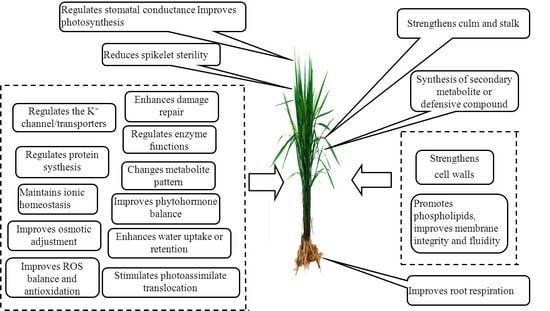
The Critical Role of Potassium in Plant Stress Response
Abstract
:1. Introduction
2. The Role of Potassium in Biotic Stress Resistance
3. The Role of Potassium in Abiotic Stress Resistance
3.1. Potassium and Drought Stress
3.1.1. Cell Elongation and Cell Membrane Stability
3.1.2. Aquaporins and Water Uptake
3.1.3. Osmotic Adjustment
3.1.4. Stomatal Regulation
3.1.5. Detoxification of Reactive Oxygen Species
3.2. Potassium and Salt Stress
3.3. Potassium and Low-Temperature Stress
3.4. Potassium and Waterlogging Stress
4. Implications
- Investigating more details about the molecular mechanisms of K in plant stress resistance.
- Examining the role of K on plant resistance to biotic and abiotic stresses in differentiated cells, tissues and organs and connecting the data relevantly.
- Identifying the common or specific response of K to distinct stress and the role of K on long-term plant responses under multiple stress conditions in nature.
- Understanding the relationship between K and other nutrients in relation to plant adaptation to stresses in different agroecological systems.
- Developing models for better K recommendations based on soil, plant and environmental factors.
- Investigating more researcher on the importance of K on crop production, nutritional quality and human and animal health.
Acknowledgements
References
- United States Census Bureau. International Data Base—Total Midyear Population for the World: 1950–2050. Available online: http://www.census.gov/population/international/data/idb/worldpoptotal.php (accessed on 6 June 2012).
- Oerke, E.C. Crop losses to pests. J. Agri. Sci 2006, 144, 31–43. [Google Scholar]
- Bray, E.A.; Bailey-Serres, J.; Weretilnyk, E. Responses to Abiotic Stresses. In Biochemistry and Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 1158–1203. [Google Scholar]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed; Academic Press: London, UK, 2012; pp. 178–189. [Google Scholar]
- Amtmann, A.; Troufflard, S.; Armengaud, P. The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plantarum 2008, 133, 682–691. [Google Scholar]
- Romheld, V.; Kirkby, E.A. Research on potassium in agriculture: Needs and prospects. Plant Soil 2010, 335, 155–180. [Google Scholar]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci 2005, 168, 521–530. [Google Scholar]
- Kant, S.; Kafkafi, U. Potassium and Abiotic Stresses in Plants. In Potassium for Sustainable Crop Production; Pasricha, N.S., Bansal, S.K., Eds.; Potash Institute of India: Gurgaon, India, 2002; pp. 233–251. [Google Scholar]
- Pettigrew, W.T. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plantarum 2008, 133, 670–681. [Google Scholar]
- Dong, H.; Kong, X.; Li, W.; Tang, W.; Zhang, D. Effects of plant density and nitrogen and potassium fertilization on cotton yield and uptake of major nutrients in two fields with varying fertility. Field Crop Res 2010, 119, 106–113. [Google Scholar]
- Shabala, S.; Pottosin, I.I. Potassium and potassium-permeable channels in plant salt tolerance. Signal. Commun. Plants 2010, 87–110. [Google Scholar]
- White, P.; Karley, A. Potassium. In Cell Biology of Metals and Nutrients; Hell, R., Mendel, R.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 199–224. [Google Scholar]
- Oerke, E.C.; Dehne, H.W. Safeguarding production-losses in major crops and the role of crop protection. Crop Prot 2004, 23, 275–285. [Google Scholar]
- Sarwar, M. Effects of potassium fertilization on population build up of rice stem borers (lepidopteron pests) and rice (Oryza sativa L.) yield. J. Cereals Oilseeds 2012, 3, 6–9. [Google Scholar]
- Holzmueller, E.J.; Jose, S.; Jenkins, M.A. Influence of calcium, potassium, and magnesium on Cornus florida L. density and resistance to dogwood anthracnose. Plant Soil 2007, 290, 189–199. [Google Scholar]
- Williams, J.; Smith, S.G. Correcting potassium deficiency can reduce rice stem diseases. Better Crops 2001, 85, 7–9. [Google Scholar]
- Perrenoud, S. Potassium and Plant Health, 2nd ed; International Potash Institute: Bern, Switzerland, 1990; pp. 8–10. [Google Scholar]
- Prabhu, A.S.; Fageria, N.K.; Huber, D.M. Potassium Nutrition and Plant Diseases. In Mineral Nutrition and Plant Disease; Datnoff, L.E., Elmer, W.H., Huber, D.M., Eds.; American Phytopathological Society: Saint Paul, MN, USA, 2007; pp. 57–78. [Google Scholar]
- Nam, M.H.; Jeong, S.K.; Lee, Y.S.; Choi, J.M.; Kim, H.G. Effects of nitrogen, phosphorus, potassium and calcium nutrition on strawberry anthracnose. Plant Pathol 2006, 55, 246–249. [Google Scholar]
- Ashley, M.K.; Grant, M.; Grabov, A. Plant responses to potassium deficiencies: A role for potassium transport proteins. J. Exp. Bot 2006, 57, 425–436. [Google Scholar]
- Mengel, K. Principles of Plant Nutrition, 5th ed; Kluwer Academic Publishers: Dordrecht, the Netherlands, 2001; pp. 481–509. [Google Scholar]
- DeDatta, J.G.; Mikkelson, D.S. Potassium Nutrition in Rice; American Society of Agronomy: Madison, WI, USA, 1985; pp. 665–699. [Google Scholar]
- Hardter, R. Potassium and Biotic Stress of Plants. In Feed the Soil to Feed the People: The Role of Potash in Sustainable Agriculture; Johnston, A.E., Ed.; International Potash Institute: Basel, Switzerland, 2003; pp. 345–362. [Google Scholar]
- Pervez, H.; Ashraf, M.; Makhdum, M.I.; Mahmood, T. Potassium nutrition of cotton (Gossypium hirsutum L.) in relation to cotton leaf curl virus disease in aridisols. Pak. J. Bot 2007, 39, 529–539. [Google Scholar]
- Prasad, D.; Singh, R.; Singh, A. Management of sheath blight of rice with integrated nutrients. Indian Phytopathol 2010, 63, 11–15. [Google Scholar]
- Foyer, C.H.; Vanacker, H.; Gomez, L.D.; Harbinson, J. Regulation of photosynthesis and antioxidant metabolism in maize leaves at optimal and chilling temperatures: Review. Plant Physiol. Biochem 2002, 40, 659–668. [Google Scholar]
- Kirkby, E.A.; LeBot, J.; Adamowicz, S.; Römheld, V. Nitrogen in Physiology—An Agronomic Perspective and Implications for the Use of Different Nitrogen Forms; International Fertiliser Society: Cambridge, York, UK, 2009. [Google Scholar]
- Egilla, J.N.; Davies, F.T.; Drew, M.C. Effect of potassium on drought resistance of Hibiscus rosa-sinensis cv. Leprechaun: Plant growth, leaf macro- and micronutrient content and root longevity. Plant Soil 2001, 229, 213–224. [Google Scholar]
- Lindhauer, M.G. Influence of K nutrition and drought on water relations and growth of sunflower (Helianthus-annuus L.). J. Plant Nutr. Soil Sci 1985, 148, 654–669. [Google Scholar]
- Bajji, M.; Kinet, J.M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul 2002, 36, 61–70. [Google Scholar]
- Wang, Z.L.; Huang, B.R. Physiological recovery of kentucky bluegrass from simultaneous drought and heat stress. Crop Sci 2004, 44, 1729–1736. [Google Scholar]
- Premachandra, G.S.; Saneoka, H.; Ogata, S. Cell membrane stability and leaf water relations as affected by potassium nutrition of water-stressed maize. J. Exp. Bot 1991, 42, 739–745. [Google Scholar]
- Heinen, R.B.; Ye, Q.; Chaumont, F. Role of aquaporins in leaf physiology. J. Exp. Bot 2009, 60, 2971–2985. [Google Scholar]
- Maurel, C.; Chrispeels, M.J. Aquaporins: A molecular entry into plant water relations. Plant Physiol 2001, 125, 135–138. [Google Scholar]
- Lian, H.L.; Yu, X.; Ye, Q.; Ding, X.S.; Kitagawa, Y.; Kwak, S.S.; Su, W.A.; Tang, Z.C. The role of aquaporin RWC3 in drought avoidance in rice. Plant Cell Physiol 2004, 45, 481–489. [Google Scholar]
- Tyerman, S.D.; Niemietz, C.M.; Bramley, H. Plant aquaporins: Multifunctional water and solute channels with expanding roles. Plant Cell Environ 2002, 25, 173–194. [Google Scholar]
- Galmes, J.; Pou, A.; Alsina, M.M.; Tomas, M.; Medrano, H.; Flexas, J. Aquaporin expression in response to different water stress intensities and recovery in Richter-110 (Vitis sp.): Relationship with ecophysiological status. Planta 2007, 226, 671–681. [Google Scholar]
- Kaldenhoff, R.; Ribas-Carbo, M.; Flexas, J.; Lovisolo, C.; Heckwolf, M.; Uehlein, N. Aquaporins and plant water balance. Plant Cell Environ 2008, 31, 658–666. [Google Scholar]
- Smart, L.B.; Moskal, W.A.; Cameron, K.D.; Bennett, A.B. MIP genes are down-regulated under drought stress in Nicotiana glauca. Plant Cell Physiol 2001, 42, 686–693. [Google Scholar]
- Alexandersson, E.; Fraysse, L.; Sjovall-Larsen, S.; Gustavsson, S.; Fellert, M.; Karlsson, M.; Johanson, U.; Kjellbom, P. Whole gene family expression and drought stress regulation of aquaporins. Plant Mol. Biol 2005, 59, 469–484. [Google Scholar]
- Liu, H.Y.; Sun, W.N.; Su, W.A.; Tang, Z.C. Co-regulation of water channels and potassium channels in rice. Physiol. Plantarum 2006, 128, 58–69. [Google Scholar]
- Cuéllar, T.; Pascaud, F.; Verdeil, J.L.; Torregrosa, L.; Adam-Blondon, A.F.; Thibaud, J.B.; Sentenac, H.; Gaillard, I. A grapevine Shaker inward K+ channel activated by the calcineurin B-like calcium sensor 1-protein kinase CIPK23 network is expressed in grape berries under drought stress conditions. Plant J 2010, 61, 58–69. [Google Scholar]
- Kanai, S.; Moghaieb, R.E.; El-Shemy, H.A.; Panigrahi, R.; Mohapatra, P.K.; Ito, J.; Nguyen, N.T.; Saneoka, H.; Fujita, K. Potassium deficiency affects water status and photosynthetic rate of the vegetative sink in green house tomato prior to its effects on source activity. Plant Sci 2011, 180, 368–374. [Google Scholar]
- Sahr, T.; Voigt, G.; Paretzke, H.G.; Schramel, P.; Ernst, D. Caesium-affected gene expression in Arabidopsis thaliana. New Phytol 2005, 165, 747–754. [Google Scholar]
- Tazawa, M.; Sutou, E.; Shibasaka, M. Onion root water transport sensitive to water channel and K+ channel Inhibitors. Plant Cell Physiol 2001, 42, 28–36. [Google Scholar]
- Guo, S.W.; Shen, Q.R.; Brueck, H. Effects of local nitrogen supply on water uptake of bean plants in a split root system. J. Integr. Plant Biol 2007, 49, 472–480. [Google Scholar]
- Oddo, E.; Inzerillo, S.; La Bella, F.; Grisafi, F.; Salleo, S.; Nardini, A. Short-term effects of potassium fertilization on the hydraulic conductance of laurus nobilis L. Tree Physiol 2011, 31, 131–138. [Google Scholar]
- Zwieniecki, M.A.; Melcher, P.J.; Holbrook, N.M. Hydrogel control of xylem hydraulic resistance in plants. Science 2001, 291, 1059–1062. [Google Scholar]
- DaCosta, M.; Huang, B.R. Osmotic adjustment associated with variation in bentgrass tolerance to drought stress. J. Am. Soc. Hortic. Sci 2006, 131, 338–344. [Google Scholar]
- Shabala, S.N.; Lew, R.R. Turgor regulation in osmotically stressed Arabidopsis epidermal root cells. Direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol 2002, 129, 290–299. [Google Scholar]
- Egilla, J.N.; Davies, F.T.; Boutton, T.W. Drought stress influences leaf water content, photosynthesis, and water-use efficiency of hibiscus rosa-sinensis at three potassium concentrations. Photosynthetica 2005, 43, 135–140. [Google Scholar]
- Jin, S.H.; Huang, J.Q.; Li, X.Q.; Zheng, B.S.; Wu, J.S.; Wang, Z.J.; Liu, G.H.; Chen, M. Effects of potassium supply on limitations of photosynthesis by mesophyll diffusion conductance in Carya cathayensis. Tree Physiol 2011, 31, 1142–1151. [Google Scholar]
- Tomemori, H.; Hamamura, K.; Tanabe, K. Interactive effects of sodium and potassium on the growth and photosynthesis of spinach and komatsuna. Plant Prod. Sci 2002, 5, 281–285. [Google Scholar]
- Pervez, H.; Ashraf, M.; Makhdum, M.I. Influence of potassium nutrition on gas exchange characteristics and water relations in cotton (Gossypium hirsutum L.). Photosynthetica 2004, 42, 251–255. [Google Scholar]
- Benlloch-Gonzalez, M.; Arquero, O.; Fournier, J.M.; Barranco, D.; Benlloch, M. K+ starvation inhibits water-stress-induced stomatal closure. J. Plant Physiol 2008, 165, 623–630. [Google Scholar]
- Benlloch-Gonzalez, M.; Romera, J.; Cristescu, S.; Harren, F.; Fournier, J.M.; Benlloch, M. K+ starvation inhibits water-stress-induced stomatal closure via ethylene synthesis in sunflower plants. J. Exp. Bot 2010, 61, 1139–1145. [Google Scholar]
- Tsonev, T.; Velikova, V.; Yildiz-Aktas, L.; Gurel, A.; Edreva, A. Effect of water deficit and potassium fertilization on photosynthetic activity in cotton plants. Plant Biosyst 2011, 145, 841–847. [Google Scholar]
- Shin, R.; Schachtman, D.P. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc. Natl. Acad. Sci. USA 2004, 101, 8827–8832. [Google Scholar]
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Nakajima, N.; Kondo, N.; Hasezawa, S. Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol 2005, 138, 2337–2343. [Google Scholar]
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Nakajima, N.; Kondo, N.; Hasezawa, S. Cytokinin and auxin inhibit abscisic acid-induced stomatal closure by enhancing ethylene production in Arabidopsis. J. Exp. Bot 2006, 57, 2259–2266. [Google Scholar]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav 2008, 3, 156–165. [Google Scholar]
- Fu, J.M.; Huang, B.R. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot 2001, 45, 105–114. [Google Scholar]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol 2004, 161, 1189–1202. [Google Scholar]
- Dat, J.; Vandenabeele, S.; Vranova, E.; van Montagu, M.; Inze, D.; van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci 2000, 57, 779–795. [Google Scholar]
- Vranova, E.; Inze, D.; van Breusegem, F. Signal transduction during oxidative stress. J. Exp. Bot 2002, 53, 1227–1236. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 2002, 7, 405–410. [Google Scholar]
- Cakmak, I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol 2000, 146, 185–205. [Google Scholar]
- Abdel Wahab, A.M.; Abd-Alla, M.H. The role of potassium fertilizer in nodulation and nitrogen fixation of faba bean (Vicia faba L.) plants under drought stress. Biol. Fert. Soils 1995, 20, 147–150. [Google Scholar]
- Peuke, A.D.; Jeschke, W.D.; Hartung, W. Flows of elements, ions and abscisic acid in Ricinus communis and site of nitrate reduction under potassium limitation. J. Exp. Bot 2002, 53, 241–250. [Google Scholar]
- Jiang, M.; Zhang, J. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage. Plant Cell Physiol 2001, 42, 1265–1273. [Google Scholar]
- Lin, C.C.; Kao, C.H. Abscisic acid induced changes in cell wall peroxidase activity and hydrogen peroxide level in roots of rice seedlings. Plant Sci 2001, 160, 323–329. [Google Scholar]
- Marschner, H.; Cakmak, I. Highlight intensity enhances chlorosis and necrosis in leaves of zinc, potassium, and magnesium deficient bean (Phaseolus vulgaris) plants. J. Plant Physiol 1989, 134, 308–315. [Google Scholar]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol 2005, 167, 645–663. [Google Scholar]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotox. Environ. Safe 2005, 60, 324–349. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol 2008, 59, 651–681. [Google Scholar]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Phys 2000, 51, 463–499. [Google Scholar]
- Yang, Y.; Zheng, Q.; Liu, M.; Long, X.; Liu, Z.; Shen, Q.; Guo, S. Difference in sodium spatial distribution in the shoot of two canola cultivars under saline stress. Plant Cell Physiol 2012, 53, 1083–1092. [Google Scholar]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plantarum 2008, 133, 651–669. [Google Scholar]
- Botella, M.A.; Martinez, V.; Pardines, J.; Cerda, A. Salinity induced potassium deficiency in maize plants. J. Plant Physiol 1997, 150, 200–205. [Google Scholar]
- Coskun, D.; Britto, D.T.; Kronzucker, H.J. Regulation and mechanism of potassium release from barley roots: an in planta 42K+ analysis. New Phytol 2010, 188, 1028–1038. [Google Scholar]
- Mian, A.; Oomen, R.J.; Isayenkov, S.; Sentenac, H.; Maathuis, F.J.; Very, A.A. Over-expression of an Na+-and K+-permeable HKT transporter in barley improves salt tolerance. Plant J 2011, 68, 468–479. [Google Scholar]
- Platten, J.D.; Cotsaftis, O.; Berthomieu, P.; Bohnert, H.; Davenport, R.J.; Fairbairn, D.J.; Horie, T.; Leigh, R.A.; Lin, H.X.; Luan, S.; et al. Nomenclature for HKT transporters, key determinants of plant salinity tolerance. Trends Plant Sci 2006, 11, 372–374. [Google Scholar]
- Byrt, C.S.; Platten, J.D.; Spielmeyer, W.; James, R.A.; Lagudah, E.S.; Dennis, E.S.; Tester, M.; Munns, R. HKT1;5-like cation transporters linked to Na+ exclusion loci in wheat, Nax2 and Kna1. Plant Physiol 2007, 143, 1918–1928. [Google Scholar]
- Horie, T.; Hauser, F.; Schroeder, J.I. HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci 2009, 14, 660–668. [Google Scholar]
- Degl’Innocenti, E.; Hafsi, C.; Guidi, L.; Navari-Izzo, F. The effect of salinity on photosynthetic activity in potassium-deficient barley species. J. Plant Physiol 2009, 166, 1968–1981. [Google Scholar]
- Qu, C.X.; Liu, C.; Gong, X.L.; Li, C.X.; Hong, M.M.; Wang, L.; Hong, F.S. Impairment of maize seedling photosynthesis caused by a combination of potassium deficiency and salt stress. Environ. Exp. Bot 2012, 75, 134–141. [Google Scholar]
- Qu, C.X.; Liu, C.; Ze, Y.G.; Gong, X.L.; Hong, M.M.; Wang, L.; Hong, F.S. Inhibition of nitrogen and photosynthetic carbon assimilation of maize seedlings by exposure to a combination of salt stress and potassium-deficient stress. Biol. Trace. Elem. Res 2011, 144, 1159–1174. [Google Scholar]
- Chen, Z.H.; Zhou, M.X.; Newman, I.A.; Mendham, N.J.; Zhang, G.P.; Shabala, S. Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Funct. Plant Biol 2007, 34, 150–162. [Google Scholar]
- Walker, D.J.; Leigh, R.A.; Miller, A.J. Potassium homeostasis in vacuolate plant cells. Proc. Natl. Acad. Sci. USA 1996, 93, 10510–10514. [Google Scholar]
- Gong, X.; Chao, L.; Zhou, M.; Hong, M.; Luo, L.; Wang, L.; Ying, W.; Jingwei, C.; Songjie, G.; Fashui, H. Oxidative damages of maize seedlings caused by exposure to a combination of potassium deficiency and salt stress. Plant Soil 2011, 340, 443–452. [Google Scholar]
- Shabala, S. Salinity and programmed cell death: Unravelling mechanisms for ion specific signalling. J. Exp. Bot 2009, 60, 709–711. [Google Scholar]
- Devi, B.S.R.; Kim, Y.J.; Selvi, S.K.; Gayathri, S.; Altanzul, K.; Parvin, S.; Yang, D.U.; Lee, O.R.; Lee, S.; Yang, D.C. Influence of potassium nitrate on antioxidant level and secondary metabolite genes under cold stress in Panax ginseng. Russ. J. Plant Physiol 2012, 59, 318–325. [Google Scholar]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plantarum 2006, 126, 45–51. [Google Scholar]
- Xiong, L.M.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14, S165–S183. [Google Scholar]
- Zhu, J.K. Cell signaling under salt, water and cold stresses. Curr. Opin. Plant Biol 2001, 4, 401–406. [Google Scholar]
- Bogdevitch, I. IPI Internal Report; International Potash Institute: Basel, Switzerland, 2000. [Google Scholar]
- Webster, D.E.; Ebdon, J.S. Effects of nitrogen and potassium fertilization on perennial ryegrass cold tolerance during deacclimation in late winter and early spring. Hortscience 2005, 40, 842–849. [Google Scholar]
- Wang, X.M.; Li, W.Q.; Li, M.Y.; Welti, R. Profiling lipid changes in plant response to low temperatures. Physiol. Plantarum 2006, 126, 90–96. [Google Scholar]
- McKersie, B.D.; Leshem, Y.Y. Stress and Stress Coping in Cultivated Plants; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 181–193. [Google Scholar]
- Haque, M.Z. Effect of nitrogen, phosphorus and potassium on spikelet sterility induced by low temperature at the reproductive stage of rice. Plant Soil 1988, 109, 31–36. [Google Scholar]
- Hakerlerler, H.; Oktay, M.; Eryuce, N.; Yagmur, B. Effect of Potassium Sources on the Chilling Tolerance of Some Vegetable Seedlings Grown in Hotbeds. In Food Security in the WANA Region, the Essential Need for Balanced Fertilization; Johnston, A.E., Ed.; International Potash Institute: Basel, Switzerland, 1997; pp. 353–359. [Google Scholar]
- Setter, T.L.; Waters, I. Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 2003, 253, 1–34. [Google Scholar]
- Zhou, M. Improvement of Plant Waterlogging Tolerance. In Waterlogging Signalling and Tolerance in Plants; Mancuso, S., Shabala, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 267–285. [Google Scholar]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol 2008, 59, 313–339. [Google Scholar]
- Wegner, L. Oxygen Transport in Waterlogged Plants. In Waterlogging Signalling and Tolerance in Plants; Mancuso, S., Shabala, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–22. [Google Scholar]
- Shabala, S. Physiological and cellular aspects of phytotoxicity tolerance in plants: The role of membrane transporters and implications for crop breeding for waterlogging tolerance. New Phytol 2011, 190, 289–298. [Google Scholar]
- Colmer, T.D.; Greenway, H. Ion transport in seminal and adventitious roots of cereals during O2 deficiency. J. Exp. Bot 2011, 62, 39–57. [Google Scholar]
- Kirmizi, S.; Bell, R.W. Responses of barley to hypoxia and salinity during seed germination, nutrient uptake, and early plant growth in solution culture. J. Plant Nutr. Soil Sci 2012, 175, 630–640. [Google Scholar]
- Pang, J.Y.; Newman, I.; Mendham, N.; Zhou, M.; Shabala, S. Microelectrode ion and O2 fluxes measurements reveal differential sensitivity of barley root tissues to hypoxia. Plant Cell Environ 2006, 29, 1107–1121. [Google Scholar]
- Teakle, N.L.; Bazihizina, N.; Shabala, S.; Colmer, T.D.; Barrett-Lennard, E.G.; Rodrigo-Moreno, A.; Läuchli, A.E. Differential tolerance to combined salinity and O2 deficiency in the halophytic grasses Puccinellia ciliata and Thinopyrum ponticum: The importance of K+ retention in roots. Environ. Exp. Bot 2013, 87, 69–78. [Google Scholar]
- Mancuso, S.; Marras, A.M. Adaptative response of Vitis root to anoxia. Plant Cell Physiol 2006, 47, 401–409. [Google Scholar]
- Mugnai, S.; Marras, A.M.; Mancuso, S. Effect of hypoxic acclimation on anoxia tolerance in Vitis roots: Response of metabolic activity and K+ fluxes. Plant Cell Physiol 2011, 52, 1107–1116. [Google Scholar]
- Fiedler, S.; Vepraskas, M.J.; Richardson, J.L. Soil redox potential: Importance, field measurements, and observations. Adv. Agron 2007, 94, 1–54. [Google Scholar]
- Erlejman, A.G.; Verstraeten, S.V.; Fraga, C.G.; Oteiza, P.I. The interaction of flavonoids with membranes: Potential determinant of flavonoid antioxidant effects. Free Radical Res 2004, 38, 1311–1320. [Google Scholar]
- Pang, J.Y.; Cuin, T.; Shabala, L.; Zhou, M.X.; Mendham, N.; Shabala, S. Effect of secondary metabolites associated with anaerobic soil conditions on ion fluxes and electrophysiology in barley roots. Plant Physiol 2007, 145, 266–276. [Google Scholar]
- Else, M.A.; Coupland, D.; Dutton, L.; Jackson, M.B. Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap. Physiol. Plantarum 2001, 111, 46–54. [Google Scholar]
- Ashraf, M.A. Waterlogging stress in plants: A review. Afr. J. Agri. Res 2012, 7, 1976–1981. [Google Scholar]
- Ashraf, M.A.; Ahmad, M.S.A.; Ashraf, M.; Al-Qurainy, F.; Ashraf, M.Y. Alleviation of waterlogging stress in upland cotton (Gossypium hirsutum L.) by exogenous application of potassium in soil and as a foliar spray. Crop Pasture Sci 2011, 62, 25–38. [Google Scholar]





| Serial number | Potassium treatments (kg ha−1) | Stem borer infestation (%) | Yield/plot (g/3 m2) | Yield (kg ha−1) | |
|---|---|---|---|---|---|
| Dead heart | White heads | ||||
| 1 | 40 kg | 3.05 b | 5.37 b | 1913.00 b | 6376.66 |
| 2 | 50 kg | 2.64 bc | 3.58 c | 287.00 a | 7623.33 |
| 3 | 60 kg | 2.40 c | 3.37 c | 2317.00 a | 7723.33 |
| 4 | Control | 4.33 a | 7.12 a | 1690.00 c | 5633.33 |
| LSD value | 0.619 | 0.561 | 219.4 | ||
| Decrease in disease | Increase in disease | No effect | Total | |
|---|---|---|---|---|
| Fungi | 89 | 33 | 8 | 130 |
| Bacteria | 18 | 5 | 0 | 23 |
| Viruses | 9 | 5 | 3 | 17 |
| Nematode | 3 | 6 | 1 | 10 |
| Treatment | K+ content in leaves (μmol/gFW) | Stomatal conductance (mmol/m2/s) |
|---|---|---|
| Normal K: Irrigated | 133.6 ± 7.3 | 456 ± 5.6 |
| Normal K: Drought | 119.4 ± 3.8 | 281 ± 27.9 |
| Low K: Irrigated | 36.3 ± 1.4 | 462 ± 4.0 |
| Low K: Drought | 25.7 ± 0.8 | 351 ± 15.2 |
| Parameter | Grain yield | K+ flux | Shoot biomass | Survival rate | Plant height | [CO2]ass | TSW | Tillering |
|---|---|---|---|---|---|---|---|---|
| K+ flux | 0.67 ** | - | - | - | - | - | - | - |
| Shoot biomass | 0.96 ** | 0.69 ** | - | - | - | - | - | - |
| Survival rate | 0.65 ** | 0.70 ** | 0.74 ** | - | - | - | - | - |
| Plant height | 0.70 ** | 0.69 ** | 0.61 ** | 0.51 ** | - | - | - | - |
| [CO2]ass | 0.68 ** | 0.69 ** | 0.65 ** | 0.48 ** | 0.50 ** | - | - | - |
| TSW | 0.72 ** | 0.70 ** | 0.70 ** | 0.63 ** | 0.74 ** | 0.48 ** | - | - |
| Tillering | 0.48 ** | 0.26 * | 0.51 ** | 0.16 | 0.23 | 0.25 * | 0.33 * | - |
| Germination | 0.29 * | 0.21 | 0.31 * | 0.33 ** | 0.16 | 0.02 | 0.38 ** | 0.20 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370-7390. https://doi.org/10.3390/ijms14047370
Wang M, Zheng Q, Shen Q, Guo S. The Critical Role of Potassium in Plant Stress Response. International Journal of Molecular Sciences. 2013; 14(4):7370-7390. https://doi.org/10.3390/ijms14047370
Chicago/Turabian StyleWang, Min, Qingsong Zheng, Qirong Shen, and Shiwei Guo. 2013. "The Critical Role of Potassium in Plant Stress Response" International Journal of Molecular Sciences 14, no. 4: 7370-7390. https://doi.org/10.3390/ijms14047370
APA StyleWang, M., Zheng, Q., Shen, Q., & Guo, S. (2013). The Critical Role of Potassium in Plant Stress Response. International Journal of Molecular Sciences, 14(4), 7370-7390. https://doi.org/10.3390/ijms14047370