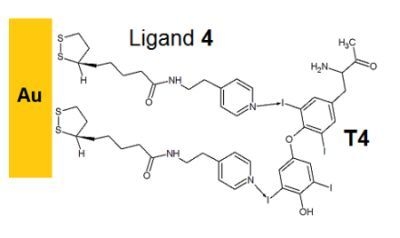
Self-Assembly of Pyridine-Modified Lipoic Acid Derivatives on Gold and Their Interaction with Thyroxine (T4)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Self-Assembly of the Lipoates and Thin Layer Formation
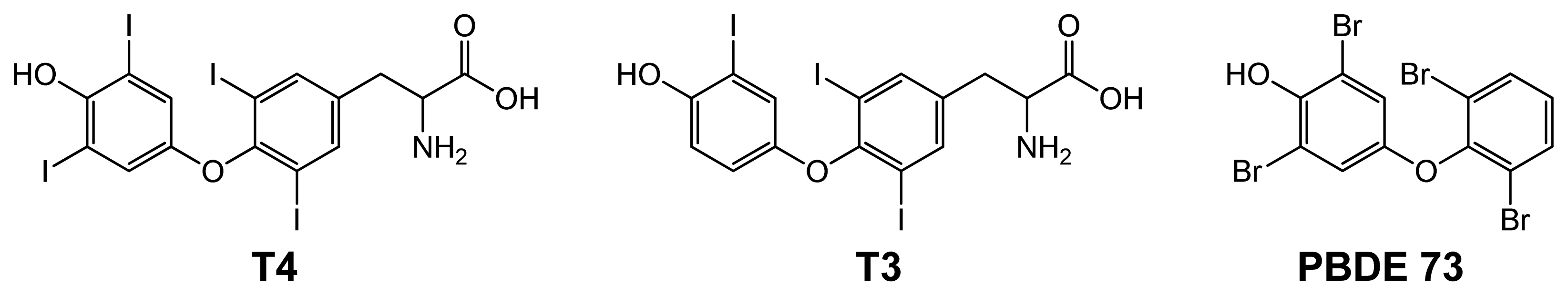
2.2. Binding of T4 to Self-Assembled Lipoate Layers
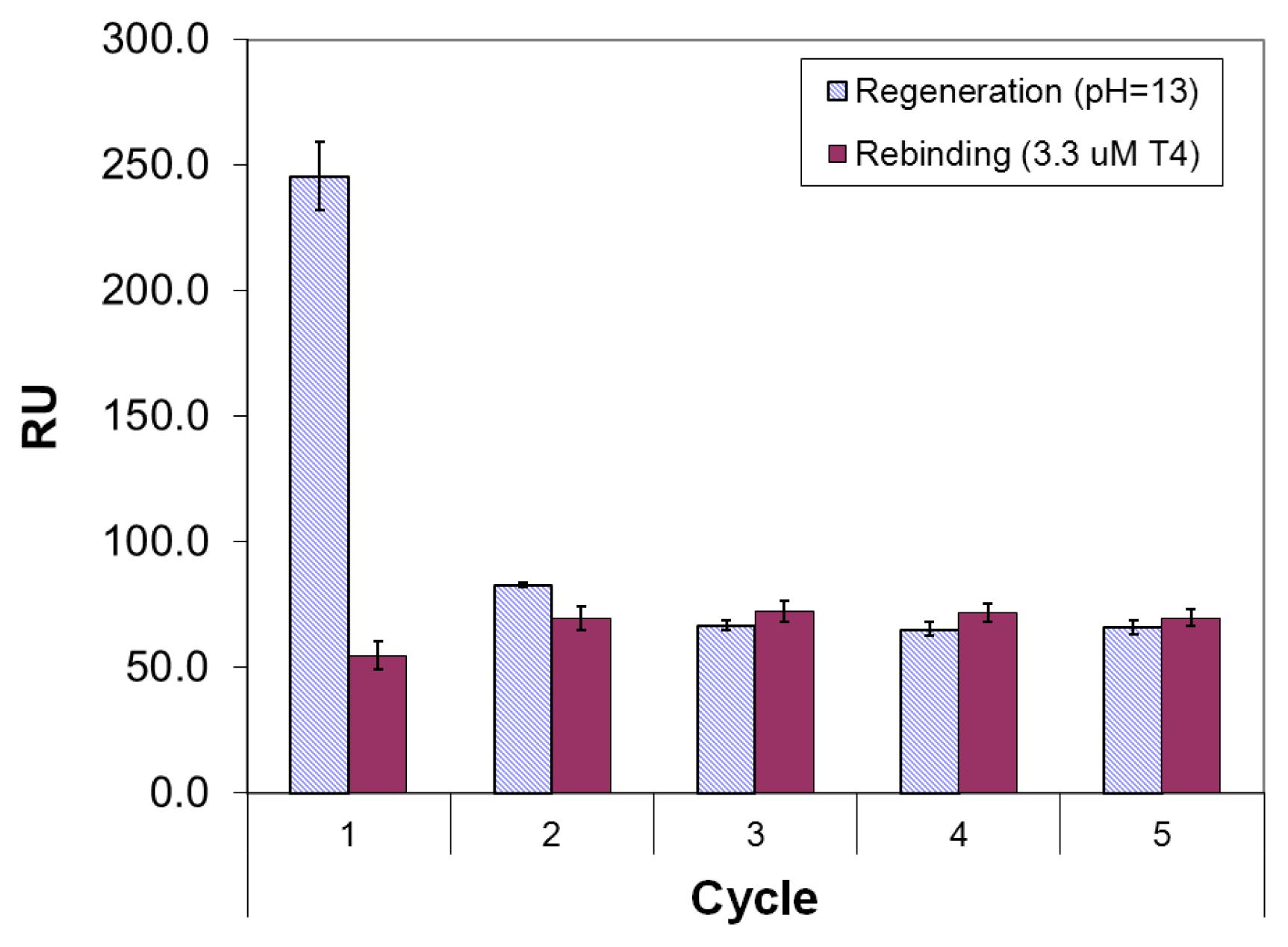
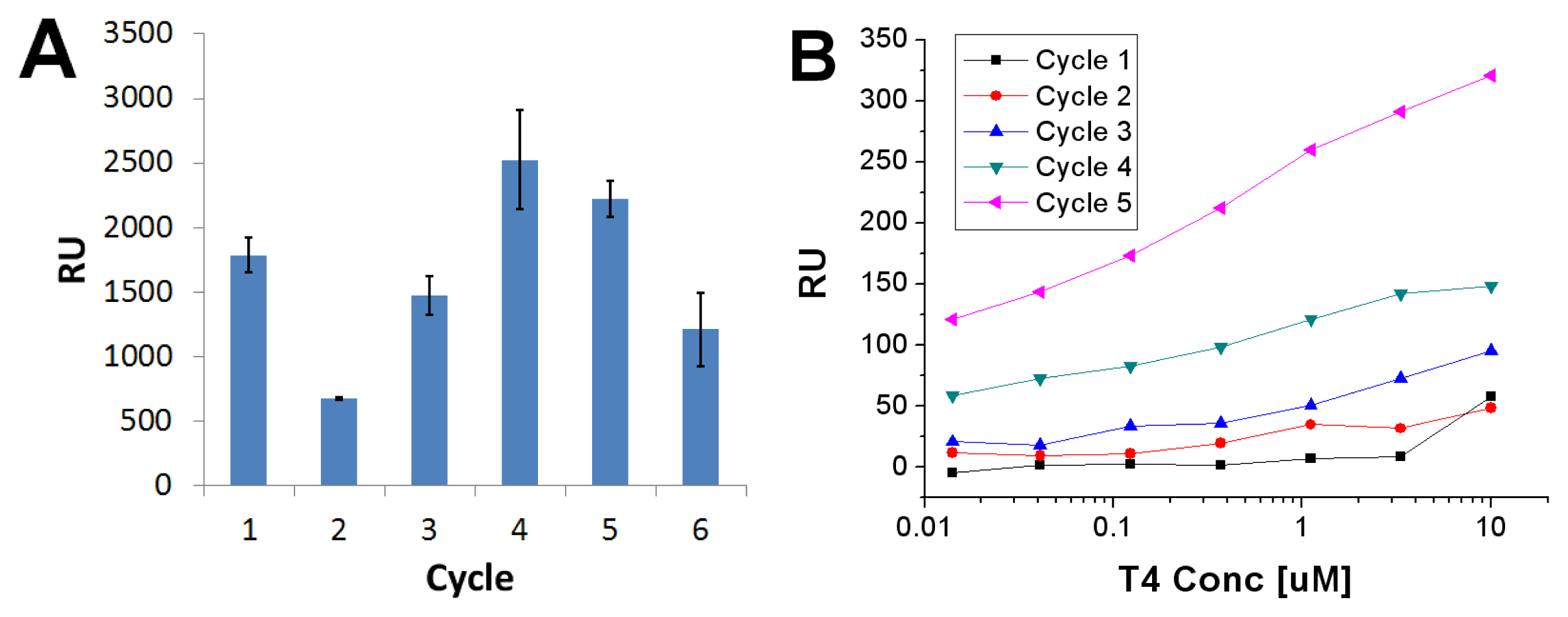
2.3. Imprinted Self-Assembled Layers with T4
3. Experimental Section
3.1. Materials and Methods
3.2. Preparation of Lipoamide Derivatives
5-(1,2-Dithiolan-3-yl)-N-[(2-phenyl-ethyl)]pentanamide (1, phenylethyl lipoamide, Mr = 309.49)
5-(1,2-Dithiolan-3-yl)-N-[2-(pyridin-2-yl)ethyl]pentanamide (2, 2-pyridylethyl lipoamide, Mr = 310.48)
5-(1,2-Dithiolan-3-yl)-N-[2-(pyridin-3-yl)ethyl]pentanamide (3, 3-pyridylethyl lipoamide, Mr = 310.48)
5-(1,2-Dithiolan-3-yl)-N-[2-(pyridin-4-yl)ethyl]pentanamide (4, 4-pyridylethyl lipoamide, Mr = 310.48)
5-(1,2-Dithiolan-3-yl)-N,N-bis(2-hydroxy-ethyl)-pentanamide (5, di-(2-hydroxyethylamino)-lipo-amide, Mr = 293.45)
3.3. Preparation of the Imprinted Films and Titration Experiments
4. Conclusions
Acknowledgments
References and Notes
- Yen, P.M. Physiological and molecular basis of thyroid hormone action. Physiolog. Rev 2001, 81, 1097–1142. [Google Scholar]
- Schimmel, M.; Utiger, R.D. Thyroidal and peripheral production of thyroid hormones. Review of recent findings and their clinical implications. Ann. Intern. Med 1977, 87, 760–768. [Google Scholar]
- Sandler, B.; Webb, P.; Apriletti, J.W.; Huber, B.R.; Togashi, M.; Cunha, S.T.; Juric, S.; Nilsson, S.; Wagner, R.; Fletterick, R.J.; et al. Thyroxine-thyroid hormone receptor interactions. J. Biol. Chem 2004, 279, 55801–55808. [Google Scholar]
- Wagner, R.L.; Apriletti, J.W.; McGrath, M.E.; West, B.L.; Baxter, J.D.; Fletterick, R.J. A structural role for hormone in the thyroid hormone receptor. Nature 1995, 378, 690–697. [Google Scholar]
- Midgley, J.E.M. Direct and indirect free thyroxine assay methods: Theory and practice. Clin. Chem 2010, 7, 1353–1363. [Google Scholar]
- Zhou, A.; Wei, Z.; Read, R.J.; Carrell, R.W. Structural mechanism for the carriage and release of thyroxine in the blood. Proc. Natl. Acad. Sci. USA 2006, 103, 13321–13326. [Google Scholar]
- Miyata, M.; Sato, T.; Kai, H. Role of the glutamic acid 54 residue in transthyretin stability and thyroxine binding. Biochemistry 2010, 49, 114–123. [Google Scholar]
- Eneqvist, T.; Lundberg, E.; Karlsson, A.; Huang, S.; Santos, C.R.A.; Power, D.M.; Sauer-Eriksson, A.E. High resolution crystal structures of piscine transthyretin reveal different binding modes for triiodothyronine and thyroxine. J. Biol. Chem 2004, 279, 26411–26416. [Google Scholar]
- Metrangolo, P.; Meyer, F.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen bonding in supramolecular chemistry. Angew. Chem. Int. Ed 2008, 47, 6114–6127. [Google Scholar]
- Crisp, T.M.; Clegg, E.D.; Cooper, R.L.; Wood, W.P.; Anderson, D.G.; Baetcke, K.P.; Hoffmann, J.L.; Morrow, M.S.; Rodier, D.J.; Schaeffer, J.E.; et al. Environmental endocrine disruption: An effects assessment and analysis. Environ. Health Perspect 1998, 106, 11–56. [Google Scholar]
- Wu, F.-B.; Han, S.-Q.; Xu, T.; He, Y.-F. Sensitive time-resolved fluoroimmunoassay for simultaneous detection of serum thyroid-stimulating hormone and total thyroxin with Eu and Sm as labels. Anal. Biochem 2003, 314, 87–96. [Google Scholar]
- Marchesini, G.R.; Meulenberg, E.; Haasnoot, W.; Mizuguchi, M.; Irth, H. Biosensor recognition of thyroid-disrupting chemicals using transport proteins. Anal. Chem 2006, 78, 1107–1114. [Google Scholar]
- Marchesini, G.R.; Koopal, K.; Meulenberg, E.; Haasnoot, W.; Irth, H. Spreeta-based biosensor assays for endocrine disruptors. Biosens. Bioelectron 2007, 22, 1908–1915. [Google Scholar]
- Mastichiadis, C.; Petrou, P.S.; Christofidis, I.; Misiakos, K.; Kakabakos, S.E. Bulk fluorescence light blockers to improve homogeneous detection in capillary-waveguide fluoroimmunosensors. Biosens. Bioelectron 2009, 24, 2735–2739. [Google Scholar]
- Shahgaldian, P.; Hegner, M.; Pieles, U. Cyclodextrin self-assembled monolayer (SAM) based surface plasmon resonance (SPR) sensor for enantioselective analysis of thyroxine. J. Incl. Phenom. Macrocycl. Chem 2005, 53, 35–39. [Google Scholar]
- Piletsky, S.; Turner, A. Molecular Imprinting of Polymers. Available online: http://www.landesbioscience.com/books/iu/id/864/?nocache=175958218 accessed on 31 January 2013.
- Vasapollo, G.; Del Sole, R.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly imprinted polymers: Present and future prospective. Int. J. Mol. Sci 2011, 12, 5908–5945. [Google Scholar]
- Tappura, K.; Vikholm-Lundin, I.; Albers, W.M. Lipoate-based imprinted self-assembled molecular thin films for biosensor applications. Biosens. Bioelectron 2007, 22, 912–919. [Google Scholar]
- Corradi, E.; Meille, S.V.; Messina, M.T.; Metrangolo, P.; Resnati, G. Halogen bonding versus hydrogen bonding in driving self-assembly processes. Angew. Chem. Int. Ed 2000, 39, 1782–1786. [Google Scholar]
- Albers, W.M.; Vikholm-Lundin, I. Surface Plasmon Resonance on Nano-Scale Organic Films. In Nano-Bio-Sensing; Carrara, S., Ed.; Springer Verlag: New York, NY, USA, 2010. [Google Scholar]
- ACD/ChemSketch Reference Manual version 12.0. Available online: http://exordio.qfb.umich.mx/CHEMSK_R.pdf accessed on 31 January 2013.
- Packing of the ligands was assessed with the OPLS force field with standard Lennard-Jones potentials and electrostatic charges calculated by PM3 [23,24].
- Kaminski, G.A.; Friesner, R.A.; Tirado-Rives, J.; Jorgensen, W.L. Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J. Phys. Chem. B 2001, 105, 6474–6487. [Google Scholar]
- Stewart, J.J.P. Optimization of parameters for semi-empirical methods 1. Method. J. Comp. Chem 1989, 10, 209–220. [Google Scholar]
- Biacore 3000 Instrument Handbook. Available online: http://labs.idi.harvard.edu/springer/uploads/Biacore3000InstrumentHandbookweb.pdf accessed on 31 January 2013.
- Sips, R. J. J. Combined form of Langmuir and Freundlich equations. Chem. Phys 1948, 16, 490–495. [Google Scholar]
- Liang, H.; Miranto, H.; Granqvist, N.; Sadowski, J.W.; Viitala, T.; Wang, B.; Yliperttula, M. Surface plasmon resonance instrument as a refractometer for liquids and ultrathin films. Sens. Act. B 2010, 149, 212–220. [Google Scholar]

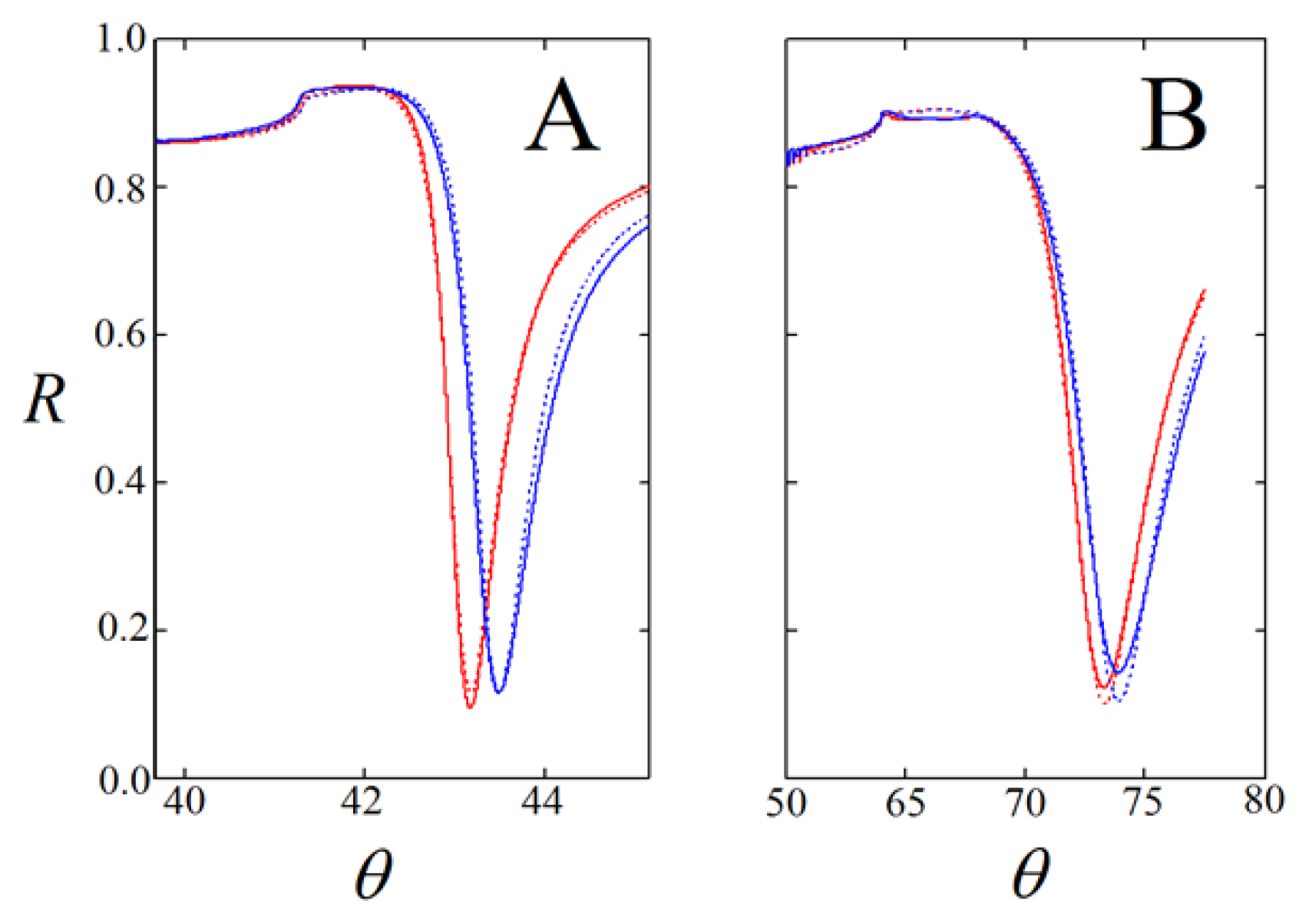
 ) and onto self-assembled layers of the pyridyl-lipoate conjugates 1 (
) and onto self-assembled layers of the pyridyl-lipoate conjugates 1 (
 ), 2 (
), 2 (
 ), 3 (
), 3 (
 ), and 4 (
), and 4 (
 ). Measurements were performed in PBS (150 mM NaCl, 50 mM phosphate buffer, pH = 9.0) supplemented with 0.1% DMSO.
). Measurements were performed in PBS (150 mM NaCl, 50 mM phosphate buffer, pH = 9.0) supplemented with 0.1% DMSO.
 ) and onto self-assembled layers of the pyridyl-lipoate conjugates 1 (
) and onto self-assembled layers of the pyridyl-lipoate conjugates 1 (
 ), 2 (
), 2 (
 ), 3 (
), 3 (
 ), and 4 (
), and 4 (
 ). Measurements were performed in PBS (150 mM NaCl, 50 mM phosphate buffer, pH = 9.0) supplemented with 0.1% DMSO.
). Measurements were performed in PBS (150 mM NaCl, 50 mM phosphate buffer, pH = 9.0) supplemented with 0.1% DMSO.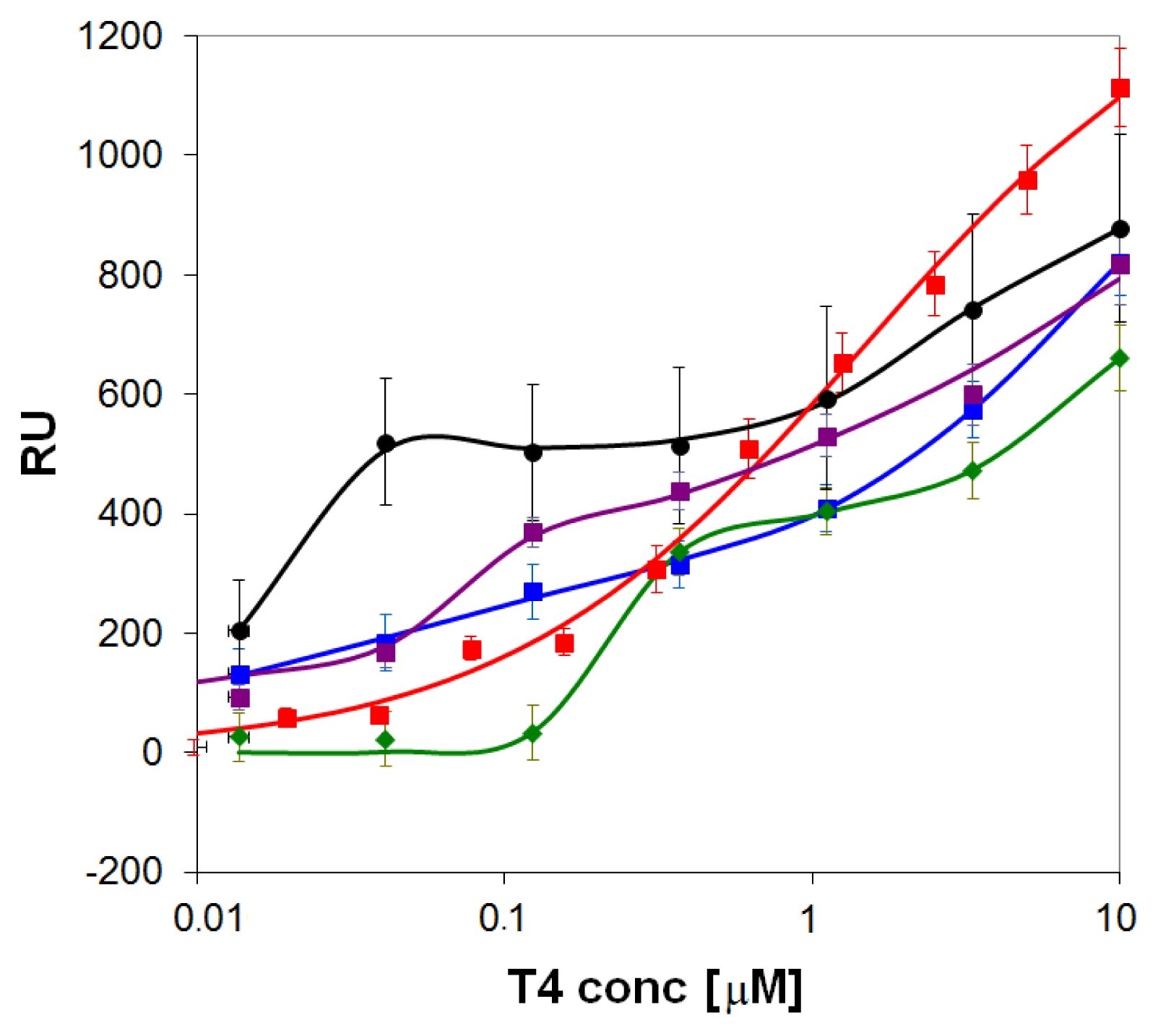

| Compound | Mr | Aa | nb | d (nm) | ρ (g/cm3) c | Γ (ng/cm2) | Φ d |
|---|---|---|---|---|---|---|---|
| 1 | 309.49 | 90.8 | 1.48 ±0.04 | 2.2 ±0.4 | 0.96 ±0.06 | 221 ±33 | 1.6 |
| 2 | 310.48 | 88.9 | 1.51 ±0.02 | 4.2 ±0.3 | 1.04 ±0.04 | 451 ±16 | 3.2 |
| 3 | 310.48 | 88.9 | 1.47 ±0.01 | 5.7 ±0.13 | 0.99 ±0.01 | 560 ±11 | 4.0 |
| 4 | 310.48 | 88.9 | 1.51 ±0.02 | 3.1 ±0.23 | 1.05 ±0.03 | 322 ±14 | 2.3 |
| 5 | 293.45 | 78.7 | 1.47 ±0.02 | 1.8 ±0.1 | 1.03 ±0.04 | 180 ±4 | 1.8 |
| T4 | 776.87 | 123.0 | 1.47 ±0.02 | 1.9 ±0.4 | 1.75 ±0.11 | 320 ±95 | -- |
Supplementary Files
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Albers, W.M.; Milani, R.; Tappura, K.; Munter, T.; Resnati, G.; Metrangolo, P. Self-Assembly of Pyridine-Modified Lipoic Acid Derivatives on Gold and Their Interaction with Thyroxine (T4). Int. J. Mol. Sci. 2013, 14, 3500-3513. https://doi.org/10.3390/ijms14023500
Albers WM, Milani R, Tappura K, Munter T, Resnati G, Metrangolo P. Self-Assembly of Pyridine-Modified Lipoic Acid Derivatives on Gold and Their Interaction with Thyroxine (T4). International Journal of Molecular Sciences. 2013; 14(2):3500-3513. https://doi.org/10.3390/ijms14023500
Chicago/Turabian StyleAlbers, Willem M., Roberto Milani, Kirsi Tappura, Tony Munter, Giuseppe Resnati, and Pierangelo Metrangolo. 2013. "Self-Assembly of Pyridine-Modified Lipoic Acid Derivatives on Gold and Their Interaction with Thyroxine (T4)" International Journal of Molecular Sciences 14, no. 2: 3500-3513. https://doi.org/10.3390/ijms14023500
APA StyleAlbers, W. M., Milani, R., Tappura, K., Munter, T., Resnati, G., & Metrangolo, P. (2013). Self-Assembly of Pyridine-Modified Lipoic Acid Derivatives on Gold and Their Interaction with Thyroxine (T4). International Journal of Molecular Sciences, 14(2), 3500-3513. https://doi.org/10.3390/ijms14023500