Exploration of the Protection of Riboflavin Laurate on Oral Mucositis Induced by Chemotherapy or Radiotherapy at the Cellular Level: What Is the Leading Contributor?
Abstract
:1. Introduction
2. Results and Discussion
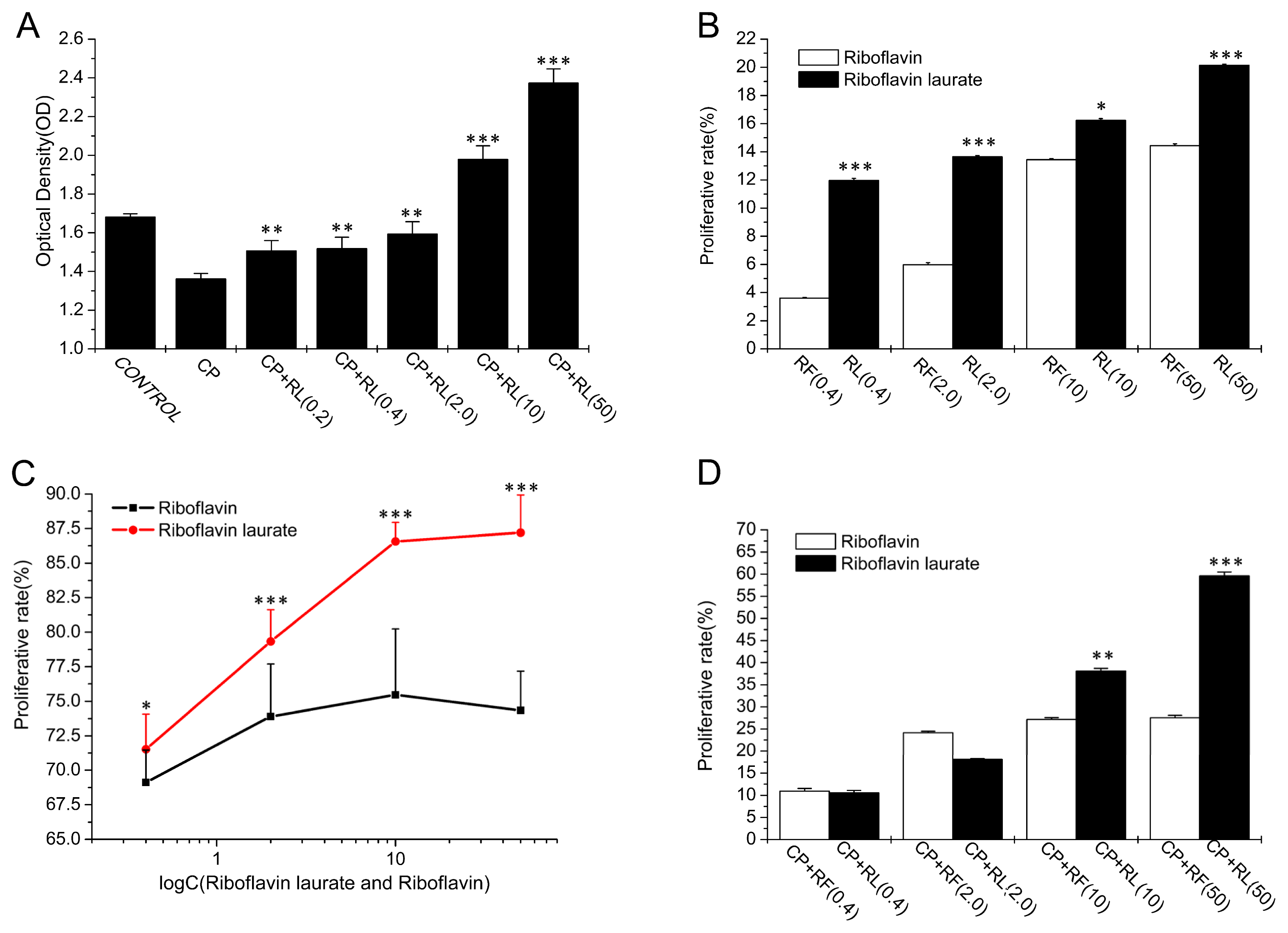
2.1. The Protective Properties of Chemotherapy, Radiotherapy-Induced Toxicity on Helf Cells
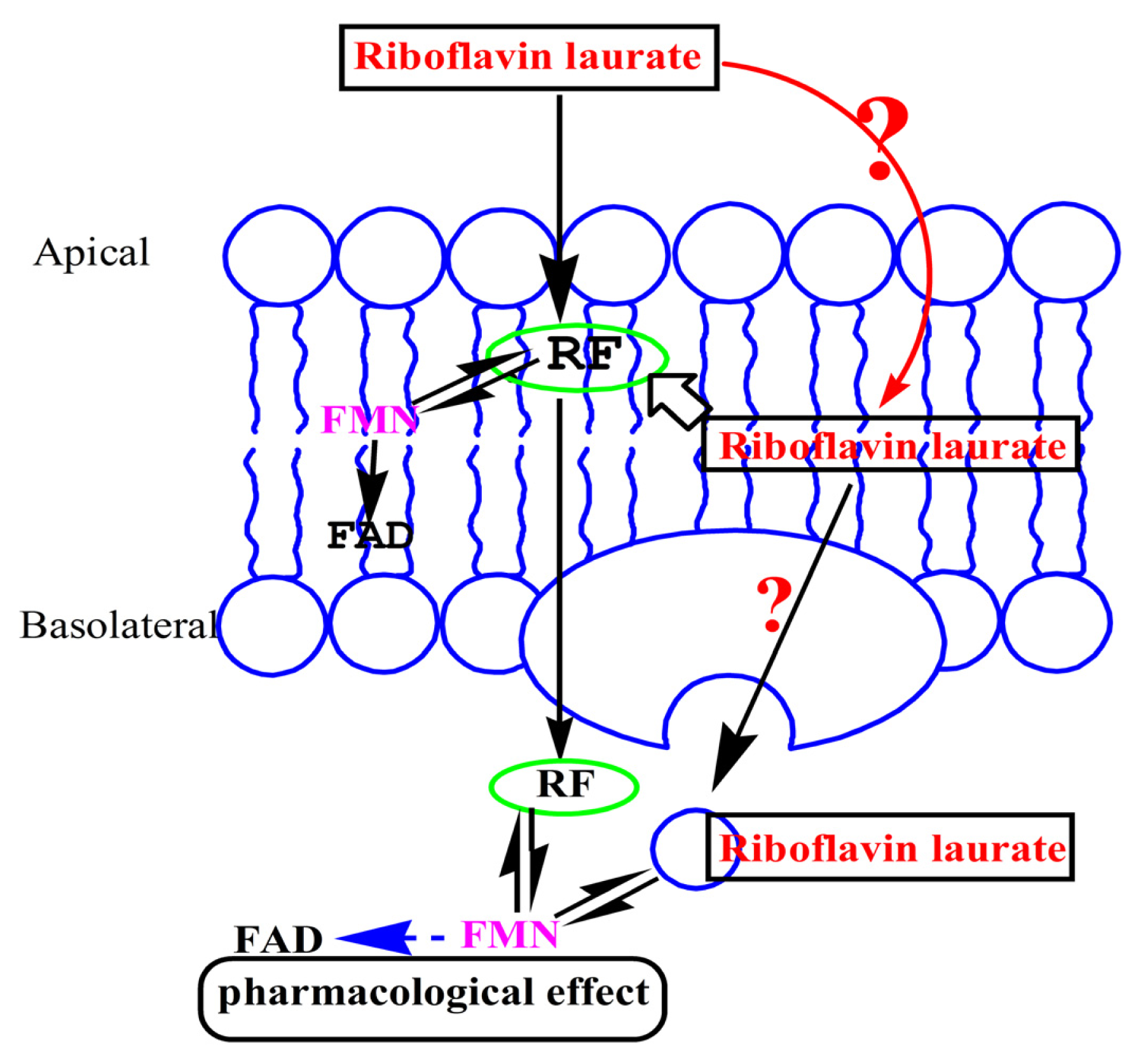
2.2. Transport Characteristics of Riboflavin Laurate by Caco-2 Cell Monolayer
2.2.1. Measurements of Caco-2 Cell Monolayer Integrity
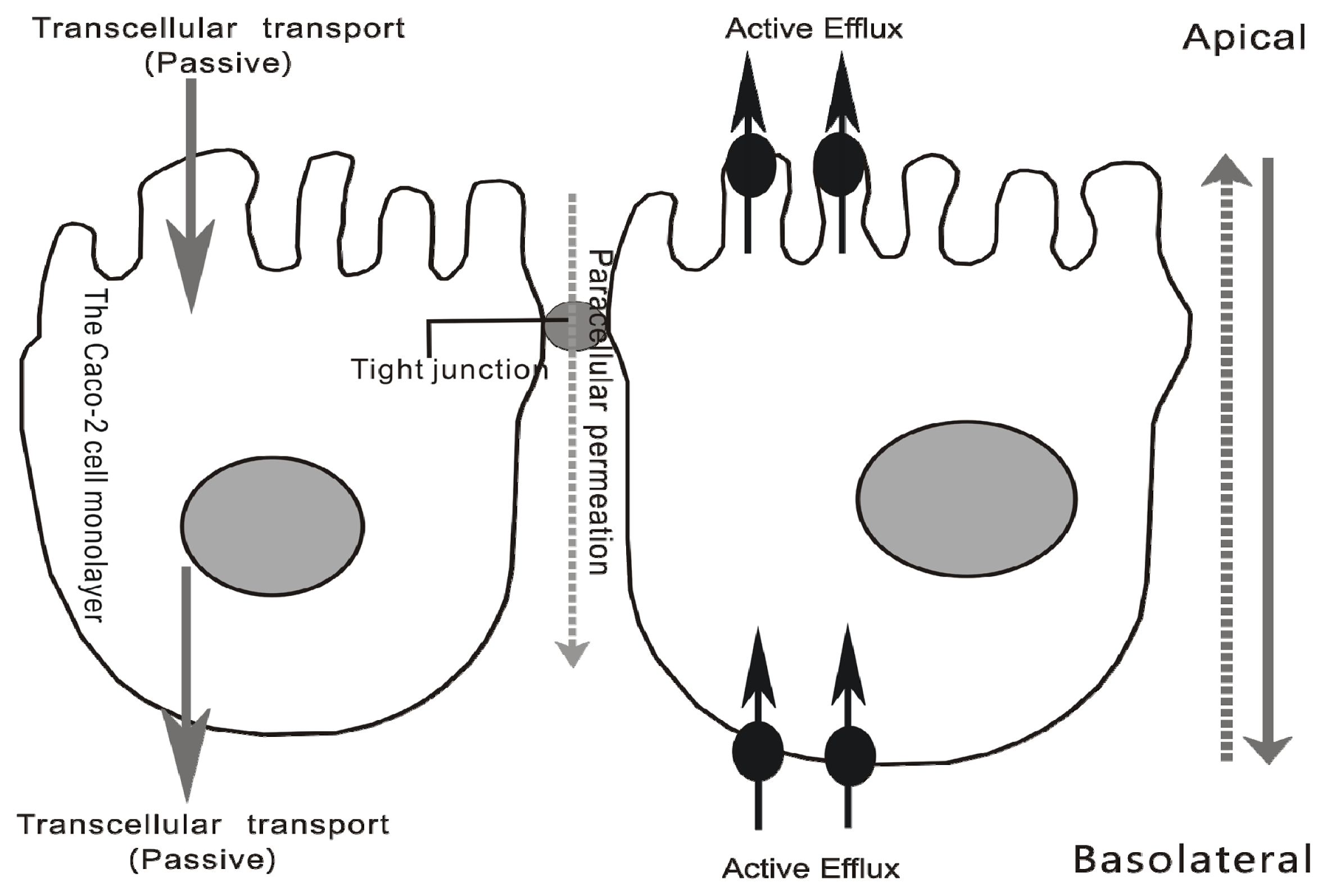
2.2.2. Transport across Caco-2 Cell Monolayer
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Riboflavin Laurate Ameliorates Chemotherapy, Radiotherapy-Induced Toxicities
3.2.2. Transport Studies Performed by Caco-2 cell Monolayer
3.3. Statistics
4. Conclusions
Acknowledgments
References
- Sinigaglia-Coimbra, R.; Lopes, A.C.; Coimbra, C.G. Riboflavin Deficiency, Brain Function, and Health. In Handbook of Behavior, Food and Nutrition, 153 ed.; Preedy, V.R., Ed.; US Determent of Agriculture: Washington, DC, USA, 2011; pp. 2427–2449. [Google Scholar]
- Wojcieszynska, D.; Hupert-Kocurek, K.; Guzik, U. Flavin-dependent enzymes in cancer prevention. Int. J. Mol. Sci 2012, 13, 16751–16768. [Google Scholar]
- Vasilaki, A.T.; McMillan, D.C.; Kinsella, J.; Duncan, A.; O’Reilly, D.S.; Talwar, D. Relation between riboflavin, flavin mononucleotide and flavin adenine dinucleotide concentrations in plasma and red cells in patients with critical illness. Clin. Chim. Acta 2010, 411, 1750–1755. [Google Scholar]
- Serrano, A.; Frago, S.; Velazquez-Campoy, A.; Medina, M. Role of Key Residues at the Flavin Mononucleotide(FMN): Adenylyltransferase Catalytic Site of the Bifunctional Riboflavin Kinase/Flavin Adenine Dinucleotide (FAD) Synthetase from Corynebacterium ammoniagenes. Int. J. Mol. Sci 2012, 13, 14492–14517. [Google Scholar]
- Northrop-Clewes, C.A.; Thurnham, D.I. The Discovery and Characterization of Riboflavin. Ann. Nutr. Metab 2012, 61, 224–230. [Google Scholar]
- Powers, H.J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr 2003, 77, 1352–1360. [Google Scholar]
- Blanck, H.M.; Bowman, B.A.; Serdula, M.K.; Khan, L.K.; Kohn, W.; Woodruff, B.A. Bhutanese Refugee Investigation Group. Angular stomatitis and riboflavin status among adolescent Bhutanese refugees living in southeastern Nepal. Am. J. Clin. Nutr 2002, 76, 430–435. [Google Scholar]
- Fu, Q.Y.; Deng, F.C.; Zhang, Z.H. Curative effect and influence of serum IL-2 on patients with recurrent oral ulcers using riboflavine laurate [Chinese]. J. Hainan Med. Coll 2009, 15, 1303–1304. [Google Scholar]
- Wang, Y.B.; Wang, Q.; Zhang, H.; Ren, J.P.; Wang, F.; Xu, Q.S. Protective effects of riboflavin laurate on oral mucosa during chemotherapy in rats [Chinese]. Infect. Inflamm. Rep 2007, 8, 26–29. [Google Scholar]
- Wu, N.H.; Qu, S.Q.; Luan, Z.; Xu, S.X.; Gong, X.J.; Hu, X.H.; Tang, X.F. Prevention of drug adverse effects by use of riboflavin laurate in high-dose methotrexate chemotherapy [In Chinese]. J. China Pediatr. Blood Cancer 2006, 11, 73–75. [Google Scholar]
- Zielinska-Dawidziak, M.; Grajek, K.; Olejnik, A.; Czaczyk, K.; Grajek, W. Transport of High Concentration of Thiamin, Riboflavin and Pyridoxine across Intestinal Epithelial Cells Caco-2. J. Nutr. Sci. Vitaminol 2008, 54, 423–429. [Google Scholar]
- Said, H.M.; Ma, T.Y. Mechanism of riboflavine uptake by Caco-2 human intestinal epithelial cells. Am. J. Physiol 1994, 266, G15–G21. [Google Scholar]
- Plevova, P. Prevention and treatment of chemotherapy- and radiotherapy- induced oral mucositis review. Oral. Oncol 1999, 35, 453–470. [Google Scholar]
- Caillot, E.; Denis, F. Radio-induced oral and pharyngeal mucositis: Management updates. Cancer Radiother 2012, 16, 358–363. [Google Scholar]
- Zur, E. Oral mucositis: Etiology, and clinical and pharmaceutical management. Int. J. Pharm. Compd 2012, 16, 22–33. [Google Scholar]
- Nilgün, K.N.; Ozlem, O.; Alper, S. Evaluation of the effect of cryotherapy in preventing oral mucositis associated with chemotherapy—A randomized controlled trial. Eur. J. Oncol. Nurs 2012, 16, 339–344. [Google Scholar]
- Bodiga, V.L.; Bodiga, S.; Surampudi, S.; Boindala, S.; Putcha, U.; Nagalla, B.; Subramaniam, K.; Manchala, R. Effect of vitamin supplementation on cisplatin-induced intestinal epithelial cell apoptosis in Wistar/NIN rats. J. Nutr 2012, 28, 572–580. [Google Scholar]
- Broeders, J.J.; van Eijkeren, J.C.; Blaauboer, B.J.; Hermens, J.L. Transport of chlorpromazine in the Caco-2 cell permeability assay: a kinetic study. Chem. Res. Toxicol 2012, 25, 1442–1451. [Google Scholar]
- Miller, M.; Taylor, A.; Kearney, N.; Paterson, G.; Wells, M.; Roe, L.; Hagen, S.; Maguire, R. Evaluation of the feasibility and acceptability of an oral care diary by patients during chemotherapy. Int. J. Nurs. Stud 2007, 44, 693–701. [Google Scholar]
- Nakano, E.; Mushtaq, S.; Heath, P.R.; Lee, E.S.; Bury, J.P.; Riley, S.A.; Powers, H.J.; Corfe, B.M. Riboflavin depletion impairs cell proliferation in adult human duodenum: Identification of potential effectors. Dig. Dis. Sci 2011, 56, 1007–1019. [Google Scholar]
- Hassan, I.; Chibber, S.; Khan, A.A.; Naseem, I. Riboflavin ameliorates cisplatin induced toxicities under photoillumination. PLoS One 2012, 7, e36273. [Google Scholar]
- Sugano, K.; Kansy, M.; Artursson, P.; Avdeef, A.; Bendels, S.; Di, L.; Ecker, G.F.; Faller, B.; Fischer, H.; Gerebtzoff, G.; et al. Coexistence of passive and carrier-mediated processes in drug transport. Nat. Rev. Drug. Discov 2010, 9, 597–614. [Google Scholar]
- Barrington, R.; Williamson, G.; Bennett, R.N.; Davis, B.D.; Brodbelt, J.S.; Kroon, P.A. Absorption, Conjugation and Efflux of the Flavonoids, Ampere and Galantine, Using the Intestinal CACO-2/ATC Cell Model. J. Funct. Foods 2009, 1, 74–87. [Google Scholar]
- Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; Hillgren, K.M.; et al. International Transporter C. Membrane transporters in drug development. Nat. Rev. Drug. Discov. 2010, 9, 215–236. [Google Scholar]
- Teng, Z.H.; Yuan, C.J.; Zhang, F.; Huan, M.L.; Cao, W.D.; Li, K.C.; Yang, J.Y.; Cao, D.Y.; Zhou, S.Y.; Mei, Q.B. Intestinal Absorption and First-Pass Metabolism of Polyphenol Compounds in Rat and Their Transport Dynamics in Caco-2 Cells. PLoS One 2012, 7, e29647. [Google Scholar]
- Kratz, J.M.; Teixeira, M.R.; Koester, L.S.; Simões, C.M.O. An HPLC-UV method for the measurement of permeability of marker drugs in the Caco-2 cell assay. Braz. J. Med. Biol. Res 2011, 44, 531–537. [Google Scholar]
- Petteys, B.J.; Frank, E.L. Rapid determination of vitamin B(2) (riboflavin) in plasma by HPLC. Clin. Chim. Acta 2011, 412, 38–43. [Google Scholar]
- Youdim, K.A.; Avdeef, A.; Abbott, N.J. In vitro trans-monolayer permeability calculations: often forgotten assumptions. Drug. Discov. Today 2003, 8, 997–1003. [Google Scholar]
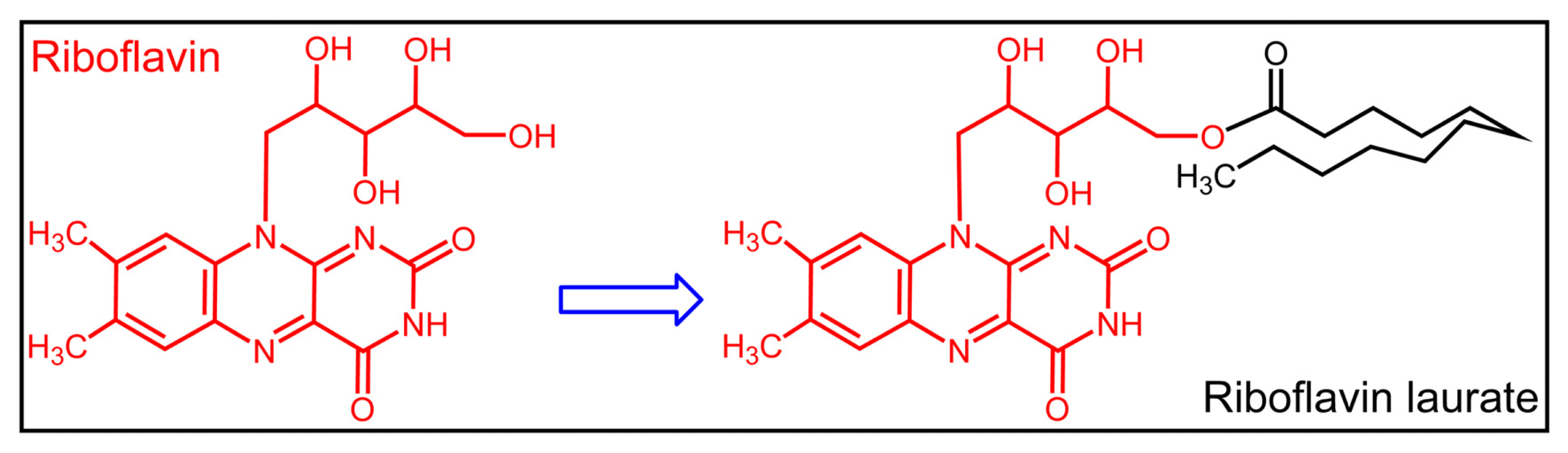
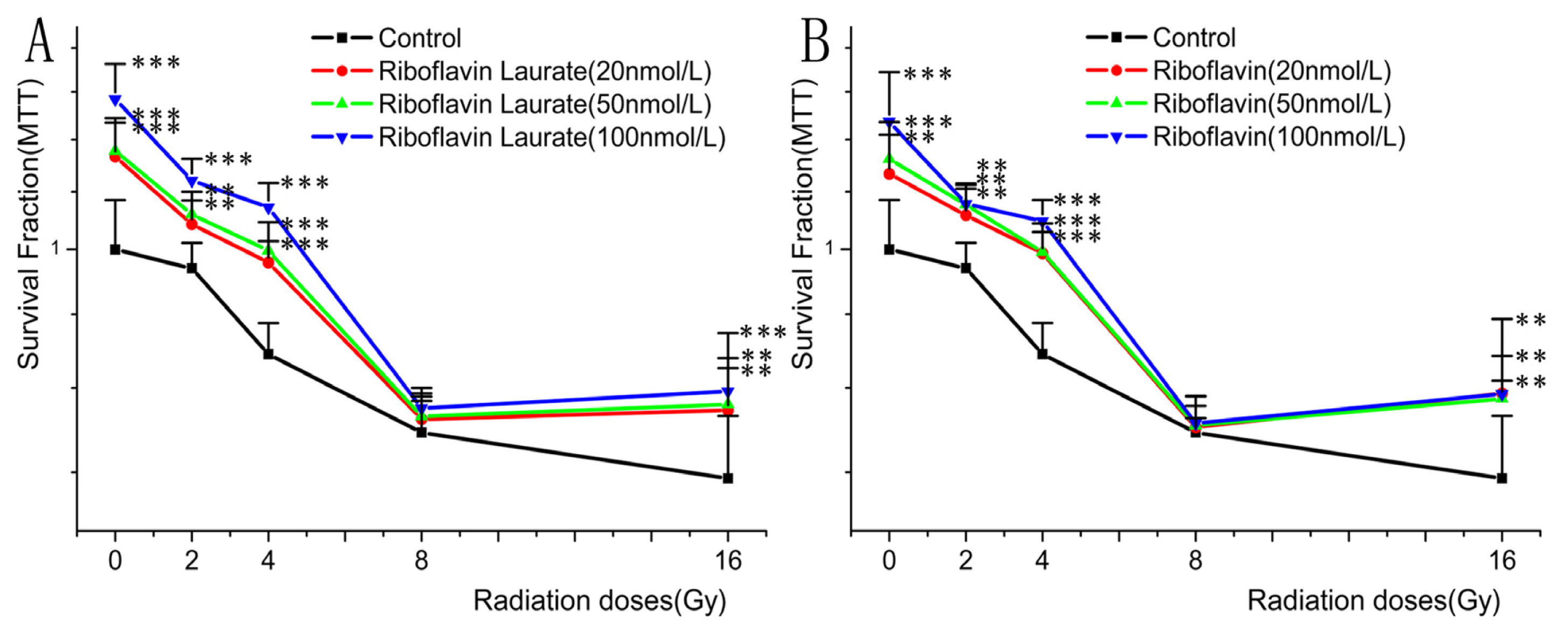
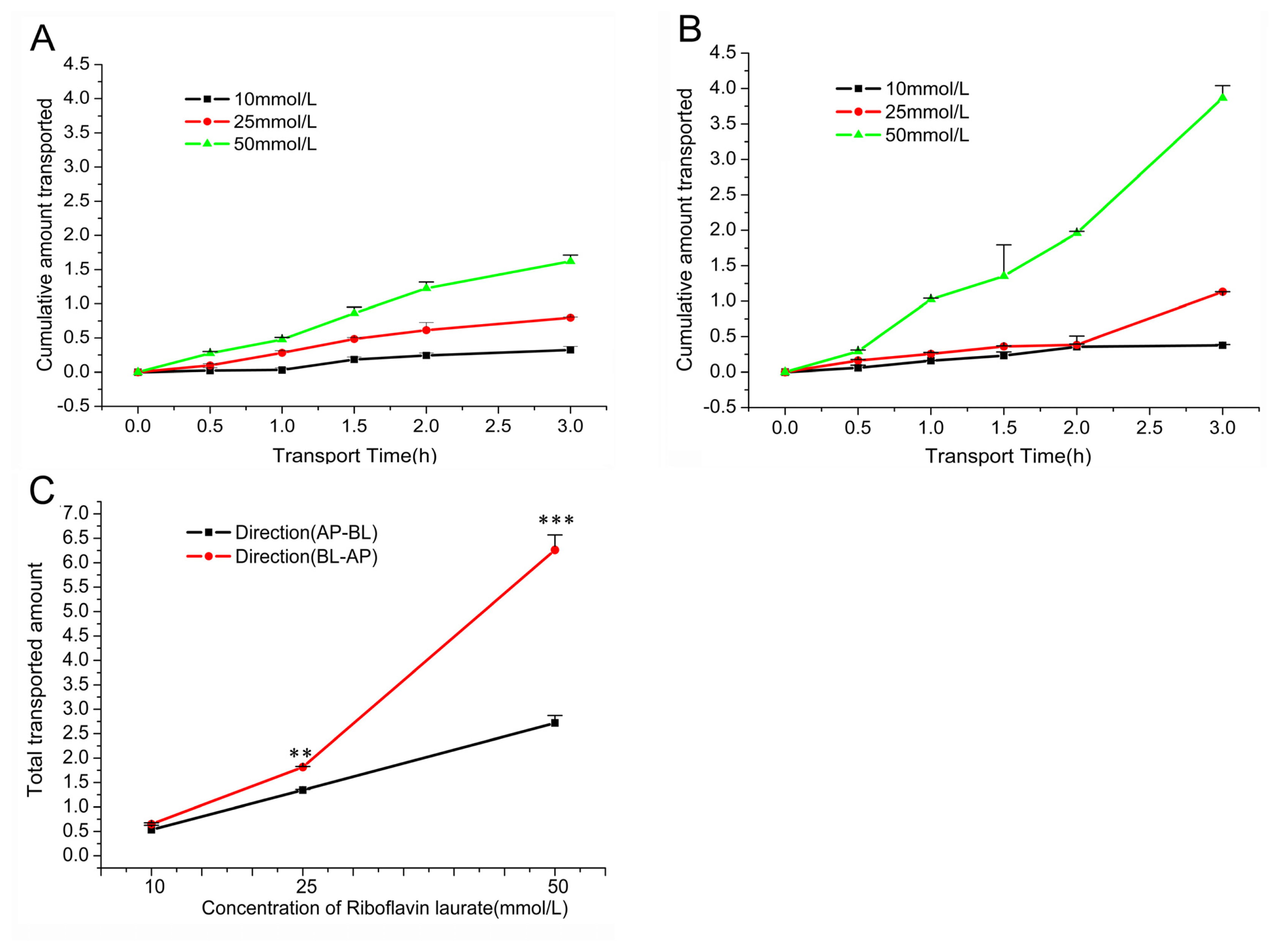
| Group | y = ax + b | D90 (Gy) | DRF |
|---|---|---|---|
| Control | y = −0.0218x + 0.965 | 2.982 | 1.000 |
| Riboflavin (20 nmol/L) | y = −0.0249x + 1.090 | 7.631 | 2.559 |
| Riboflavin (50 nmol/L) | y = −0.0271x + 1.112 | 7.823 | 2.624 |
| Riboflavin (100 nmol/L) | y = −0.0305x + 1.163 | 8.623 | 2.892 |
| Riboflavin laurate (20 nmol/L) | y = −0.0270x + 1.100 | 7.407 | 2.484 |
| Riboflavin laurate (50 nmol/L) | y = −0.0276x + 1.116 | 7.826 | 2.625 |
| Riboflavin laurate (100 nmol/L) | y = −0.0335x + 1.212 | 9.313 | 3.124 |
| Concentration | AP-BL | BL-AP | EfR | ||
|---|---|---|---|---|---|
| Papp (10−6, cm/s) | %T | Papp (10−6, cm/s) | %T | ||
| 10(mmol/L) | 4.4173 ± 0.7591 | 5.34 ± 0.92 | 5.357 ± 0.2442 | 6.48 ± 0.30 | 1.213 |
| 25(mmol/L) | 4.4431 ± 0.0003 | 5.37 ± 0.06 | 6.004 ± 0.0440 | 7.26 ± 0.05 | 1.351 |
| 50(mmol/L) | 4.4947 ± 0.2530 | 5.44 ± 0.31 | 10.35 ± 0.5057 | 12.52 ± 0.61 | 2.303 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xuan, Z.; An, Y.; Yang, D.; Wang, S.; Xu, Q.; Yuan, S. Exploration of the Protection of Riboflavin Laurate on Oral Mucositis Induced by Chemotherapy or Radiotherapy at the Cellular Level: What Is the Leading Contributor? Int. J. Mol. Sci. 2013, 14, 4722-4733. https://doi.org/10.3390/ijms14034722
Xuan Z, An Y, Yang D, Wang S, Xu Q, Yuan S. Exploration of the Protection of Riboflavin Laurate on Oral Mucositis Induced by Chemotherapy or Radiotherapy at the Cellular Level: What Is the Leading Contributor? International Journal of Molecular Sciences. 2013; 14(3):4722-4733. https://doi.org/10.3390/ijms14034722
Chicago/Turabian StyleXuan, Zixue, Yinghong An, Dexuan Yang, Shanshan Wang, Qishou Xu, and Shoujun Yuan. 2013. "Exploration of the Protection of Riboflavin Laurate on Oral Mucositis Induced by Chemotherapy or Radiotherapy at the Cellular Level: What Is the Leading Contributor?" International Journal of Molecular Sciences 14, no. 3: 4722-4733. https://doi.org/10.3390/ijms14034722
APA StyleXuan, Z., An, Y., Yang, D., Wang, S., Xu, Q., & Yuan, S. (2013). Exploration of the Protection of Riboflavin Laurate on Oral Mucositis Induced by Chemotherapy or Radiotherapy at the Cellular Level: What Is the Leading Contributor? International Journal of Molecular Sciences, 14(3), 4722-4733. https://doi.org/10.3390/ijms14034722




