Expression and Functions of Fibroblast Growth Factor 10 in the Mouse Mammary Gland
Abstract
:1. Introduction
2. Results
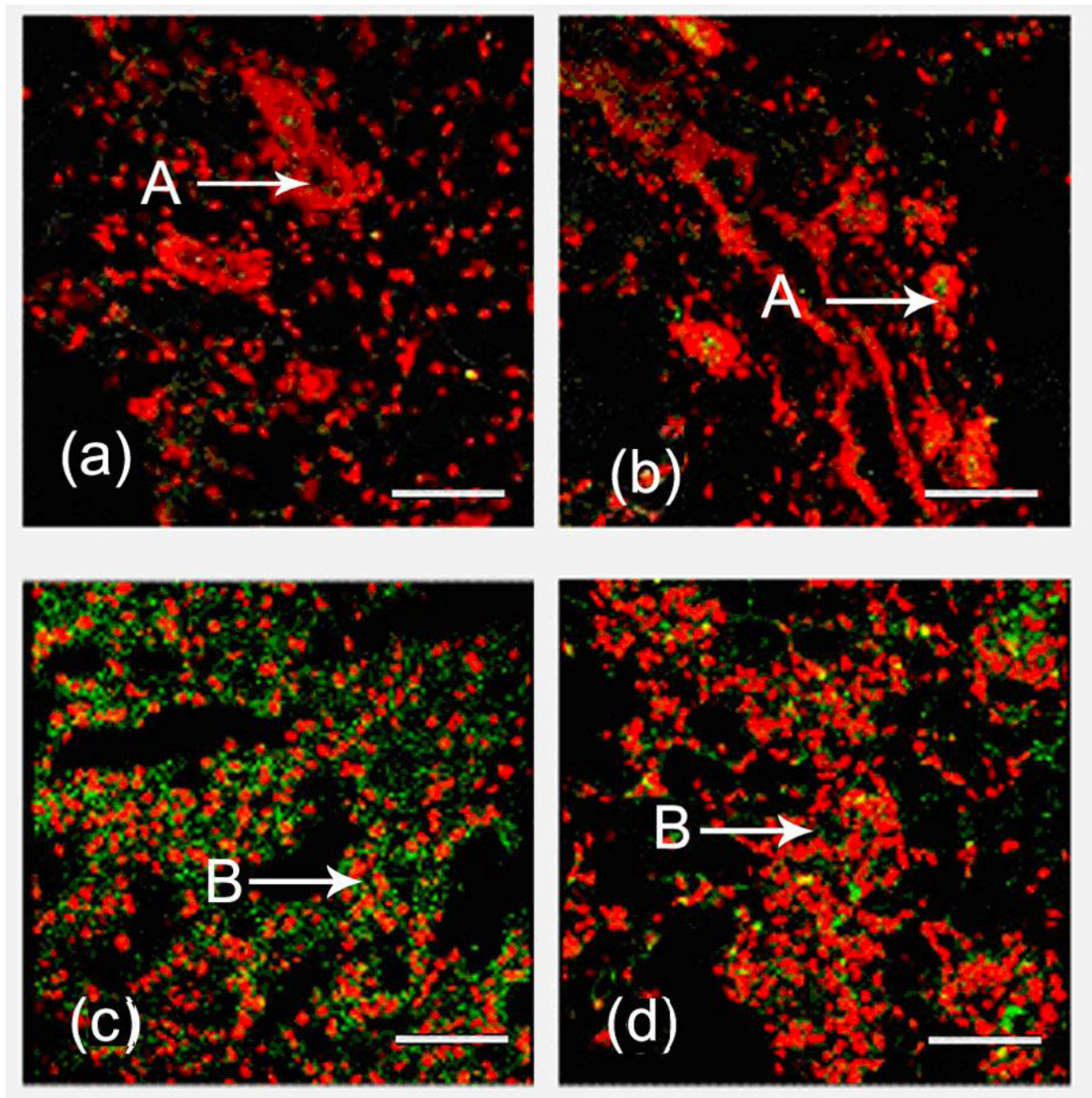
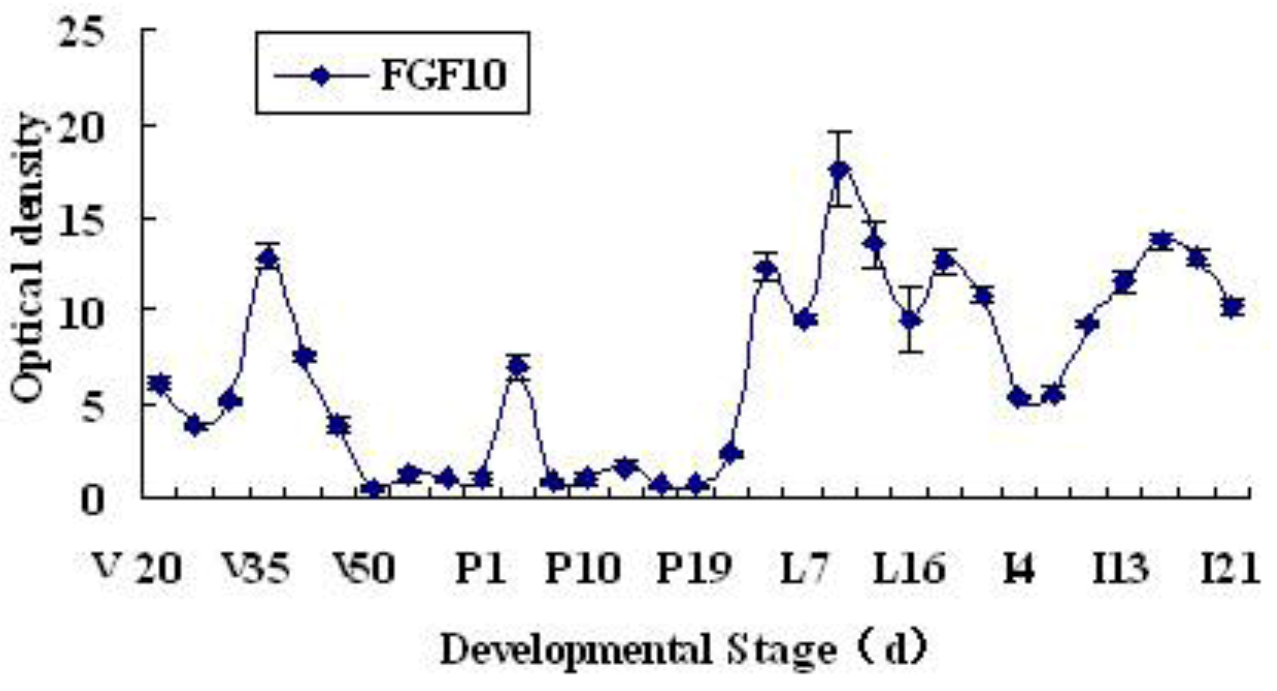
2.1. Expression and Localization of FGF10 in the Mouse Mammary Gland
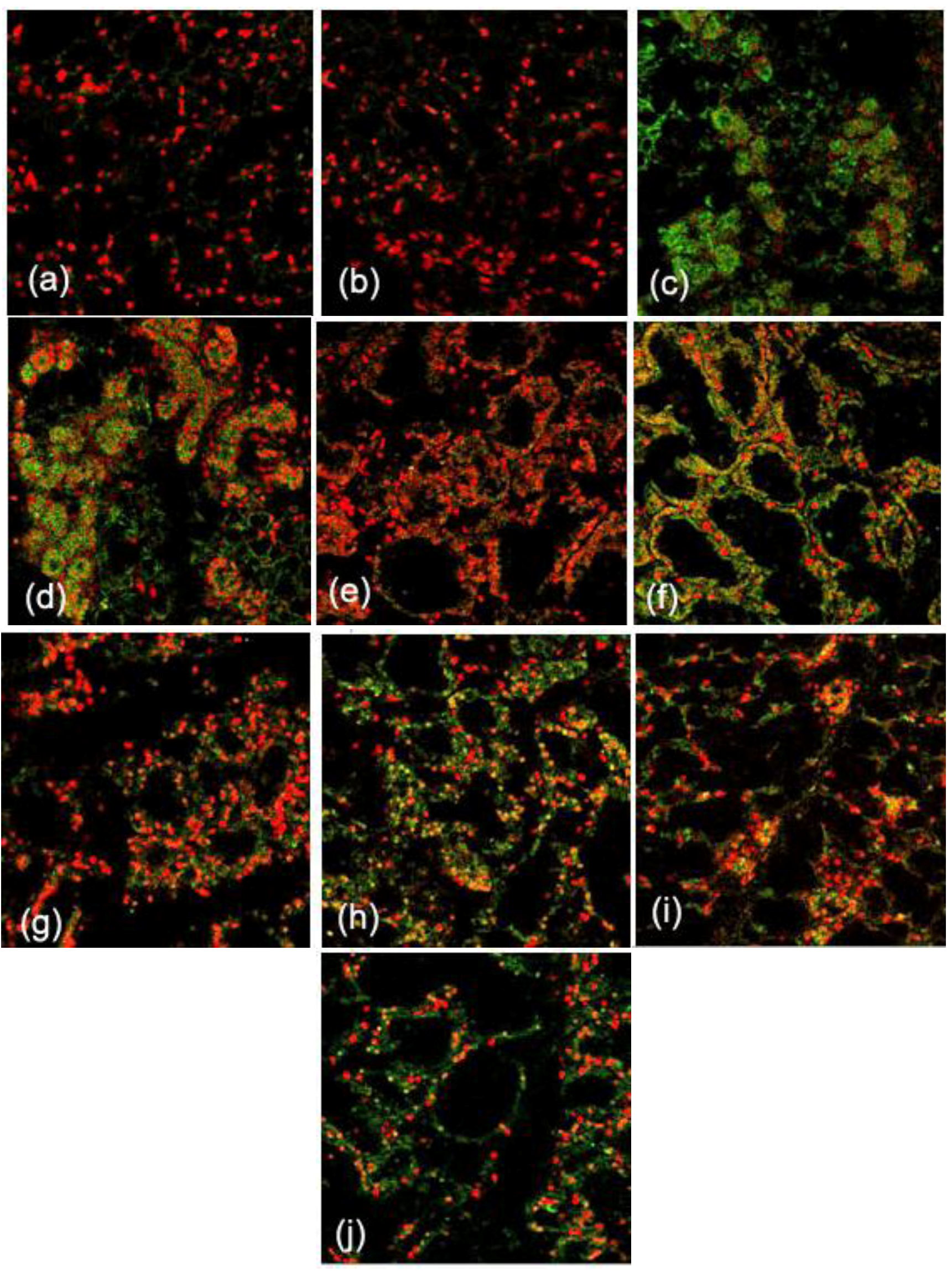
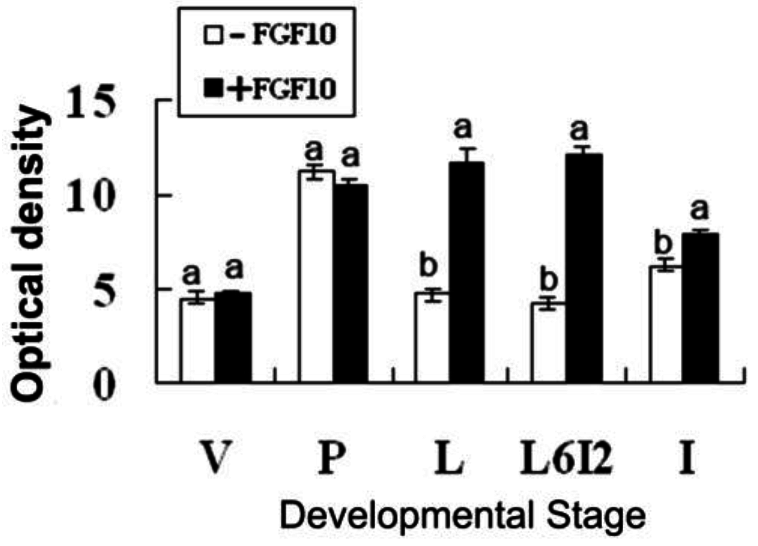
2.2. Effects of FGF10 in the Mouse Mammary Gland
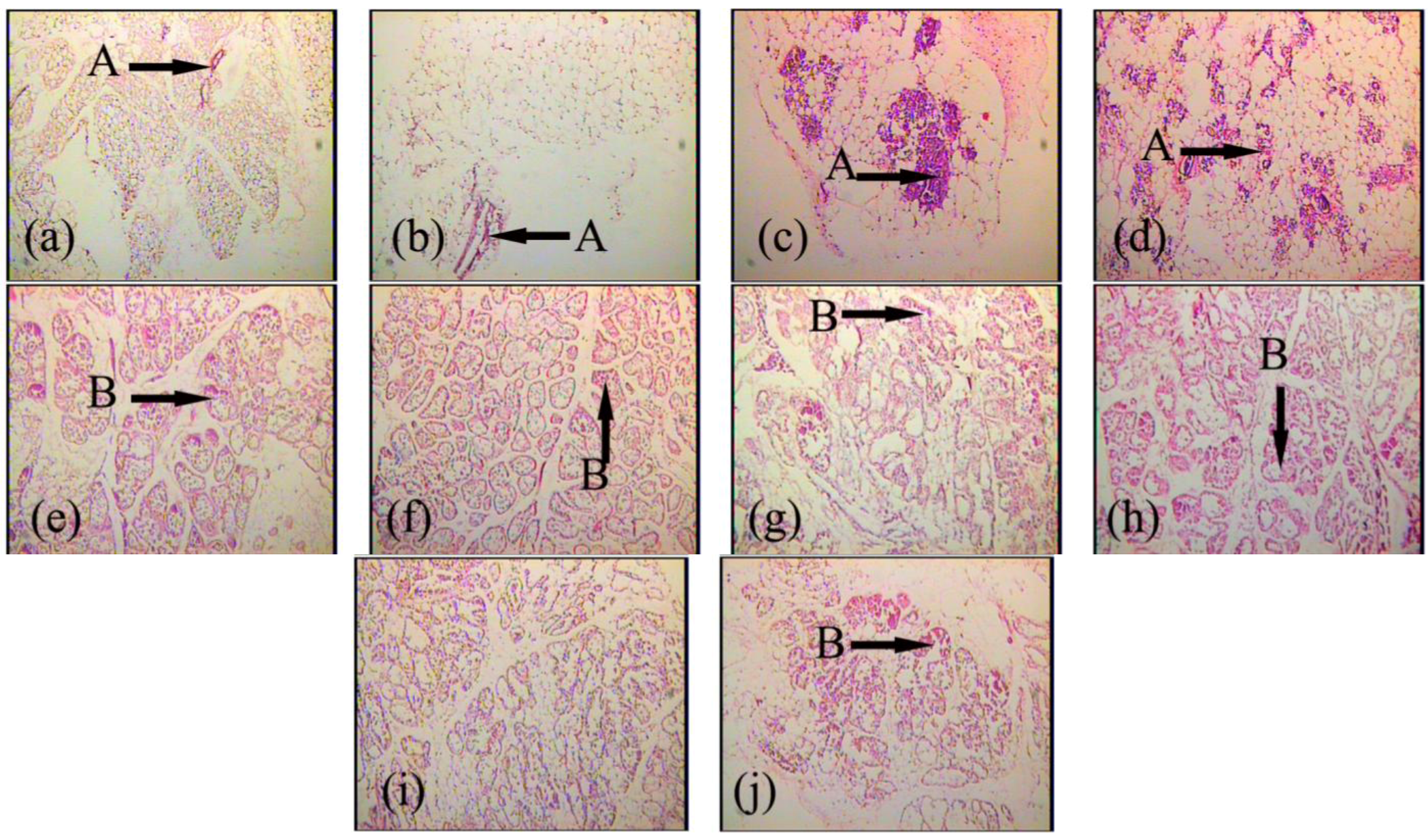
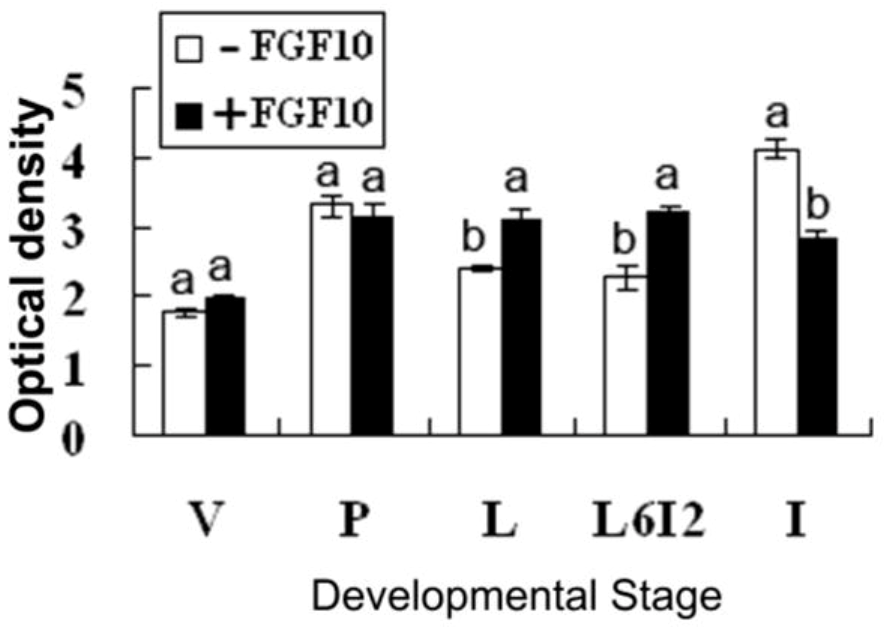
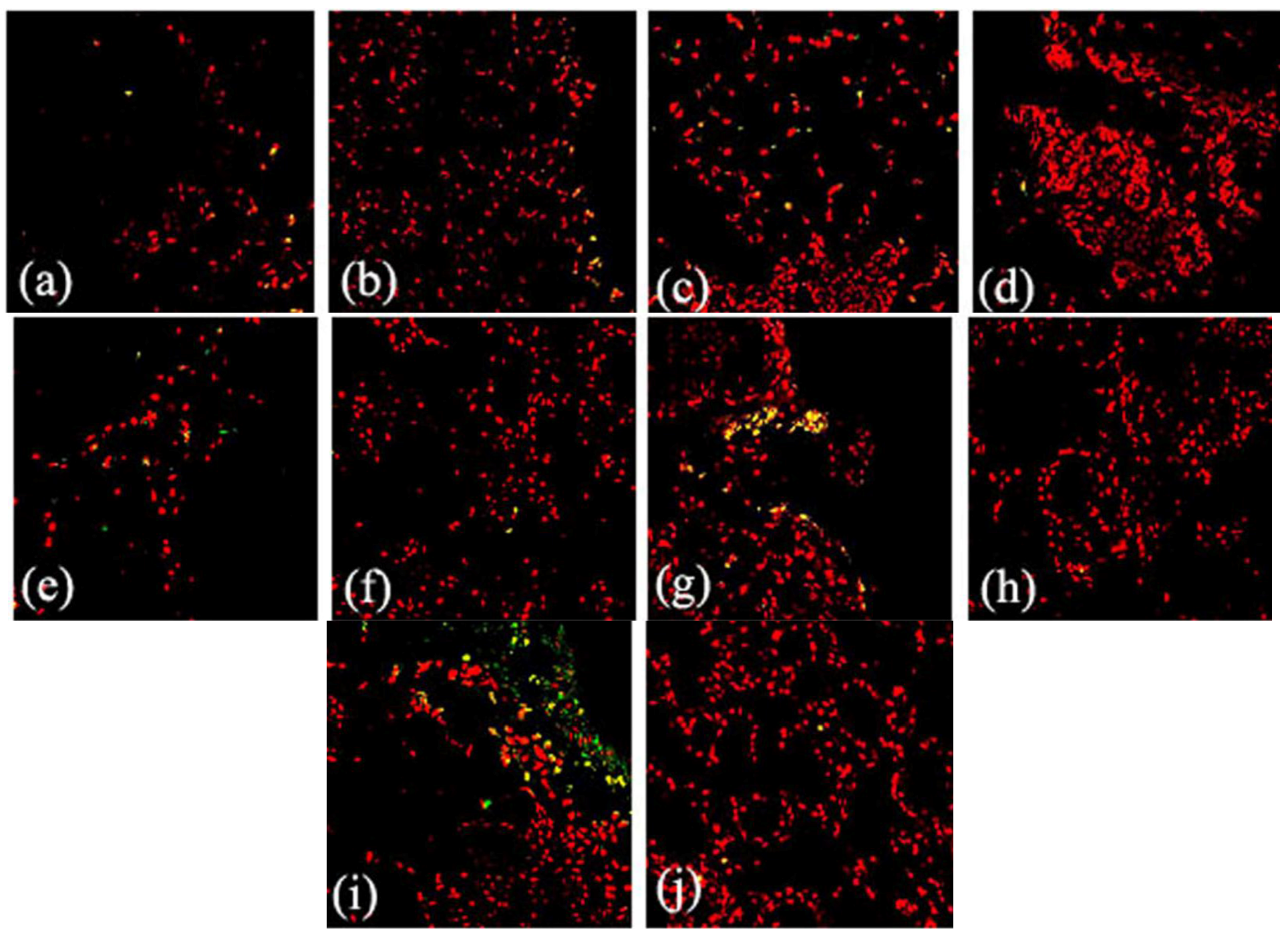
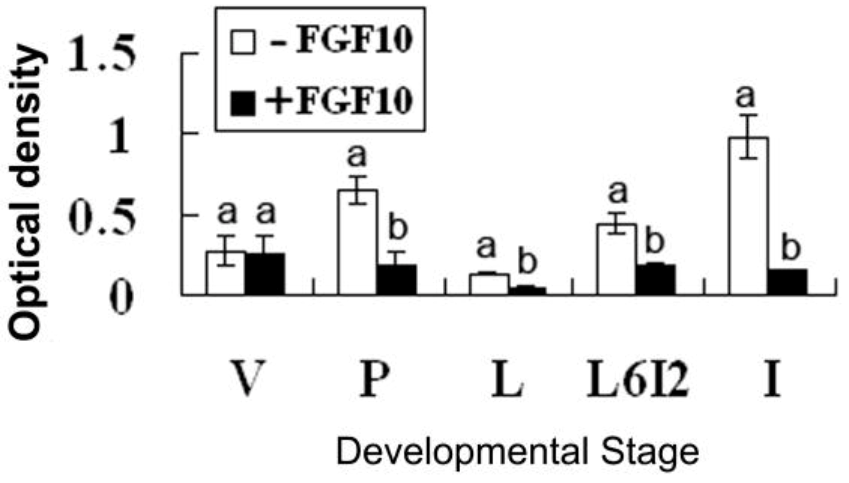
3. Discussion
3.1. Expression and Localization of FGF10 in the Mouse Mammary Gland
3.2. FGF10 and Mouse Mammary Gland Morphology
3.3. FGF10 and Apoptosis
4. Materials and Methods
4.1. Animals, Tissue Sampling, and Preparation
4.2. Materials
4.3. Mammary Explant Culture
4.4. Immunofluorescence
4.5. Apoptosis
4.6. Image Analysis
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Mailleux, A.A.; Spencer-Dene, B.; Dillon, C.; Ndiaye, D.; Savona-Baron, C.; Itoh, N.; Kato, S.; Dickson, C.; Thiery, J.P.; Bellusci, S. Role of FGF10 /FGFR2b signaling during mammary gland development in the mouse embryo. Development 2002, 129, 53–60. [Google Scholar]
- Yamasaki, M.; Miyake, A.; Tagashira, S.; Itoh, N. Structure and expression of the rat mRNA encoding a novel member of the fibroblast growth factor family. Biol. Chem. 1996, 271, 15918–15921. [Google Scholar] [CrossRef]
- Pedchenko, V.K.; Imagawa, W. Pattern of expression of the KGF receptor and its ligands KGF and FGF-10 during postnatal mouse mammary gland development. Mol. Reprod. Dev. 2000, 56, 441–447. [Google Scholar] [CrossRef]
- Steinberg, Z.; Myers, C.; Heim, V.M.; Lathrop, C.A.; Rebustini, I.T.; Stewart, J.S.; Larsen, M.; Hoffman, M.P. FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development 2005, 132, 1223–1234. [Google Scholar] [CrossRef]
- Spencer-Dene, B.; Dillon, C.; Fantl, V.; Kerr, K.; Petiot, A.; Dickson, C. Fibroblast growth factor signalling in mouse mammary gland development. Endocr. Relat. Cancer 2001, 8, 211–217. [Google Scholar] [CrossRef]
- Veltmaat, J.M.; Relaix, F.; Le, L.T.; Kratochwil, K.; Sala, F.G.; van Veelen, W.; Rice, R.; Spencer-Dene, B.; Mailleux, A.A.; Rice, D.P.; et al. Gli3-mediated somitic Fgf10 expression gradients are required for the induction and patterning of mammary epithelium along the embryonic axes. Development 2006, 133, 2325–2335. [Google Scholar] [CrossRef]
- Parsa, S.; Ramasamy, S.K.; de Langhe, S.; Gupte, V.V.; Haigh, J.J.; Medina Dand Bellusci, S. Terminal end bud maintenance in mammary gland is dependent upon FGFR2b signaling. Dev. Biol. 2008, 317, 121–131. [Google Scholar] [CrossRef]
- Schwertfeger, K.L. Fibroblast growth factors in development and cancer: Insights from the mammary and prostate glands. Curr. Drug Targets 2009, 7, 632–644. [Google Scholar]
- Huijts, P.E.; van Dongen, M.; de Goeij, M.C.; van Moolenbroek, A.J.; Blanken, F.; Vreeswijk, M.P.; de Kruijf, E.M.; Mesker, W.E.; van Zwet, E.W.; Tollenaar, R.A.; et al. Tumor-derived fibroblasts expressed eight times more FGFR2 mRNA than the corresponding fibroblasts from normal breast tissue. Breast Cancer Res. 2011, 4, R72. [Google Scholar]
- Bellusci, S.; Grindley, J.; Emoto, H.; Itoh, N.; Hogan, B.L. Fibroblast growth factor 10 (FGF10 ) and branching morphogenesis in the embryonic mouse lung. Development 1997, 124, 4867–4878. [Google Scholar]
- Thomson, A.A.; Cunha, G. Prostatic growth and development are regulated by FGF-10. Development 1999, 126, 3693–3701. [Google Scholar]
- Hyatt, B.A.; Shangguan, X.; Shannon, J.M. FGF-10 induces SP-C and Bmp4 and regulates proximal-distal patterning in embryonic tracheal epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L1116–L1126. [Google Scholar] [CrossRef]
- Warburton, D.; Lee, M.K. Current concepts on lung development. Curr. Opin. Pediatr. 1999, 11, 188–192. [Google Scholar] [CrossRef]
- Min, H.; Danilenko, D.M.; Scully, S.A.; Bolon, B.; Ring, B.D.; Tarpley, J.E.; DeRose, M.; Simonet, W.S. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998, 12, 3156–3161. [Google Scholar] [CrossRef]
- Sekine, K.; Ohuchi, H.; Fujiwara, M.; Yamasaki, M.; Yoshizawa, T.; Sato, T.; Yagishita, N.; Matsui, D.; Koga, Y.; Itoh, N.; et al. Fgf10 is essential for limb and lung formation. Nat. Genet. 1999, 21, 138–141. [Google Scholar] [CrossRef]
- Hishikawa, Y.; Tamaru, N.; Ejima, K.; Hayashi, T.; Koji, T. Expression of keratinocyte growth factor and its receptor in human breast cancer: Its inhibitory role in the induction of apoptosis possibly through the overexpression of Bcl-2. Arch. Histol. Cytol. 2004, 67, 455–464. [Google Scholar] [CrossRef]
- Pardo, O.E.; Arcaro, A.; Salerno, G.; Raguz, S.; Downward, J.; Seckl, M.J. Fibroblast growth factor-2 induces translational regulation of Bcl-XL and Bcl-2 via a MEK-dependent pathway:correlation with resistance to etoposide-induced apoptosis. J. Biol. Chem. 2002, 277, 12040–12046. [Google Scholar]
- Upadhyay, D.; Panduri, V.; Kamp, D.W. Fibroblast growth factor-10 prevents asbestos-induced alveolar epithelial cell apoptosis by a mitogen-activated protein kinase-dependent mechanism. Am. J. Respir. Cell Mol. Biol. 2005, 32, 232–238. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cui, Y.; Li, Q. Expression and Functions of Fibroblast Growth Factor 10 in the Mouse Mammary Gland. Int. J. Mol. Sci. 2013, 14, 4094-4105. https://doi.org/10.3390/ijms14024094
Cui Y, Li Q. Expression and Functions of Fibroblast Growth Factor 10 in the Mouse Mammary Gland. International Journal of Molecular Sciences. 2013; 14(2):4094-4105. https://doi.org/10.3390/ijms14024094
Chicago/Turabian StyleCui, Yingjun, and Qingzhang Li. 2013. "Expression and Functions of Fibroblast Growth Factor 10 in the Mouse Mammary Gland" International Journal of Molecular Sciences 14, no. 2: 4094-4105. https://doi.org/10.3390/ijms14024094



