2268. [Google Scholar]
- Fu, Z.; Schroeder, M.J.; Shabanowitz, J.; Kaldis, P.; Togawa, K.; Rustgi, A.K.; Hunt, D.F.; Sturgill, T.W. Activation of a nuclear Cdc2-related kinase within a mitogen-activated protein kinase-like TDY motif by autophosphorylation and cyclin-dependent protein kinase-activating kinase. Mol. Cell. Biol 2005, 25, 6047–6064. [Google Scholar]
- Fu, Z.; Kim, J.; Vidrich, A.; Sturgill, T.W.; Cohn, S.M. Intestinal cell kinase, a MAP kinase-related kinase, regulates proliferation and G1 cell cycle progression of intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol 2009, 297, G632–G640. [Google Scholar]
- Xia, L.; Robinson, D.; Ma, A.H.; Chen, H.C.; Wu, F.; Qiu, Y.; Kung, H.J. Identification of human male germ cell-associated kinase, a kinase transcriptionally activated by androgen in prostate cancer cells. J. Biol. Chem 2002, 277, 35422–35433. [Google Scholar]
- Ma, A.H.; Xia, L.; Desai, S.J.; Boucher, D.L.; Guan, Y.; Shih, H.M.; Shi, X.B.; deVere White, R.W.; Chen, H.W.; Tepper, C.G.; et al. Male germ cell-associated kinase, a male-specific kinase regulated by androgen, is a coactivator of androgen receptor in prostate cancer cells. Cancer Res 2006, 66, 8439–8447. [Google Scholar]
- Vallesi, A.; di Pretoro, B.; Ballarini, P.; Apone, F.; Luporini, P. A novel protein kinase from the ciliate Euplotes raikovi with close structural identity to the mammalian intestinal and male-germ cell kinases: Characterization and functional implications in the autocrine pheromone signaling loop. Protist 2010, 161, 250–263. [Google Scholar]
- Vallesi, A.; Giuli, G.; Bradshaw, R.A.; Luporini, P. Autocrine mitogenic activity of pheromone produced by the protozoan ciliate Euplotes raikovi. Nature 1995, 376, 522–524. [Google Scholar]
- Valbonesi, A.; Luporini, P. Description of two new species of Euplotes and Euplotes rariseta from Antarctica. Polar Biol 1990, 11, 47–53. [Google Scholar]
- Di Giuseppe, G.; Erra, F.; Dini, F.; Alimenti, C.; Vallesi, A.; Pedrini, B.; Wüthrich, K.; Luporini, P. Antarctic and Arctic populations of the ciliate Euplotes nobilii show common pheromone-mediated cell-cell signaling and cross-mating. Proc. Natl. Acad. Sci. USA 2011, 108, 3181, –3186.. [Google Scholar]
- Vallesi, A.; di Giuseppe, G.; Dini, F.; Luporini, P. Pheromone evolution in the protozoan ciliate, Euplotes: The ability to synthesize diffusibile forms is ancestral and secondarily lost. Mol. Phylogenet. Evol 2008, 47, 439–442. [Google Scholar]
- Jiang, J.; Zhang, Q.; Warren, A.; Al-Rasheid, K.A.; Song, W. Morphology and SSUrRNA gene-based phylogeny of two marine Euplotes species, E. orientalis spec. nov. and E. raikovi (Ciliophora, Euplotida). Eur. J. Protisotol 2010, 46, 121–132. [Google Scholar]
- Klobutcher, L.A.; Gygax, S.E.; Podoloff, J.D.; Vermeesch, J.R.; Price, C.M.; Tebeau, C.M.; Jahn, C.L. Conserved DNA sequences adjacent to chromosome fragmentation and telomere addition sites in Euplotes crassus. Nucleic Acids Res 1998, 26, 4230, –4240.. [Google Scholar]
- Jahn, C.L.; Klobutcher, L.A. Genome remodeling in ciliated protozoa. Annu. Rev. Microbiol 2002, 56, 489, –520.. [Google Scholar]
- Baird, S.E.; Klobutcher, L.A. Characterization of chromosome fragmentation in two protozoans and identification of a candidate fragmentation sequence in Euplotes crassus. Genes Dev 1989, 3, 585–597. [Google Scholar]
- Klobutcher, L.A.; Farabaugh, P.J. Shifty ciliates: Frequent programmed translational frameshifting in euplotids. Cell 2002, 111, 763–766. [Google Scholar]
- Klobutcher, L.A. Sequencing of random Euplotes crassus macronuclear genes supports a high frequency of +1 translational frameshifting. Eukaryot. Cell 2005, 4, 2098–2105. [Google Scholar]
- Chasin, L. Searching for Splicing Motifs. In Alternative Splicing in the Postgenomic Era; Blencowe, B.J., Graveley, B.R., Eds.; Landes Biosciences: Austin, TX, USA, 2007; pp. 85–106. [Google Scholar]
- Dobri, N.; Oumarou, E.E.; Alimenti, C.; Ortenzi, C.; Luporini, P.; Vallesi, A. Methionine sulfoxide reduction in ciliates: Characterization of the ready-to-use methionine sulfoxide-R-reductase genes in Euplotes. Gene 2013, 515, 110–116. [Google Scholar]
- Scheeff, E.D.; Bourne, P.E. Structural evolution of the protein kinase-like superfamily. PLoS Comput. Biol 2005, 1, e49. [Google Scholar]
- Kannan, N.; Taylor, S.S.; Zhai, Y.; Venter, J.C.; Manning, G. Structural and functional diversity of the microbial kinome. PLoS Biol 2007, 5, e17. [Google Scholar]
- Janitza, P.; Ullrich, K.K.; Quint, M. Toward a comprehensive phylogenetic reconstruction of the evolutionary history of mitogen-activated protein kinases in the plant kingdom. Front. Plant Sci 2012, 3, 271. [Google Scholar]

| Denominations | Sequences (5′–3′) |
|---|---|
| mapk-fw1 a | GGWGAYGGTACWTAYGGWTC |
| mapk-fw2 | GCTACCAACAAGAAGACAAACG |
| mapk-fw3 | TATGGTAAGAATGATGATAAGGATG |
| mapk-fw4 | CAAACGTTAGAAGGTTTGGCCTAT |
| mapk-5′fw | CTTATAAAAATGGACAGGTACAAAATTAT |
| mapk-rv1 a | AAGYTTRTAWCCYTCWGGCCA |
| mapk-rv2 a | CCTTGCTTRTTYTGRTARTAYTTTC |
| mapk-rv3 a | TGTCTRTTWGAAACRTTTGGRAARTG |
| mapk-rv4 | CTCAAACACGAAGTATAGGTCATC |
| mapk-3′rv | TGAGCATACACTATTGATTATGAATACA |
| Tel | CCCCAAAACCCCAAAA |
| Oligo(dT)-linker | ACTAGTCTCGAGTTTTTTTTTTTTTTTTTT |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Candelori, A.; Luporini, P.; Alimenti, C.; Vallesi, A. Characterization and Expression of the Gene Encoding En-MAPK1, an Intestinal Cell Kinase (ICK)-like Kinase Activated by the Autocrine Pheromone-Signaling Loop in the Polar Ciliate, Euplotes nobilii. Int. J. Mol. Sci. 2013, 14, 7457-7467. https://doi.org/10.3390/ijms14047457
Candelori A, Luporini P, Alimenti C, Vallesi A. Characterization and Expression of the Gene Encoding En-MAPK1, an Intestinal Cell Kinase (ICK)-like Kinase Activated by the Autocrine Pheromone-Signaling Loop in the Polar Ciliate, Euplotes nobilii. International Journal of Molecular Sciences. 2013; 14(4):7457-7467. https://doi.org/10.3390/ijms14047457
Chicago/Turabian StyleCandelori, Annalisa, Pierangelo Luporini, Claudio Alimenti, and Adriana Vallesi. 2013. "Characterization and Expression of the Gene Encoding En-MAPK1, an Intestinal Cell Kinase (ICK)-like Kinase Activated by the Autocrine Pheromone-Signaling Loop in the Polar Ciliate, Euplotes nobilii" International Journal of Molecular Sciences 14, no. 4: 7457-7467. https://doi.org/10.3390/ijms14047457





