Vascular Endothelial Growth Factor Receptor Family in Ascidians, Halocynthia roretzi (Sea Squirt). Its High Expression in Circulatory System-Containing Tissues
Abstract
:Abbreviation
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
| S. sq. | Sea squirt |
| B. schlosseri | Botryllus schlosseri |
| H. roretzi | Halocynthia roretzi |
| C. intestinalis | Ciona intestinalis |
1. Introduction
2. Results and Discussion
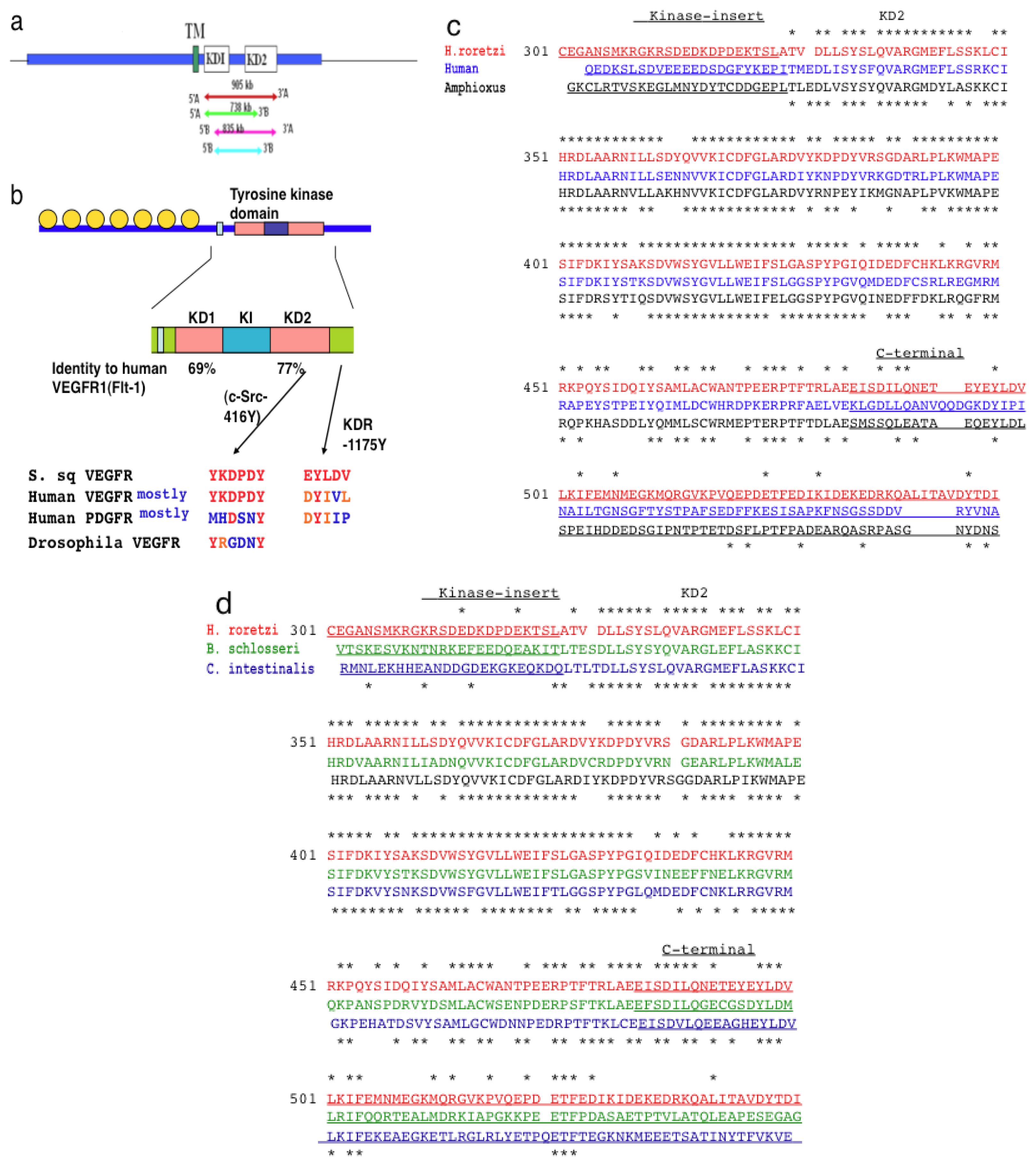
2.1. Isolation of VEGFR-Like Tyrosine Kinase Gene in H. roretzi by Using Degeneration-Primer Method
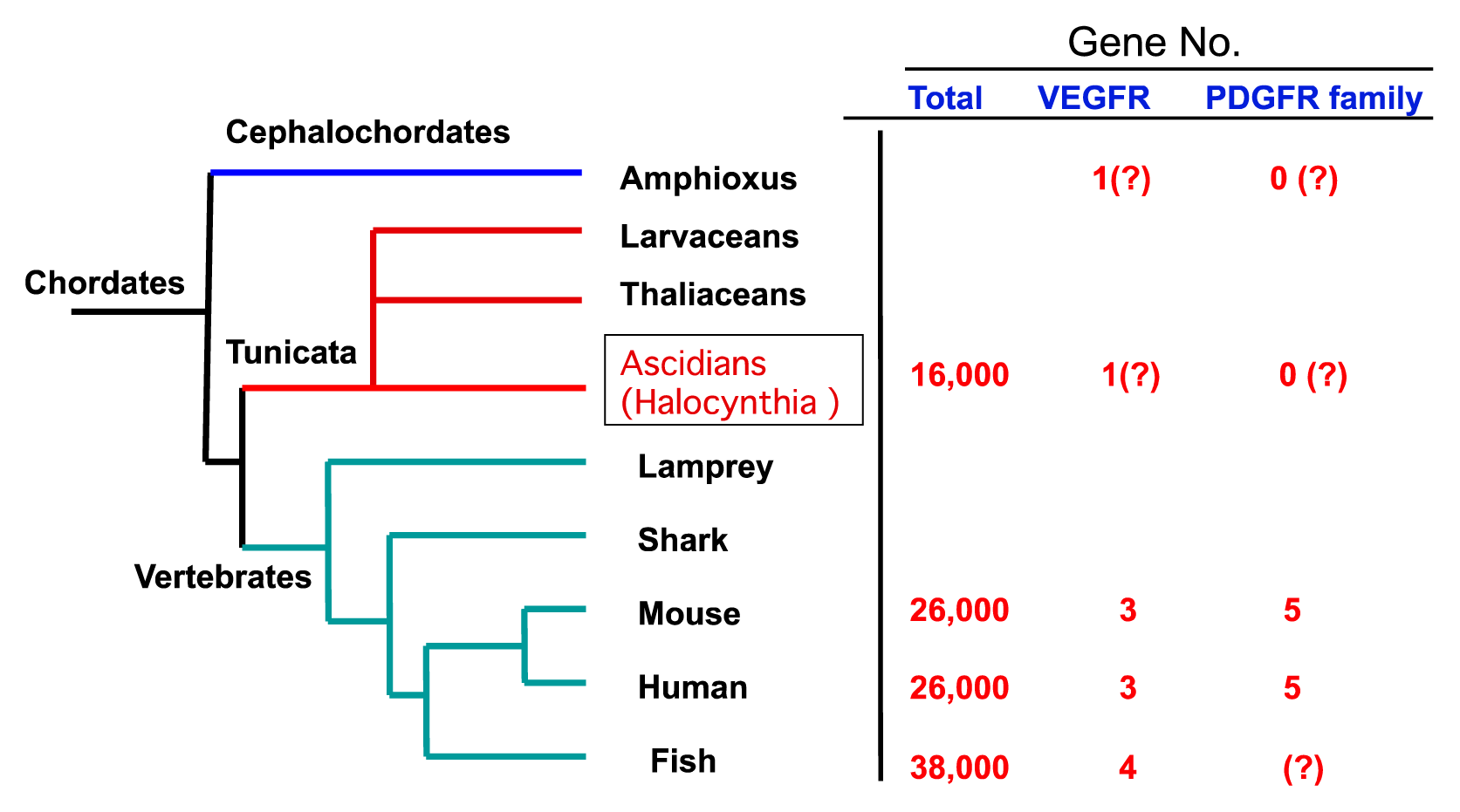
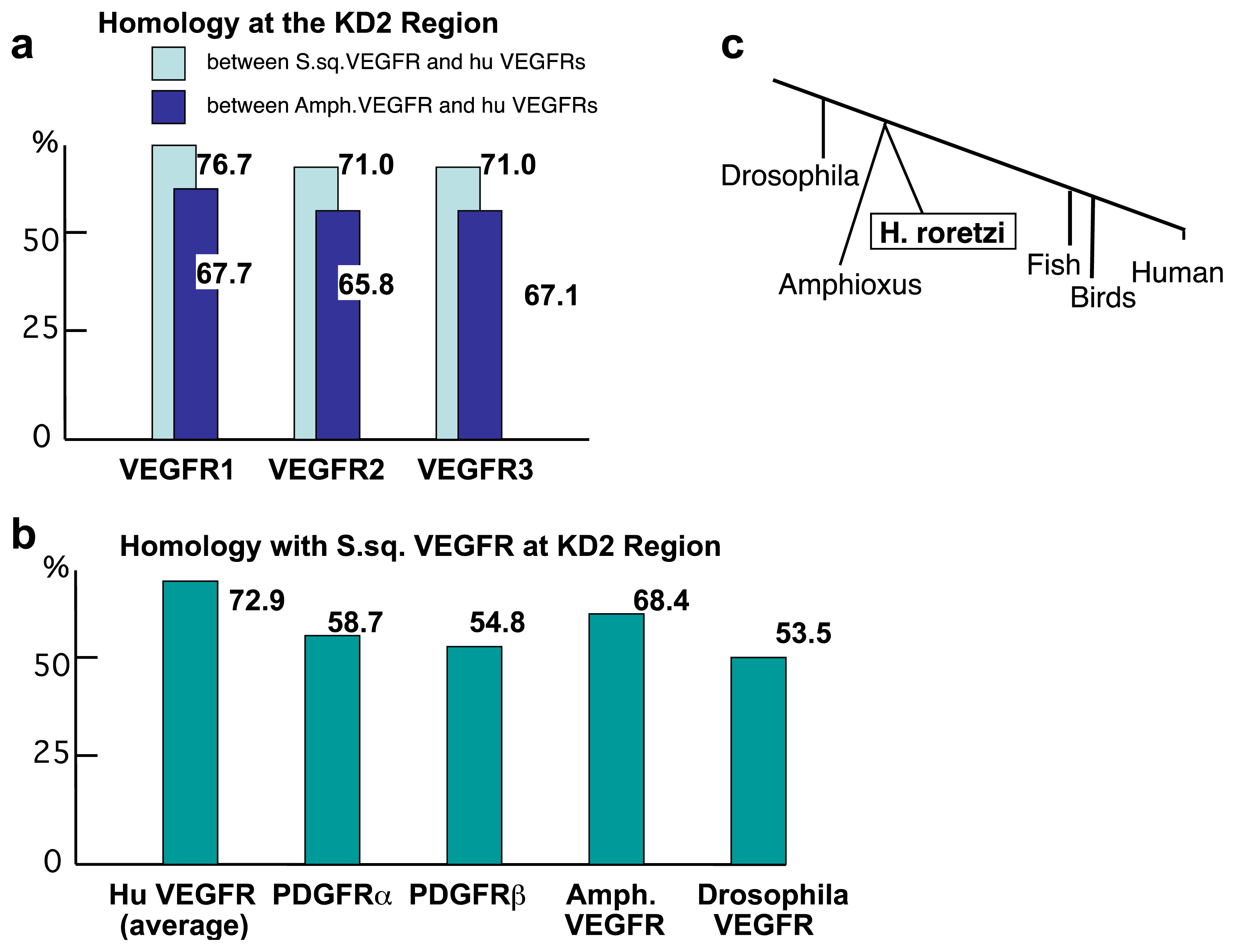
2.2. The Structure of S. sq VEGFR Is Closer to VEGFR Family than PDGFR Family in Humans
2.3. Amino Acid Sequence Critical for the Intracellular Signaling of Human VEGFR Is Conserved in S. sq VEGFR
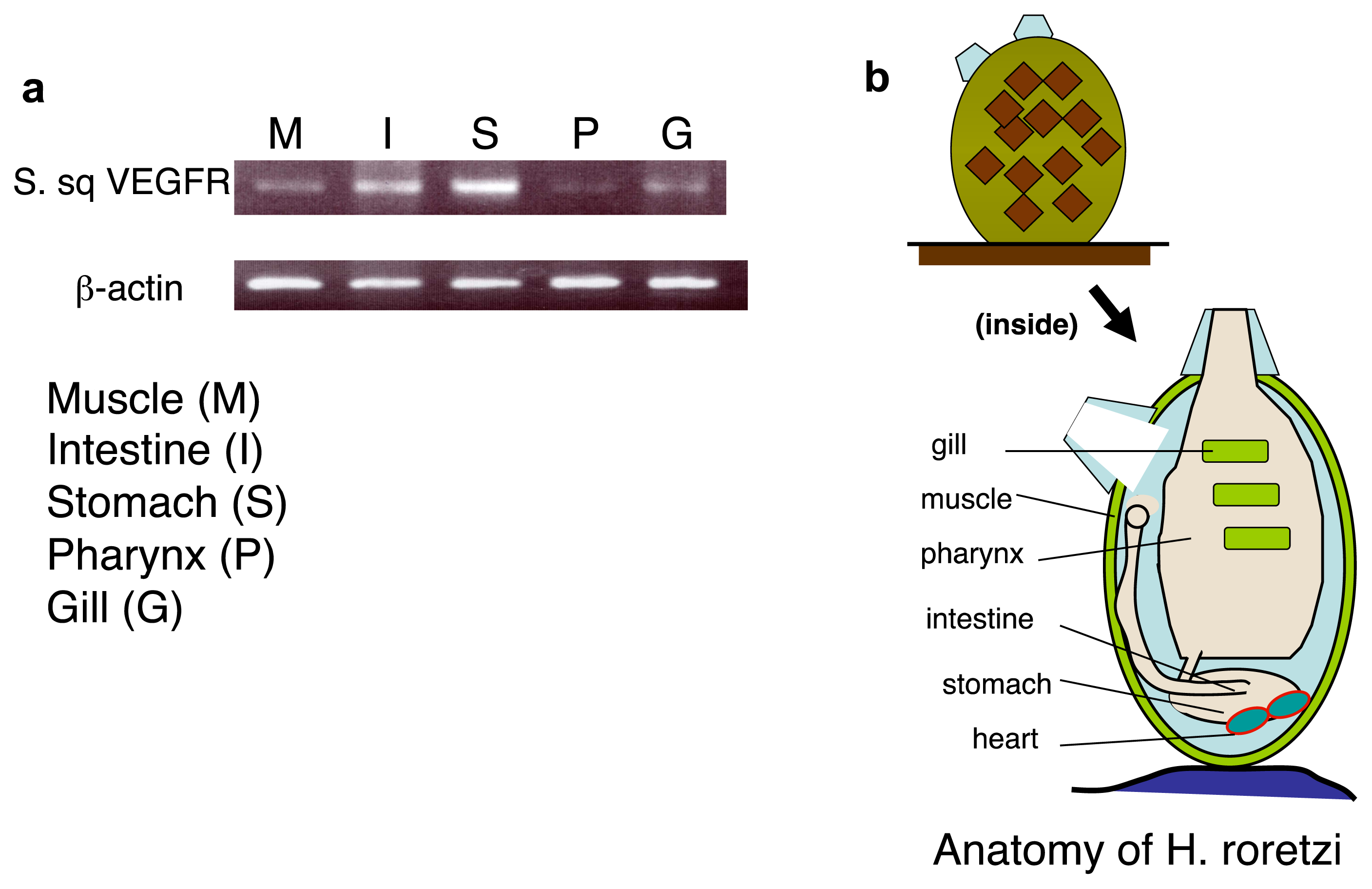
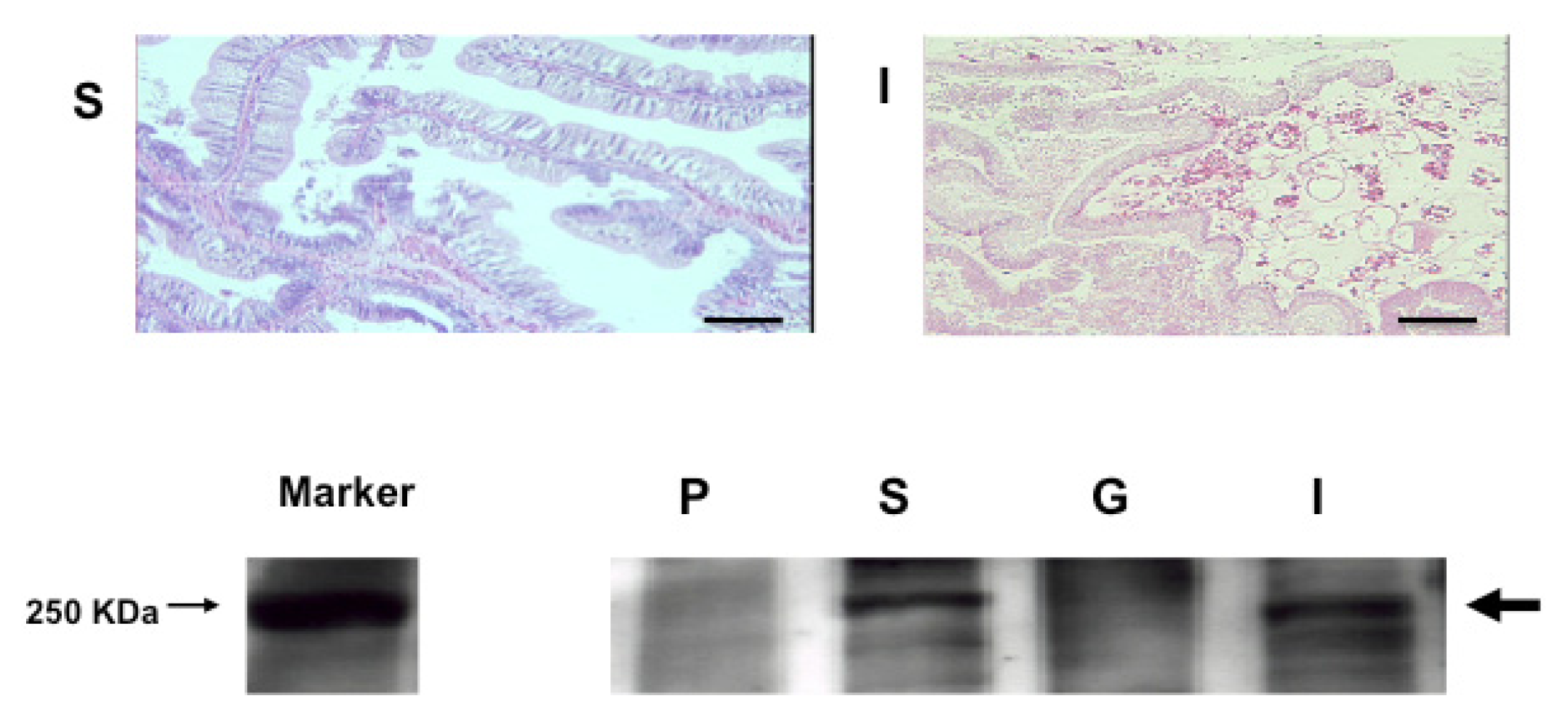
2.4. An Intimate Relationship between Expression of S. sq VEGFR mRNA and the Circulatory System in H. roretzi
3. Experimental Section
3.1. Animals
3.2. The Acid Guanidium-Phenol-Chloroform (AGPC) Method
3.3. cDNA Synthesis
3.4. Degenerated Oligonucleotide Primers
(A) Primers for isolation of a VEGFR-like tyrosine kinase gene in H. roretzi
(B) Primers for detection of β-actin mRNA in H. roretzi
3.5. PCR
3.6. Restriction Enzymes
3.7. Histological Analysis
3.8. RT-PCR Detection of S. sq VEGFR mRNA
3.9. Generation of Antibody
3.10. Western Blotting
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Dehal, P.; Satou, Y.; Campbell, R.K.; Chapman, J.; Degnan, B.; de Tomaso, A.; Davidson, B.; di Gregorio, A.; Gelpke, M.; Goodstein, D.M.; et al. The draft genome of Ciona intestinalis: Insights into chordate and vertebrate origins. Science 2002, 298, 2157–2167. [Google Scholar]
- Holland, L.Z.; Gibson-Brown, J.J. The Ciona intestinalis genome: When the constraints are off. BioEssays 2003, 25, 529–532. [Google Scholar]
- Johnson, D.S.; Davidson, B.; Brown, C.D.; Smith, W.C.; Sidow, A. Noncoding regulating sequences of ciona exhibit strong coreesespondence between evolutionary constraint and functional importance. Genome Res 2004, 14, 2448–2456. [Google Scholar]
- Leveugle, M.; Prat, K.; Popovici, C.; Birnbaum, D.; Coulier, F. Phylogenetic analysis of Ciona intestinalis gene super-families supports the hypothesis of successive gene expansion. J. Mol. Evol 2004, 58, 168–181. [Google Scholar]
- Satou, Y.; Mineta, K.; Ogasawara, M.; Sasakura, Y.; Shoguchi, E.; Ueno, K.; Yamada, L.; Matsumoto, J.; Wasserscheid, J.; Dewar, K.; et al. Improved genome assembly and evidence-based global gene model set for the chordate Ciona intestinalis: New insight into intron and operon populations. Genome Biol 2008, 9, R152. [Google Scholar]
- Zhan, A.; Macisaac, H.J.; Cristescu, M.E. Invasion genetics of the Ciona intestinalis species complex: From regional endemism to global homogeneity. Mol. Ecol 2010, 19, 4678–4694. [Google Scholar]
- Wada, S.; Tokuoka, M.; Shoguchi, E.; Kobayashi, K.; di Gregorio, A.; Spagnuolo, A.; Branno, M.; Kohara, Y.; Rokhsar, D.; Levine, M.; et al. A genomewide survey of developmentally relevant genes in Ciona intestinalis. II. Genes for homeobox transcription factors. Dev. Genes Evol 2003, 213, 222–234. [Google Scholar]
- Shoguchi, E.; Hamada, M.; Fujie, M.; Satoh, N. Direct examination of chromosomal clustering of organ-specific genes in the chordate Ciona intestinalis. Genesis 2011, 49, 662–672. [Google Scholar]
- Munoz-Chapuli, R.; Carmona, R.; Guadix, J.A.; Macias, D.; Perez-Pomares, J.M. The origin of the endothelial cells: An evo-devo approach for the invertebrate/vertebrate transition of the circulatory system. Evol. Dev 2005, 7, 351–358. [Google Scholar]
- Gasparini, F.; Longo, F.; Manni, L.; Burighel, P.; Zaniolo, G. Tubular sprouting as a mode of vascular formation in a colonial ascidian (Tunicata). Dev. Dyn 2007, 236, 719–731. [Google Scholar]
- Gasparini, F.; Burighel, P.; Manni, L.; Zaniolo, G. Vascular regeneration and angiogenic-like sprouting mechanism in a compound ascidian is similar to vertebrates. Evol. Dev 2008, 10, 591–605. [Google Scholar]
- Tiozzo, S.; Voskoboynik, A.; Brown, F.D.; de Tomaso, A.W. A conserved role of the VEGF pathway in angiogenesis of an ectodermally-derived vasculature. Dev. Biol 2008, 315, 243–255. [Google Scholar]
- Stolfi, A.; Gainous, T.B.; Young, J.J.; Mori, A.; Levine, M.; Christiaen, L. Early chordate origins of the vertebrate second heart field. Science 2010, 329, 565–568. [Google Scholar]
- Munoz-Chapuli, R. Evolution of angiogenesis. Int. J. Dev. Biol 2011, 55, 345–351. [Google Scholar]
- Shibuya, M.; Yamaguchi, S.; Yamane, A.; Ikeda, T.; Tojo, A.; Matsushime, H.; Sato, M. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 1990, 5, 519–524. [Google Scholar]
- Shibuya, M.; Claesson-Welsh, L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res 2006, 312, 549–560. [Google Scholar]
- Shibuya, M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J. Biochem. Mol. Biol 2006, 39, 469–478. [Google Scholar]
- De Vries, C.; Escobedo, J.A.; Ueno, H.; Houck, K.; Ferrara, N.; Williams, L.T. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992, 255, 989–991. [Google Scholar]
- Alitalo, K.; Carmeliet, P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 2002, 1, 219–227. [Google Scholar]
- Takahashi, T.; Yamaguchi, S.; Chida, K.; Shibuya, M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-γ and DNA synthesis in vascular endothelial cells. EMBO J 2001, 20, 2768–2778. [Google Scholar]
- Sakurai, Y.; Ohgimoto, K.; Kataoka, Y.; Yoshida, N.; Shibuya, M. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 1076–1081. [Google Scholar]
- Holland, P.W.; Garcia-Fernandez, J.; Williams, N.A.; Sidow, A. Gene duplications and the origins of vertebrate development. Development 1994, 1994, 125–133. [Google Scholar]
- Hirano, T.; Nishida, H. Developmental fates of larval tissues after metamorphosis in ascidian Halocynthia roretzi. I. Origin of mesodermal tissues of the juvenile. Dev. Biol 1997, 192, 199–210. [Google Scholar]
- Hoch, R.V.; Soriano, P. Roles of PDGF in animal development. Development 2003, 130, 4769–4784. [Google Scholar]
- Hoffmann, F.G.; Opazo, J.C. Evolution of the relaxin/insulin-like gene family in placental mammals: Implications for its early evolution. J. Mol. Evol 2011, 72, 72–79. [Google Scholar]
- Suga, H.; Hoshiyama, D.; Kuraku, S.; Katoh, K.; Kubokawa, K.; Miyata, T. Protein tyrosine kinase cDNAs from amphioxus, hagfish, and lamprey: Isoform duplications around the divergence of cyclostomes and gnathostomes. J. Mol. Evol 1999, 49, 601–608. [Google Scholar]
- Duchek, P.; Somogyi, K.; Jekely, G.; Beccari, S.; Rorth, P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 2001, 107, 17–26. [Google Scholar]
- Duloquin, L.; Lhomond, G.; Gache, C. Localized VEGF signaling from ectoderm to mesenchyme cells controls morphogenesis of the sea urchin embryo skeleton. Development 2007, 134, 2293–2302. [Google Scholar]
- Wu, Y.; Brock, A.R.; Wang, Y.; Fujitani, K.; Ueda, R.; Galko, M.J. A blood-borne PDGF/VEGF-like ligand initiates wound-induced epidermal cell migration in Drosophila larvae. Curr. Biol 2009, 19, 1473–1477. [Google Scholar]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem 1987, 162, 156–159. [Google Scholar]
- Satou, Y.; Imai, K.S.; Satoh, N. FGF genes in the basal chordate Ciona intestinalis. Dev. Genes Evol 2002, 212, 432–438. [Google Scholar]
- Wada, H.; Satoh, N. Details of the evolutionary history from invertebrates to vertebrates, as deduced from the sequences of 18S rDNA. Proc. Natl. Acad. Sci. USA 1994, 91, 1801–1804. [Google Scholar]
- Tsagkogeorga, G.; Turon, X.; Galtier, N.; Douzery, E.J.; Delsuc, F. Accelerated evolutionary rate of housekeeping genes in tunicates. J. Mol. Evol 2010, 71, 153–167. [Google Scholar]
- Oda, H.; Wada, H.; Tagawa, K.; Akiyama-Oda, Y.; Satoh, N.; Humphreys, T.; Zhang, S.; Tsukita, S. A novel amphioxus cadherin that localizes to epithelial adherens junctions has an unusual domain organization with implications for chordate phylogeny. Evol. Dev 2002, 4, 426–434. [Google Scholar]
- Cañestro, C.; Bassham, S.; Postlethwait, J.H. Seeing chordate evolution through the Ciona genome sequence. Genome Biol 2003, 4, 208. [Google Scholar]
- Gee, H. Evolution: Careful with that amphioxus. Nature 2006, 439, 923–924. [Google Scholar]
- Lemaire, P. Evolutionary crossroads in developmental biology: The tunicates. Development 2011, 138, 2143–2152. [Google Scholar]
- Ishimaru, S.; Ueda, R.; Hinohara, Y.; Ohtani, M.; Hanafusa, H. PVR plays a critical role via JNK activation in thorax closure during Drosophila metamorphosis. EMBO J 2004, 23, 3984–3994. [Google Scholar]
- Heino, T.I.; Karpanen, T.; Wahlstrom, G.; Pulkkinen, M.; Eriksson, U.; Alitalo, K.; Roos, C. The Drosophila VEGF receptor homolog is expressed in hemocytes. Mech. Dev 2001, 109, 69–77. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Samarghandian, S.; Shibuya, M. Vascular Endothelial Growth Factor Receptor Family in Ascidians, Halocynthia roretzi (Sea Squirt). Its High Expression in Circulatory System-Containing Tissues. Int. J. Mol. Sci. 2013, 14, 4841-4853. https://doi.org/10.3390/ijms14034841
Samarghandian S, Shibuya M. Vascular Endothelial Growth Factor Receptor Family in Ascidians, Halocynthia roretzi (Sea Squirt). Its High Expression in Circulatory System-Containing Tissues. International Journal of Molecular Sciences. 2013; 14(3):4841-4853. https://doi.org/10.3390/ijms14034841
Chicago/Turabian StyleSamarghandian, Saeed, and Masabumi Shibuya. 2013. "Vascular Endothelial Growth Factor Receptor Family in Ascidians, Halocynthia roretzi (Sea Squirt). Its High Expression in Circulatory System-Containing Tissues" International Journal of Molecular Sciences 14, no. 3: 4841-4853. https://doi.org/10.3390/ijms14034841



