Natural Products as a Source for Treating Neglected Parasitic Diseases
Abstract
:1. Introduction
2. Nematodes
2.1. Medicinal Plants in the Treatment and Control of Filariasis
2.1.1. Subcutaneous Filariasis
2.1.2. Lymphatic Filariasis
2.2. Schistosomiasis
2.2.1. Currently Available Drugs for the Treatment of Schistosomiasis
2.2.1.1. Metrifonate
2.2.1.2. Oxamniquine
2.2.1.3. Praziquantel
2.2.2. Natural Products for the Control of Schistosomiasis
2.2.3. Mode of Action and Molecular Targets in Schistosoma
2.2.3.1. Compounds that Disrupt Mating
2.2.3.2. Compounds Acting on the Tegument Structure or Composition
2.2.3.3. Compounds Acting on the Parasite Nervous System
3. Trypanosomatids
4. Summary
Acknowledgements
References
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.; Biavatti, M.W.; Brun, R.; Da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V.; et al. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases—Part II. Curr. Med. Chem 2012, 19, 2176–2228. [Google Scholar]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.; Biavatti, M.W.; Brun, R.; Da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V.; et al. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases—Part I. Curr. Med. Chem 2012, 19, 2128–2175. [Google Scholar]
- Christen, P.C. Muriel plants as a source of therapeutic and health products. CHIMIA Int. J. Chem 2012, 66, 320–323. [Google Scholar]
- Ioset, J.R. Natural products for neglected diseases: A review. Curr. Organ. Chem 2008, 12, 643–666. [Google Scholar]
- Cavalier-Smith, T. A revised six-kingdom system of life. Biol. Rev. Camb. Philos. Soc 1998, 73, 203–266. [Google Scholar]
- Lambshead, P.J.D. Recent developments in marine benthic biodiversity research. Oceanis 1993, 19, 5–24. [Google Scholar]
- Hugot, J.P.; Baujard, P.; Morand, S. Biodiversity in helminths and nematodes as a field of study: An overview. Nematology 2001, 3, 199–208. [Google Scholar]
- World Health Organization, Onchocerciasis and Its Control. In Report of 512 a WHO Expert Committee on Onchocerciasis Control; Geneva; WHO Technical 513 Report Series; WHO: Geneva, Switzerland, 1995; pp. 1–104.
- Osei-Atweneboana, M.Y.; Eng, J.K.; Boakye, D.A.; Gyapong, J.O.; Prichard, R.K. Prevalence and intensity of Onchocerca volvulus infection and efficacy of ivermectin in endemic communities in Ghana: A two-phase epidemiological study. Lancet 2007, 369, 2021–2029. [Google Scholar]
- Wagman, R.J.E. The New Complete Medical and Health Encyclopedia; Lexicon Publication Inc.: New York, NY, USA, 1982; Volume 4, p. 1310. [Google Scholar]
- Navaratnam, V. Recent Advances in the Chemotheraphy of Lymphatic Filariasis. Proceeding of 34th SEAMEO TROPMED Regional Seminar: Current Status of Filariasis in Southeast Asia, Kuala Lumpur, Malaysia, 26–27 June 1992.
- World Health Organization, Global programme to eliminate lymphatic filariasis. In Wkly. Epidemiol. Rec.; 2009; pp. 437–444.
- Gyapong, J.O.; Kumaraswami, V.; Biswas, G.; Ottesen, E.A. Treatment strategies underpinning the global programme to eliminate lymphatic filariasis. Expert Opin. Pharmacother 2005, 6, 179–200. [Google Scholar]
- Bockarie, M.J.; Taylor, M.J.; Gyapong, J.O. Current practices in the management of lymphatic filariasis. Expert Rev. Anti-Infect. Ther 2009, 7, 595–605. [Google Scholar]
- Liang, J.L.; King, J.D.; Ichimori, K.; Handzel, T.; Pa'au, M.; Lammie, P.J. Impact of five annual rounds of mass drug administration with diethylcarbamazine and albendazole on Wuchereria bancrofti infection in American Samoa. Am. J. Trop. Med. Hyg 2008, 78, 924–928. [Google Scholar]
- The Carter Center. Available online: http://www.cartercenter.org/health/lf/index.html accessed on 29 December 2012.
- The Center of Disease Control and Prevention. Available online: http://www.cartercenter.org/health/lf/index.html accessed on 29 December 2012.
- Winnen, M.; Plaisier, A.P.; Alley, E.S.; Nagelkerke, N.J.; van Oortmarssen, G.; Boatin, B.A.; Habbema, J.D. Can ivermectin mass treatments eliminate onchocerciasis in Africa? Bull World Health Organ 2002, 80, 384–391. [Google Scholar]
- Kaplan, R.M. Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol 2004, 20, 477–481. [Google Scholar]
- Howell, S.B.; Burke, J.M.; Miller, J.E.; Terrill, T.H.; Valencia, E.; Williams, M.J.; Williamson, L.H.; Zajac, A.M.; Kaplan, R.M. Prevalence of anthelmintic resistance on sheep and goat farms in the southeastern United States. J. Am. Vet. Med. Assoc 2008, 233, 1913–1919. [Google Scholar]
- Moussala, M.; Fobi, G.; Zogo, O.; Hiag, B.L.A.; Bengono, G.; McMoli, T.E. Survenue d’hémorragies rétiniennes lors du traitement de l’onchocercose par l’invermectine chez une patiente co-infectée par la loase. J. Français d’Ophtalmologie 2004, 27, 63–66. [Google Scholar]
- Melo, A.C.F.L.; Reis, I.F.; Bevilaqua, C.M.L.; Vieira, L.S.; Echevarria, F.M.; Melo, L.M. Nematodeos resistentes a anti-helminticos em rebanhos de ovinos ecaprinos do estado do Ceará, Brasil. Ciencia Rural 2003, 33, 339–344. [Google Scholar]
- Sullivan, K.; Shealy, C.N. Complete Natural Home Remedies; Element Books Limited: Shafterburg, UK, 1997. [Google Scholar]
- Robinson, M.M.; Zhang, X. Traditional Medicines: Global Situation, Issues and Challenges; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Achukwi, M.D.; Harnett, W.; Renz, A. Onchocerca ochengi transmission dynamics and the correlation of O. ochengi microfilaria density in cattle with the transmission potential. Vet. Res 2000, 31, 611–621. [Google Scholar]
- Nyasse, B.; Ngantchou, I.; Nono, J.J.; Schneider, B. Antifilarial activity in vitro of polycarpol and 3-O-acetyl aleuritolic acid from cameroonian medicinal plants against Onchocerca gutturosa. Nat. Prod. Res 2006, 20, 391–397. [Google Scholar]
- Cho-Ngwa, F.; Abongwa, M.; Ngemenya, M.N.; Nyongbela, K.D. Selective activity of extracts of Margaritaria discoidea and Homalium africanum on Onchocerca ochengi. BMC Complement. Altern. Med 2010, 10, 62. [Google Scholar]
- Ndjonka, D.; Agyare, C.; Luersen, K.; Djafsia, B.; Achukwi, D.; Nukenine, E.N.; Hensel, A.; Liebau, E. In vitro activity of Cameroonian and Ghanaian medicinal plants on parasitic (Onchocerca ochengi) and free-living (Caenorhabditis elegans) nematodes. J. Helminthol 2011, 85, 304–312. [Google Scholar]
- Ndjonka, D.; Ajonina-Ekoti, I.; Djafsia, B.; Luersen, K.; Abladam, E.; Liebau, E. Anogeissus leiocarpus extract on the parasite nematode Onchocerca ochengi and on drug resistant mutant strains of the free-living nematode Caenorhabditis elegans. Vet. Parasitol 2012, 190, 136–142. [Google Scholar]
- Smith, R.A.; Pontiggia, L.; Waterman, C.; Lichtenwalner, M.; Wasserman, J. Comparison of motility, recovery, and methyl-thiazolyl-tetrazolium reduction assays for use in screening plant products for anthelmintic activity. Parasitol. Res 2009, 105, 1339–1343. [Google Scholar]
- Hoste, H.; Brunet, S.; Paolini, V.; Bahuaud, D.; Chauveau, S.; Fouraste, I.; Lefrileux, Y. Compared in vitro anthelmintic effects of eight tannin-rich plants browsed by goats in the southern part of France. Option Méditerrenéennes 2009, 431–436. [Google Scholar]
- Thomsen, H.; Reider, K.; Franke, K.; Wessjohann, L.A.; Keiser, J.; Dagne, E.; Arnold, N. Characterization of constituents and anthelmintic properties of Hagenia abyssinica. Sci. Pharm 2012, 80, 433–446. [Google Scholar]
- Katiki, L.M.; Ferreira, J.F.; Gonzalez, J.M.; Zajac, A.M.; Lindsay, D.S.; Chagas, A.C.; Amarante, A.F. Anthelmintic effect of plant extracts containing condensed and hydrolyzable tannins on Caenorhabditis elegans, and their antioxidant capacity. Vet. Parasitol 2012, 18, 218–227. [Google Scholar]
- Lakshmi, V.; Kumar, R.; Gupta, P.; Varshney, V.; Srivastava, M.N.; Dikshit, M.; Murthy, P.K.; Misra-Bhattacharya, S. The antifilarial activity of a marine red alga, Botryocladia leptopoda, against experimental infections with animal and human filariae. Parasitol. Res 2004, 93, 468–474. [Google Scholar]
- Fujimaki, Y.; Kamachi, T.; Yanagi, T.; Caceres, A.; Maki, J.; Aoki, Y. Macrofilaricidal and microfilaricidal effects of Neurolaena lobata, a Guatemalan medicinal plant, on Brugia pahangi. J. Helminthol 2005, 79, 23–28. [Google Scholar]
- Misra, N.; Sharma, M.; Raj, K.; Dangi, A.; Srivastava, S.; Misra-Bhattacharya, S. Chemical constituents and antifilarial activity of Lantana camara against human lymphatic filariid Brugia malayi and rodent filariid Acanthocheilonema viteae maintained in rodent hosts. Parasitol. Res 2007, 100, 439–448. [Google Scholar]
- Sahare, K.N.; Anandhraman, V.; Meshram, V.G.; Meshram, S.U.; Reddy, M.V.; Tumane, P.M.; Goswami, K. Anti-microfilarial activity of methanolic extract of Vitex negundo and Aegle marmelos and their phytochemical analysis. Indian J. Exp. Biol 2008, 46, 128–131. [Google Scholar]
- Sharma, R.D.; Veerpathran, A.R.; Dakshinamoorthy, G.; Sahare, K.N.; Goswami, K.; Reddy, M.V. Possible implication of oxidative stress in anti filarial effect of certain traditionally used medicinal plants in vitro against Brugia malayi microfilariae. Pharmacogn. Res 2010, 2, 350–354. [Google Scholar]
- Gaur, R.L.; Sahoo, M.K.; Dixit, S.; Fatma, N.; Rastogi, S.; Kulshreshtha, D.K.; Chatterjee, R.K.; Murthy, P.K. Antifilarial activity of Caesalpinia bonducella against experimental filarial infections. Indian J. Med. Res 2008, 128, 65–70. [Google Scholar]
- Mathew, N.; Misra-Bhattacharya, S.; Perumal, V.; Muthuswamy, K. Antifilarial lead molecules isolated from Trachyspermum ammi. Molecules 2008, 13, 2156–2168. [Google Scholar]
- Singh, M.; Shakya, S.; Soni, V.K.; Dangi, A.; Kumar, N.; Bhattacharya, S.M. The n-hexane and chloroform fractions of Piper betle L. trigger different arms of immune responses in BALB/c mice and exhibit antifilarial activity against human lymphatic filarid Brugia malayi. Int. Immunopharmacol 2009, 9, 716–728. [Google Scholar]
- Misra, S.; Verma, M.; Mishra, S.K.; Srivastava, S.; Lakshmi, V.; Misra-Bhattacharya, S. Gedunin and photogedunin of Xylocarpus granatum possess antifilarial activity against human lymphatic filarial parasite Brugia malayi in experimental rodent host. Parasitol. Res 2011, 109, 1351–1360. [Google Scholar]
- Zaridah, M.Z.; Idid, S.Z.; Omar, A.W.; Khozirah, S. In vitro antifilarial effects of three plant species against adult worms of subperiodic Brugia malayi. J. Ethnopharmacol 2001, 78, 79–84. [Google Scholar]
- Sashidhara, K.V.; Singh, S.P.; Misra, S.; Gupta, J.; Misra-Bhattacharya, S. Galactolipids from Bauhinia racemosa as a new class of antifilarial agents against human lymphatic filarial parasite, Brugia malayi. Eur. J. Med. Chem 2012, 50, 230–235. [Google Scholar]
- Azeez, S.; Babu, R.O.; Aykkal, R.; Narayanan, R. Virtual screening and in vitro assay of potential drug like inhibitors from spices against glutathione-S-transferase of filarial nematodes. J. Mol. Model 2012, 18, 151–163. [Google Scholar]
- Kushwaha, S.; Roy, S.; Maity, R.; Mallick, A.; Soni, V.K.; Singh, P.K.; Chaurasiya, N.D.; Sangwan, R.S.; Misra-Bhattacharya, S.; Mandal, C. Chemotypical variations in Withania somnifera lead to differentially modulated immune response in BALB/c mice. Vaccine 2012, 30, 1083–1093. [Google Scholar]
- Kushwaha, S.; Soni, V.K.; Singh, P.K.; Bano, N.; Kumar, A.; Sangwan, R.S.; Misra-Bhattacharya, S. Withania somnifera chemotypes NMITLI 101R, NMITLI 118R, NMITLI 128R and withaferin A protect Mastomys coucha from Brugia malayi infection. Parasite Immunol 2012, 34, 199–209. [Google Scholar]
- Salem, M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int. Immunopharmacol 2005, 5, 1749–1770. [Google Scholar]
- Hoerauf, A.; Mand, S.; Fischer, K.; Kruppa, T.; Marfo-Debrekyei, Y.; Debrah, A.Y.; Pfarr, K.M.; Adjei, O.; Buttner, D.W. Doxycycline as a novel strategy against bancroftian filariasis-depletion of Wolbachia endosymbionts from Wuchereria bancrofti and stop of microfilaria production. Med. Microbiol. Immunol 2003, 192, 211–216. [Google Scholar]
- Taylor, M.J.; Makunde, W.H.; McGarry, H.F.; Turner, J.D.; Mand, S.; Hoerauf, A. Macrofilaricidal activity after doxycycline treatment of Wuchereria bancrofti: A double-blind, randomised placebo-controlled trial. Lancet 2005, 365, 2116–2121. [Google Scholar]
- Sahare, K.N.; Anandharaman, V.; Meshram, V.G.; Meshram, S.U.; Gajalakshmi, D.; Goswami, K.; Reddy, M.V. In vitro effect of four herbal plants on the motility of Brugia malayi microfilariae. Indian J. Med. Res 2008, 127, 467–471. [Google Scholar]
- World Health Organization. Schistosomiasis: Population requiring preventive chemotherapy and number of people treated in 2010. Wkly Epidemiol. Rec 2012, 4, 37–44.
- King, C.H. Parasites and poverty: The case of schistosomiasis. Acta Trop 2010, 113, 95–104. [Google Scholar]
- Gray, D.J.; McManus, D.P.; Li, Y.; Williams, G.M.; Bergquist, R.; Ross, A.G. Schistosomiasis elimination: Lessons from the past guide the future. Lancet Infect. Dis 2010, 10, 733–736. [Google Scholar]
- Siddiqui, A.A.; Siddiqui, B.A.; Ganley-Leal, L. Schistosomiasis vaccines. Hum. Vaccin 2011, 7, 1192–1197. [Google Scholar]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar]
- Rollinson, D.; Knopp, S.; Levitz, S.; Stothard, J.R.; Tchuente, L.A.; Garba, A.; Mohammed, K.A.; Schur, N.; Person, B.; Colley, D.G.; et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2012, in press. [Google Scholar]
- Fenwick, A.W.; J.P. Schistosomiasis: Challenges for control, treatment and drug resistance. Curr. Opin. Infect. Dis. 2006, 19, 577–582. [Google Scholar]
- Steinmann, P.; Keiser, J.; Bos, R.; Tanner, M.; Utzinger, J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis 2006, 6, 411–425. [Google Scholar]
- Fenwick, A.; Savioli, L. Schistosomiasis elimination. Lancet Infect. Dis 2011, 11, 346–347. [Google Scholar]
- Rapado, L.N.; Nakano, E.; Ohlweiler, F.P.; Kato, M.J.; Yamaguchi, L.F.; Pereira, C.A.; Kawano, T. Molluscicidal and ovicidal activities of plant extracts of the Piperaceae on Biomphalaria glabrata (Say, 1818). J. Helminthol 2011, 85, 66–72. [Google Scholar]
- Chimbari, M.J. Enhancing schistosomiasis control strategy for zimbabwe: Building on past experiences. J. Parasitol. Res. 2012, 2012. [Google Scholar] [CrossRef]
- World Health Organization, Working to Overcome the Global Impact of Neglected Tropical Diseases—First WHO Report on Neglected Tropical Diseases; WHO Press: Geneva, Switzerland, 2010; p. 184.
- Bueding, E.; Fisher, J. Factors affecting the inhibition of phosphofructokinase activity of Schistosoma mansoni by trivalent organic antimonials. Biochem. Pharmacol 1966, 15, 1197–1211. [Google Scholar]
- Cioli, D.; Pica-Mattoccia, L.; Archer, S. Antischistosomal drugs: Past, present and future? Pharmacol. Ther 1995, 68, 35–85. [Google Scholar]
- Denham, DA; H.R. The effect of metrifonate in vitro on Schistosoma haematobium and S. mansoni adults. Trans. R. Soc. Trop. Med. Hyg 1971, 65, 695–696. [Google Scholar]
- Holmstedt, B.; Nordgren, I.; Sandoz, M.; Sundwall, A. Metrifonate. Summary of toxicological and pharmacological information available. Arch. Toxicol 1978, 41, 3–29. [Google Scholar]
- Cioli, D. Chemotherapy of schistosomiasis: An update. Parasitol. Today 1998, 14, 418–422. [Google Scholar]
- Pica-Mattoccia, L.; Cioli, D. Studies on the mode of action of oxamniquine and related schistosomicidal drugs. Am. J. Trop. Med. Hyg 1985, 34, 112–118. [Google Scholar]
- Kohn, A.B.; Anderson, P.A.; Roberts-Misterly, J.M.; Greenberg, R.M. Schistosome calcium channel beta subunits. Unusual modulatory effects and potential role in the action of the antischistosomal drug praziquantel. J. Biol. Chem 2001, 276, 36873–36876. [Google Scholar]
- Jeziorski, M.C.; Greenberg, R.M. Voltage-gated calcium channel subunits from platyhelminths: Potential role in praziquantel action. Int. J. Parasitol 2006, 36, 625–632. [Google Scholar]
- Salvador-Recatala, V.; Greenberg, R.M. Calcium channels of schistosomes: Unresolved questions and unexpected answers. Wiley Interdiscip. Rev 2012, 1, 85–93. [Google Scholar]
- Day, T.A.B.J.; Pax, R.A. Praziquantel: The enigmatic antiparasitic. Parasitol. Today 1992, 8, 342–344. [Google Scholar]
- Utzinger, J.; Keiser, J.; Shuhua, X.; Tanner, M.; Singer, B.H. Combination chemotherapy of schistosomiasis in laboratory studies and clinical trials. Antimicrob. Agents Chemother 2003, 47, 1487–1495. [Google Scholar]
- Sabah, A.A.; Fletcher, C.; Webbe, G.; Doenhoff, M.J. Schistosoma mansoni: Chemotherapy of infections of different ages. Exp. Parasitol 1986, 61, 294–303. [Google Scholar]
- Fenwick, A.; Savioli, L.; Engels, D.; Robert Bergquist, N.; Todd, M.H. Drugs for the control of parasitic diseases: Current status and development in schistosomiasis. Trends Parasitol 2003, 19, 509–515. [Google Scholar]
- Doenhoff, M.J.; Hagan, P.; Cioli, D.; Southgate, V.; Pica-Mattoccia, L.; Botros, S.; Coles, G.; Tchuem Tchuente, L.A.; Mbaye, A.; Engels, D. Praziquantel: Its use in control of schistosomiasis in sub-Saharan Africa and current research needs. Parasitology 2009, 136, 1825–1835. [Google Scholar]
- Utzinger, J.; N’Goran, E.K.; Caffrey, C.R.; Keiser, J. From innovation to application: Social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop 2011, 120, S121–S137. [Google Scholar]
- Bennett, J.L.; Day, T.; Liang, F.T.; Ismail, M.; Farghaly, A. The development of resistance to anthelmintics: A perspective with an emphasis on the antischistosomal drug praziquantel. Exp. Parasitol 1997, 87, 260–267. [Google Scholar]
- Kusel, J.; Hagan, P. Praziquantel—its use, cost and possible development of resistance. Parasitol. Today 1999, 15, 352–354. [Google Scholar]
- Ismail, M.; Botros, S.; Metwally, A.; William, S.; Farghally, A.; Tao, L.F.; Day, T.A.; Bennett, J.L. Resistance to praziquantel: Direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am. J. Trop. Med. Hyg 1999, 60, 932–935. [Google Scholar]
- Cioli, D. Praziquantel: Is there real resistance and are there alternatives? Curr. Opin. Infect. Dis 2000, 13, 659–663. [Google Scholar]
- Doenhoff, M.J.; Kusel, J.R.; Coles, G.C.; Cioli, D. Resistance of Schistosoma mansoni to praziquantel: Is there a problem? Trans. R. Soc. Trop. Med. Hyg 2002, 96, 465–469. [Google Scholar]
- Doenhoff, M.; Kimani, G.; Cioli, D. Praziquantel and the control of schistosomiasis. Parasitol. Today 2000, 16, 364–366. [Google Scholar]
- Gryseels, B.; Mbaye, A.; de Vlas, S.J.; Stelma, F.F.; Guisse, F.; van Lieshout, L.; Faye, D.; Diop, M.; Ly, A.; Tchuem-Tchuente, L.A.; et al. Are poor responses to praziquantel for the treatment of Schistosoma mansoni infections in Senegal due to resistance? An overview of the evidence. Trop. Med. Int. Health 2001, 6, 864–873. [Google Scholar]
- Caffrey, C.R. Chemotherapy of schistosomiasis: Present and future. Curr. Opin. Chem. Biol 2007, 11, 433–439. [Google Scholar]
- Sabra, A.N.; Botros, S.S. Response of Schistosoma mansoni isolates having different drug sensitivity to praziquantel over several life cycle passages with and without therapeutic pressure. J. Parasitol 2008, 94, 537–541. [Google Scholar]
- Doenhoff, M.J.; Cioli, D.; Utzinger, J. Praziquantel: Mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis 2008, 21, 659–667. [Google Scholar]
- Barakat, R.; El Morshedy, H. Efficacy of two praziquantel treatments among primary school children in an area of high Schistosoma mansoni endemicity, Nile Delta, Egypt. Parasitology 2011, 138, 440–446. [Google Scholar]
- Kayser, O.; Kiderlen, A.F.; Croft, S.L. Natural products as antiparasitic drugs. Parasitol. Res 2003, 90, S55–S62. [Google Scholar]
- Da Silva Filho, A.A.; Costa, E.S.; Cunha, W.R.; Silva, M.L.; Nanayakkara, N.P.; Bastos, J.K. In vitro antileishmanial and antimalarial activities of tetrahydrofuran lignans isolated from Nectandra megapotamica (Lauraceae). Phytother. Res 2008, 22, 1307–1310. [Google Scholar]
- Moraes, J.; Nascimento, C.; Lopes, P.O.; Nakano, E.; Yamaguchi, L.F.; Kato, M.J.; Kawano, T. Schistosoma mansoni: In vitro schistosomicidal activity of piplartine. Exp. Parasitol 2011, 127, 357–364. [Google Scholar]
- Ndamba, J.; Chidimu, M.G.; Zimba, M.; Gomo, E.; Munjoma, M. An investigation of the schistosomiasis transmission status in Harare. Cent. Afr. J. Med 1994, 40, 337–342. [Google Scholar]
- Clark, T.E.; Appleton, C.C.; Kvalsvig, J.D. Schistosomiasis and the use of indigenous plant molluscicides: A rural South African perspective. Acta Trop 1997, 66, 93–107. [Google Scholar]
- Sparg, S.G.; van Staden, J.; Jager, A.K. Efficiency of traditionally used South African plants against schistosomiasis. J. Ethnopharmacol 2000, 73, 209–214. [Google Scholar]
- Molgaard, P.; Nielsen, S.B.; Rasmussen, D.E.; Drummond, R.B.; Makaza, N.; Andreassen, J. Anthelmintic screening of Zimbabwean plants traditionally used against schistosomiasis. J. Ethnopharmacol 2001, 74, 257–264. [Google Scholar]
- Bah, S.; Diallo, D.; Dembele, S.; Paulsen, B.S. Ethnopharmacological survey of plants used for the treatment of schistosomiasis in Niono District, Mali. J. Ethnopharmacol 2006, 105, 387–399. [Google Scholar]
- Cunha, N.L.U.C.; Cintra, L.S.; Souza, H.C.; Peixoto, J.A.; Silva, C.P.; Magalhães, L.G.; Groppo, M.; Rodrigues, V.; Filho, A.A.S.; Silva, M.L.A.; et al. In Vitro schistosomicidal activity of some brazilian cerrado species and their isolated compounds. Evidence-Based Complement. Alter. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Magalhaes, L.G.; Machado, C.B.; Morais, E.R.; Moreira, E.B.; Soares, C.S.; da Silva, S.H.; da Silva Filho, A.A.; Rodrigues, V. In vitro schistosomicidal activity of curcumin against Schistosoma mansoni adult worms. Parasitol. Res 2009, 104, 1197–1201. [Google Scholar]
- Magalhaes, L.G.; Kapadia, G.J.; da Silva Tonuci, L.R.; Caixeta, S.C.; Parreira, N.A.; Rodrigues, V.; da Silva Filho, A.A. In vitro schistosomicidal effects of some phloroglucinol derivatives from Dryopteris species against Schistosoma mansoni adult worms. Parasitol. Res 2010, 106, 395–401. [Google Scholar]
- Caixeta, S.C.; Melo, N.I.; Wakabayashi, K.A.L.; Aguiar, G.P.; Aguiar, D.P.; Mantovani, A.L.L.; Alves, J.M.; Oliveira, P.F.; Tavares, D.C.; Groppo, M.G.; et al. Chemical composition and in vitro schistosomicidal activity of the essential oil of Plectranthus neochilus grown in southeast Brazil. Chem. Biodivers 2011, 8, 2147–2157. [Google Scholar]
- Pereira, A.C.; Magalhaes, L.G.; Goncalves, U.O.; Luz, P.P.; Moraes, A.C.; Rodrigues, V.; da Matta Guedes, P.M.; da Silva Filho, A.A.; Cunha, W.R.; Bastos, J.K.; et al. Schistosomicidal and trypanocidal structure-activity relationships for (+/−)-licarin A and its (−)- and (+)-enantiomers. Phytochemistry 2011, 72, 1424–1430. [Google Scholar]
- Moraes, J.; Nascimento, C.; Yamaguchi, L.F.; Kato, M.J.; Nakano, E. Schistosoma mansoni: In vitro schistosomicidal activity and tegumental alterations induced by piplartine on schistosomula. Exp. Parasitol 2012, 132, 222–227. [Google Scholar]
- Jisaka, M.; Kawanaka, M.; Sugiyama, H.; Takegawa, K.; Huffman, M.A.; Ohigashi, H.; Koshimizu, K. Antischistosomal activities of sesquiterpene lactones and steroid glucosides from Vernonia amygdalina, possibly used by wild chimpanzees against parasite-related diseases. Biosci. Biotechnol. Biochem 1992, 56, 845–846. [Google Scholar]
- McIntosh, H.M.; Olliaro, P. Artemisinin derivatives for treating uncomplicated malaria. Cochrane Database Syst. Rev. 2000, CD000256. [Google Scholar]
- Le, W.J.; You, J.Q.; Yang, Y.Q.; Mei, J.Y.; Guo, H.F.; Yang, H.Z.; Zhang, C.W. Studies on the efficacy of artemether in experimental schistosomiasis (author’s transl). Yao Xue Xue Bao 1982, 17, 187–193. [Google Scholar]
- Yue, W.J.; You, J.Q.; Mei, J.Y. Effects of artemether on Schistosoma japonicum adult worms and ova. Zhongguo Yao Li Xue Bao 1984, 5, 60–63. [Google Scholar]
- Utzinger, J.; Xiao, S.; N’Goran, E.K.; Bergquist, R.; Tanner, M. The potential of artemether for the control of schistosomiasis. Int. J. Parasitol 2001, 31, 1549–1562. [Google Scholar]
- Araujo, N.; Kohn, A.; Katz, N. Activity of the artemether in experimental schistosomiasis mansoni. Mem. Inst. Oswaldo Cruz 1991, 86, 185–188. [Google Scholar]
- Utzinger, J.; N’Goran, E.K.; N’Dri, A.; Lengeler, C.; Xiao, S.; Tanner, M. Oral artemether for prevention of Schistosoma mansoni infection: Randomised controlled trial. Lancet 2000, 355, 1320–1325. [Google Scholar]
- Keiser, J.; Chollet, J.; Xiao, S.H.; Mei, J.Y.; Jiao, P.Y.; Utzinger, J.; Tanner, M. Mefloquine—An aminoalcohol with promising antischistosomal properties in mice. PLoS Negl. Trop. Dis 2009, 3, e350. [Google Scholar]
- Erasmus, D.A. A comparative study of the reproductive system of mature, immature and “unisexual” female Schistosoma mansoni. Parasitology 1973, 67, 165–183. [Google Scholar]
- Allam, G. Immunomodulatory effects of curcumin treatment on murine schistosomiasis mansoni. Immunobiology 2009, 214, 712–727. [Google Scholar]
- Cichewicz, R.H.L.; McKerrow, JH; Nair, MG. Kwanzoquinones A–G and other constituents of Hemerocallis fulva “Kwanzo” roots and their activity against the human pathogenic trematode Schistosoma mansoni. Tetrahedron 2002, 58, 8597–8606. [Google Scholar]
- El-Shenawy, N.S.; Soliman, M.F.; Abdel-Nabi, I.M. Does Cleome droserifolia have anti-schistosomiasis mansoni activity? Rev. Inst. Med. Trop. Sao Paulo 2006, 48, 223–228. [Google Scholar]
- Jatsa, H.B.; Ngo Sock, E.T.; Tchuem Tchuente, L.A.; Kamtchouing, P. Evaluation of the in vivo activity of different concentrations of Clerodendrum umbellatum Poir against Schistosoma mansoni infection in mice. Afr. J. Tradit. Complement. Altern. Med 2009, 6, 216–221. [Google Scholar]
- Jiraungkoorskul, W.; Sahaphong, S.; Sobhon, P.; Riengrojpitak, S.; Kangwanrangsan, N. Schistosoma mekongi: The in vitro effect of praziquantel and artesunate on the adult fluke. Exp. Parasitol 2006, 113, 16–23. [Google Scholar]
- Utzinger, J.; Chollet, J.; Tu, Z.; Xiao, S.; Tanner, M. Comparative study of the effects of artemether and artesunate on juvenile and adult Schistosoma mansoni in experimentally infected mice. Trans. R. Soc. Trop. Med. Hyg 2002, 96, 318–323. [Google Scholar]
- Lescano, S.Z.; Chieffi, P.P.; Canhassi, R.R.; Boulos, M.; Amato Neto, V. Antischistosomal activity of artemether in experimental Schistosomiasis mansoni. Rev. Saude Publica 2004, 38, 71–75. [Google Scholar]
- Xiao, S.; Shen, B.; Chollet, J.; Utzinger, J.; Tanner, M. Tegumental changes in adult Schistosoma mansoni harbored in mice treated with artemether. J. Parasitol 2000, 86, 1125–1132. [Google Scholar]
- De Oliveira, C.B.; Comunello, L.N.; Lunardelli, A.; Amaral, R.H.; Pires, M.G.; da Silva, G.L.; Manfredini, V.; Vargas, C.R.; Gnoatto, S.C.; de Oliveira, J.R.; Gosmann, G. Phenolic enriched extract of Baccharis trimera presents anti-inflammatory and antioxidant activities. Molecules 2012, 17, 1113–1123. [Google Scholar]
- Soliman, M.F. Evaluation of avocado/soybean unsaponifiable alone or concurrently with praziquantel in murine schistosomiasis. Acta Trop 2012, 122, 261–266. [Google Scholar]
- Lima, C.M.; Freitas, F.I.; Morais, L.C.; Cavalcanti, M.G.; Silva, L.F.; Padilha, R.J.; Barbosa, C.G.; Santos, F.A.; Alves, L.C.; de Diniz, M.F. Ultrastructural study on the morphological changes to male worms of Schistosoma mansoni after in vitro exposure to allicin. Rev. Soc. Bras. Med. Trop 2011, 44, 327–330. [Google Scholar]
- Race, G.J.; Martin, J.H.; Moore, D.V.; Larsh, J.E., Jr. Scanning and transmission electronmicroscopy of Schistosoma mansoni eggs, cercariae, and and adults. Am. J. Trop. Med. Hyg. 1971, 20, 914–924. [Google Scholar]
- Hockley, D.J. Ultrastructure of the tegument of Schistosoma. Adv Parasitol 1973, 11, 233–305. [Google Scholar]
- Mitsui, Y.; Miura, M.; Aoki, Y. In vitro effects of artesunate on the survival of worm pairs and egg production of Schistosoma mansoni. J Helminthol 2009, 83, 7–11. [Google Scholar]
- Thompson, D.P.; Klein, R.D.; Geary, T.G. Prospects for rational approaches to anthelmintic discovery. Parasitology 1996, 113, S217–S238. [Google Scholar]
- Sangster, N.C.; Song, J.; Demeler, J. Resistance as a tool for discovering and understanding targets in parasite neuromusculature. Parasitology 2005, 131, S179–S190. [Google Scholar]
- Marks, N.J.; Maule, A.G. Neuropeptides in helminths: Occurrence and distribution. Adv. Exp. Med. Biol 2010, 692, 49–77. [Google Scholar]
- Taman, A.; Ribeiro, P. Glutamate-mediated signaling in Schistosoma mansoni: A novel glutamate receptor is expressed in neurons and the female reproductive tract. Mol. Biochem. Parasitol 2011, 176, 42–50. [Google Scholar]
- Hillman, G.R.; Gibler, W.B.; Chu, S.H. Fluorescent probes of acetylcholine binding sites-indicators of drug action in Schistosoma mansoni. Biochem. Pharmacol 1976, 25, 2529–2535. [Google Scholar]
- Bizimana, N.; Tietjen, U.; Zessin, K.H.; Diallo, D.; Djibril, C.; Melzig, M.F.; Clausen, P.H. Evaluation of medicinal plants from Mali for their in vitro and in vivo trypanocidal activity. J. Ethnopharmacol 2006, 103, 350–356. [Google Scholar]
- Freiburghaus, F.; Kaminsky, R.; Nkunya, M.H.; Brun, R. Evaluation of African medicinal plants for their in vitro trypanocidal activity. J. Ethnopharmacol 1996, 55, 1–11. [Google Scholar]
- Youan, B.B.; Coulibaly, S.; Miezan, T.B.; Doua, F.; Bamba, M. In vivo evaluation of sixteen plant extracts on mice inoculated with Trypanosoma brucei gambiense. Bull. World Health Organ 1997, 75, 343–348. [Google Scholar]
- Dua, V.K.; Verma, G.; Agarwal, D.D.; Kaiser, M.; Brun, R. Antiprotozoal activities of traditional medicinal plants from the Garhwal region of North West Himalaya, India. J. Ethnopharmacol 2011, 136, 123–128. [Google Scholar]
- Mann, A.; Ifarajimi, O.R.; Adewoye, A.T.; Ukam, C.; Udeme, E.E.; Okorie, II; Sakpe, M.S.; Ibrahim, D.R.; Yahaya, Y.A.; Kabir, A.Y.; et al. In vivo antitrypanosomal effects of some ethnomedicinal plants from Nupeland of north central Nigeria. Afr. J. Tradit. Complement. Altern. Med 2011, 8, 15–21. [Google Scholar]
- Buckner, F.S.N.N. Advances in Chagas disease drug development: 2009–2010. Curr. Opin. Infect. Dis 2010, 23, 609–616. [Google Scholar]
- Andrade e Silva, M.L.; Cicarelli, R.M.; Pauletti, P.M.; Luz, P.P.; Rezende, K.C.; Januario, A.H.; da Silva, R.; Pereira, A.C.; Bastos, J.K.; de Albuquerque, S.; et al. Trypanosoma cruzi: Evaluation of (−)-cubebin derivatives activity in the messenger RNAs processing. Parasitol. Res 2011, 109, 445–451. [Google Scholar]
- Parmar, V.S.J.; Gupta, S.; Talwar, S.; Rajwanshi, V.K.K.; Azim, A.; Malhotra, S.K.; Jain, R.; Sharma, N.K.; Tyagi, O.D.L.; Errington, W.; et al. Polyphenols and alkaloids from Piper species. Phytochemistry 1998, 49, 1069–1078. [Google Scholar]
- Baldoqui, D.C.; Kato, M.J.; Cavalheiro, A.J.; Bolzani, V.S.; Young, M.C.; Furlan, M.A. chromene and prenylated benzoic acid from Piper aduncum. Phytochemistry 1999, 51, 899–902. [Google Scholar]
- Kato, M.J.; Furlan, M. Chemistry and evolution of the Piperaceae. Pure Appl. Chem 2007, 79, 529–538. [Google Scholar]
- Nour, A.M.; Khalid, S.A.; Kaiser, M.; Brun, R.; Abdallah, W.E.; Schmidt, T.J. The antiprotozoal activity of sixteen asteraceae species native to Sudan and bioactivity-guided isolation of xanthanolides from Xanthium brasilicum. Planta Med 2009, 75, 1363–1368. [Google Scholar]
- Batista, J.M., Jr; Lopes, A.A.; Ambrosio, D.L.; Regasini, L.O.; Kato, M.J.; Bolzani, V.S.; Cicarelli, R.M.; Furlan, M. Natural chromenes and chromene derivatives as potential anti-trypanosomal agents. Biol. Pharm. Bull. 2008, 31, 538–540. [Google Scholar]
- Flores, N.; Jimenez, I.A.; Gimenez, A.; Ruiz, G.; Gutierrez, D.; Bourdy, G.; Bazzocchi, I.L. Antiparasitic activity of prenylated benzoic acid derivatives from Piper species. Phytochemistry 2009, 70, 621–627. [Google Scholar]
- Flores, N.; Jimenez, I.A.; Gimenez, A.; Ruiz, G.; Gutierrez, D.; Bourdy, G.; Bazzocchi, I.L. Benzoic acid derivatives from Piper species and their antiparasitic activity. J. Nat. Prod 2008, 71, 1538–1543. [Google Scholar]
- Torres-Santos, E.C.; Moreira, D.L.; Kaplan, M.A.; Meirelles, M.N.; Rossi-Bergmann, B. Selective effect of 2′,6′-dihydroxy-4′-methoxychalcone isolated from Piper aduncum on Leishmania amazonensis. Antimicrob Agents Chemother 1999, 43, 1234–1241. [Google Scholar]
- Hermoso, A.; Jimenez, I.A.; Mamani, Z.A.; Bazzocchi, I.L.; Pinero, J.E.; Ravelo, A.G.; Valladares, B. Antileishmanial activities of dihydrochalcones from Piper elongatum and synthetic related compounds. Structural requirements for activity. Bioorg. Med. Chem 2003, 11, 3975–3980. [Google Scholar]
- Bodiwala, H.S.S.G.; Singh, R.; Dey, C.S.; Sharma, S.S.; Bhutani, K.K.; Singh, I.P. Antileishmanial amides and lignans from Piper cubeba and Piper retrofractum. J. Nat. Med 2007, 61, 418–421. [Google Scholar]
- Luize, P.S.; Ueda-Nakamura, T.; Dias Filho, B.P.; Cortez, D.A.; Nakamura, C.V. Activity of neolignans isolated from Piper regnellii (MIQ.) C.DC. var. pallescens against Trypanosoma cruzi. Biol. Pharm. Bull 2006, 29, 2126–2130. [Google Scholar]
- Martins, R.C.; Lago, J.H.; Albuquerque, S.; Kato, M.J. Trypanocidal tetrahydrofuran lignans from inflorescences of Piper solmsianum. Phytochemistry 2003, 64, 667–670. [Google Scholar]
- Ribeiro, T.S.; Freire-de-Lima, L.; Previato, J.O.; Mendonca-Previato, L.; Heise, N.; de Lima, M.E. Toxic effects of natural piperine and its derivatives on epimastigotes and amastigotes of Trypanosoma cruzi. Bioorg. Med. Chem. Lett 2004, 14, 3555–3558. [Google Scholar]
- Cotinguiba, F.R.; Bolzani, V.S.; Debonsi, H.M.; Passerini, G.D.; Cicarelli, R.M.B.; Kato, M.J.; Furlan, M. Piperamides and their derivatives as potential anti-trypanosomal agents. Med. Chem. Res 2009, 18, 703–711. [Google Scholar]
- Lopes, A.A.; Lopez, S.N.; Regasini, L.O.; Junior, J.M.; Ambrosio, D.L.; Kato, M.J.; da Silva Bolzani, V.; Cicarelli, R.M.; Furlan, M. In vitro activity of compounds isolated from Piper crassinervium against Trypanosoma cruzi. Nat. Prod. Res 2008, 22, 1040–1046. [Google Scholar]
- Ganfon, H.; Bero, J.; Tchinda, A.T.; Gbaguidi, F.; Gbenou, J.; Moudachirou, M.; Frederich, M.; Quetin-Leclercq, J. Antiparasitic activities of two sesquiterpenic lactones isolated from Acanthospermum hispidum D.C. J. Ethnopharmacol 2012, 141, 411–417. [Google Scholar]
- Nibret, E.; Ashour, M.L.; Rubanza, C.D.; Wink, M. Screening of some Tanzanian medicinal plants for their trypanocidal and cytotoxic activities. Phytother. Res 2010, 24, 945–947. [Google Scholar]
- Oliveira, M.F.; d’Avila, J.C.; Tempone, A.J.; Soares, J.B.; Rumjanek, F.D.; Ferreira-Pereira, A.; Ferreira, S.T.; Oliveira, P.L. Inhibition of heme aggregation by chloroquine reduces Schistosoma mansoni infection. J. Infect. Dis 2004, 190, 843–852. [Google Scholar]
- Jones, A.K.; Bentley, G.N.; Oliveros Parra, W.G.; Agnew, A. Molecular characterization of an acetylcholinesterase implicated in the regulation of glucose scavenging by the parasite Schistosoma. FASEB J 2002, 16, 441–443. [Google Scholar]
- Abdulla, M.H.; Ruelas, D.S.; Wolff, B.; Snedecor, J.; Lim, K.C.; Xu, F.; Renslo, A.R.; Williams, J.; McKerrow, J.H.; Caffrey, C.R. Drug discovery for schistosomiasis: Hit and lead compounds identified in a library of known drugs by medium-throughput phenotypic screening. PLoS Negl. Trop. Dis 2009, 3, e478. [Google Scholar]
- Quezada, L.A.; McKerrow, J.H. Schistosome serine protease inhibitors: Parasite defense or homeostasis? An. Acad. Bras. Cienc 2011, 83, 663–672. [Google Scholar]
- Mendonca-Silva, D.L.; Pessoa, R.F.; Noel, F. Evidence for the presence of glutamatergic receptors in adult Schistosoma mansoni. Biochem. Pharmacol 2002, 64, 1337–1344. [Google Scholar]
| Names | Family | Parts used | Solvent used for extraction | Active compounds | Activities | References |
|---|---|---|---|---|---|---|
| Polyalthia suaveolens | Annonaceae |  Polycarpol | O. gutturosa: Significant inhibitory activities on the vitality of adult male worms | [26] | ||
| Discoglypremna caloneura | Euphorbiaceae | 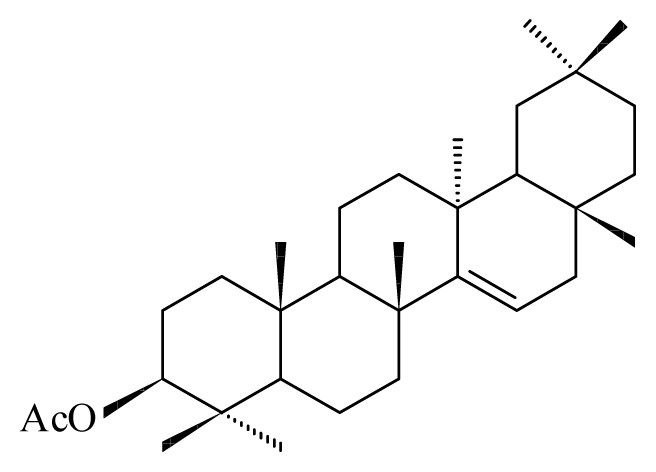 3-O-acetyl aleuritolic acid | ||||
| Homalium africanum | Salicaceae | Leaves | Hexane Methylene chloride | O. ochengi: Microfilaricide | [27] | |
| Margaritaria discoidea | Euphorbiaciaea | Roots Leaves | Hexane Methylene chloride | O. ochengi: Microfilaricide | ||
| Anogeissus leiocarpus | Combretaceae | Bark, leaves | Ethanol | 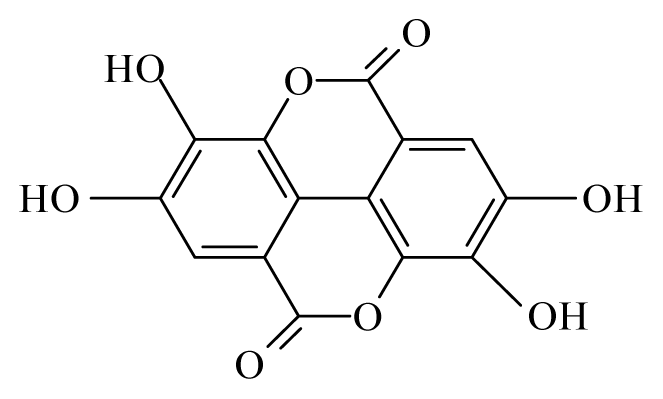 Ellagic acid | O. ochengi: Microfilaricide and macrofilaricide. C. elegans: High activity on adults and larvae | [28,29] |
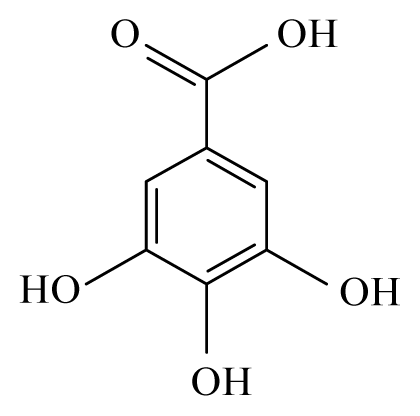 Gallic acid | ||||||
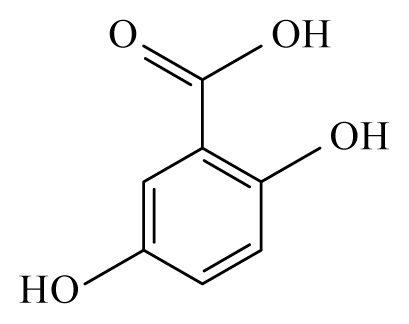 Gentisic acid | ||||||
| Khaya senegalensis | Meliaceae | Bark, leaves | Ethanol | O. ochengi: Microfilaricide and macrofilaricide. C. elegans: Moderate activity on adults and larvae | [28] | |
| Euphorbia hirta | Euphorbiaciaea | Leaves | Ethanol | C. elegans: Moderate activity on adults and larvae | ||
| Parquetina nigrescens | Asclepiadaceae | Water | [28] | |||
| Annona senegalensis | Annonaceae | Water | C. elegans: Moderate activity on adults and larvae | [28] | ||
| Hagenia abyssinica | Rosaceae | Female flowers | 80% Methanol | [32] | ||
| Acer rubrum | Aceraceae | |||||
| Rosa multiflora | Rosaceae | |||||
| Quercus alba | Fagaceae | Leaves | 70% Acetone | C. elegans: Moderate activity on adults and larvae | [33] | |
| Rhus typhina | Anacardiaceae | |||||
| Lespedeza cuneata | Fabaceae | |||||
| Salix X sepulcralis | Salicaceae | Leaves | 70% Acetone | C. elegans: Low activity on adults and larvae | ||
| Robinia pseudoacacia | Fabaceae | |||||
| Botryocladia leptopoda | Rhodymeniaceae | Whole alga | 95% Ethanol | L. sigmodontis and A. viteae: Adults B. malayi: Macrofilaricide and sterilization of female | [34] | |
| Neurolaena lobata | Asteraceae | Leaves | Ethanol | B. pahangi: Macrofilaricide and micrifilaricide | [35] | |
| Lantana camara | Verbenaceae | Stem | 95% Ethanol | A. viteae: Microfilaricide (95.04%) and sterilization of female (60.66%) | [36] | |
| B. malayi: Mastomys coucha killed 43.05% of the adult and sterilized 76% females | ||||||
| Lantana camara | Verbenaceae | Stem | Chloroform fraction | 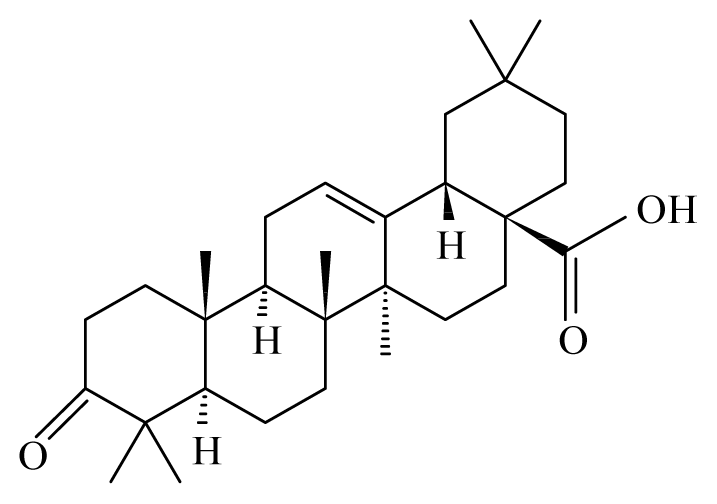 Oleanonic acid | B. malayi: in M. coucha (Meriones unguiculatus) killed 100% (80%) of the adult | [36] |
| B. malayi: Macrofilaricide | ||||||
| Butea monosperma | Fabaceae | Leaves | Water | Polyphenol | B. malayi: Strong inhibition of motility microfilariae, presence of oxidative parameters | [36–38] |
| Roots | Water | B. malayi: Strong inhibition of motility microfilariae | [36,37] | |||
| Vitex negundo | Lamiaceae | Roots | Ethanol | Alkaloids, saponin, flavonoids, polyphenol | B. malayi: Strong inhibition of motility microfilariae, presence of oxidative parameters | |
| Aegle marmelo | Rutaceae | Leaves | Ethanol | 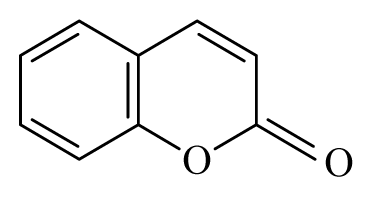 Coumarin Polyphenol | B. malayi: Strong inhibition of motility microfilariae, presence of oxidative parameters | [36–38] |
| Ricinus communis | Euphorbiaceae | Leaves | 70% Methanol | B. malayi: Moderate inhibition of motility microfilariae | ||
| Caesalpinia bonducella | Caesalpiniaceae | Seed kernel | Ethanol | L. sigmodontis in cotton rats S. hispidus: Reduction to up to 96% filariae and 100% female sterilization. Microfilaricide in B. malayi. | ||
| Butanol fraction | L. sigmodontis in cotton rats S. hispidus: Reduction to up to 73.7% microfilariae. 82.5% mortality of macrofilaria and 100% female sterilization. Microfilaricide in B. malayi. | [39] | ||||
| Aqueous fraction | L. sigmodontis in cotton rats S. hispidus: Reduction to up to 90% microfilariae. 82.5% mortality of macrofilaria and 100% female sterilization. Microfilaricide in B. malayi. | |||||
| Trachyspermum ammi | Apiaceae | Fruits | Methanol | Phenolic monoterpene | S. digitata: Macrofilaricide B. malayi: Macrofilaricide and females sterilization | [40] |
| Piper betle | Piperaceae | Leaves | Methanol | n-Hexane and chloroform fractions | B. malayi: Microfilaricide, moderate activity on macrofilariae and female sterilization. Immunomodulatory properties in mices | [41] |
| Xylocarpus granatum | Meliaceae | Fruits | 50% Ethanol | 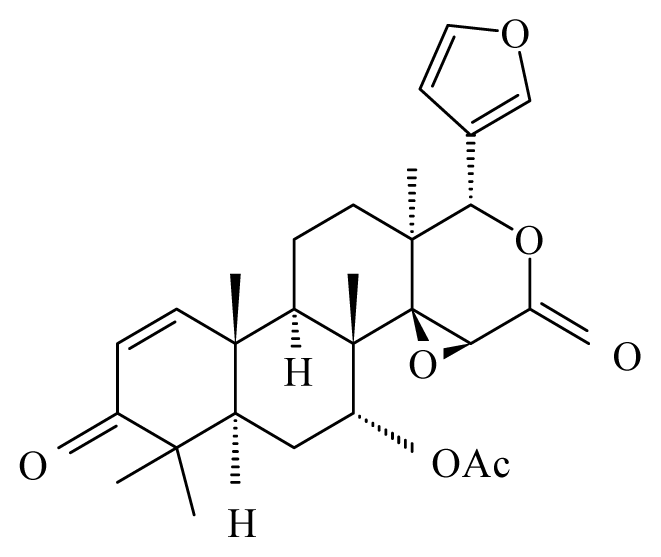 Gedunin | B. malayi: Excellent microfilaricidal and macrofilaricidal efficacies | [42,43] |
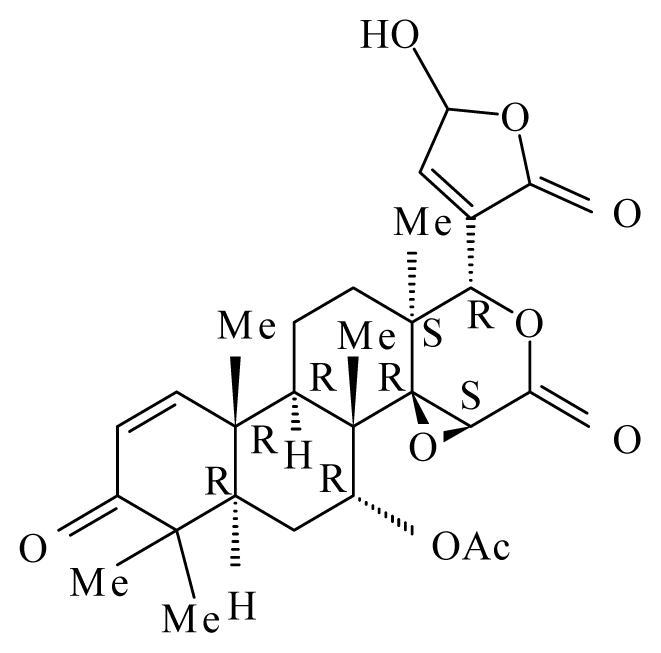 Photogedunin | ||||||
| Bauhinia racemosa | Caesalpinaeceae | Leaves | 95% Ethanol | 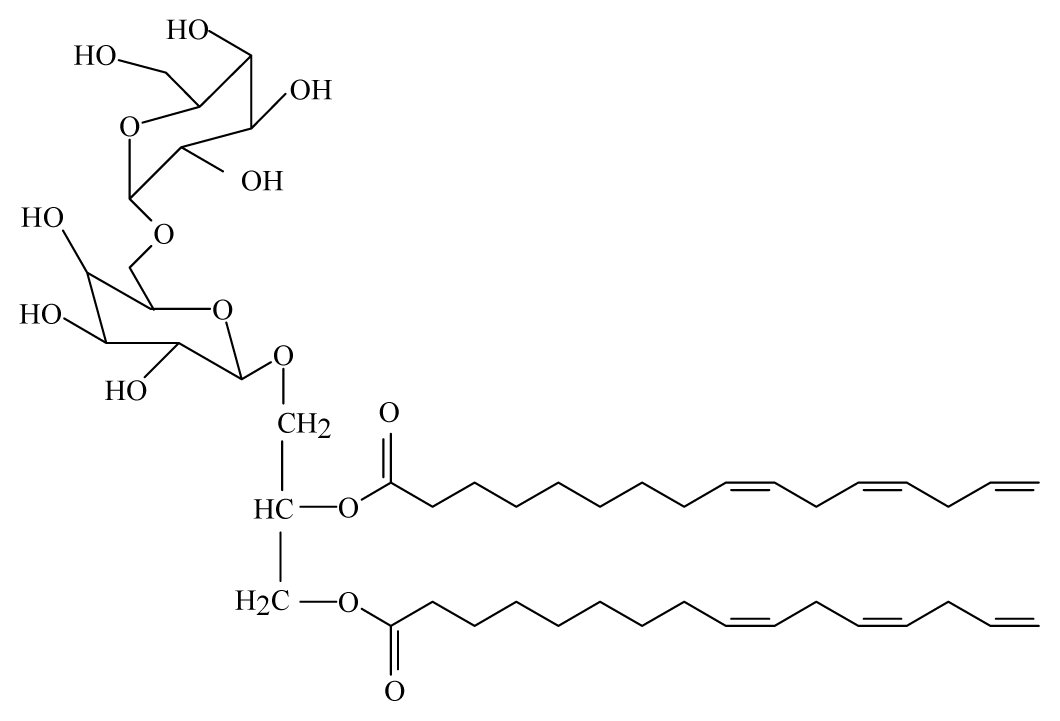 Galactolipid 1 | B. malayi: In vivo and in vitro antifilarial activity | [44] |
| Bauhinia racemosa | Caesalpinaeceae | Leaves | 95% Ethanol | 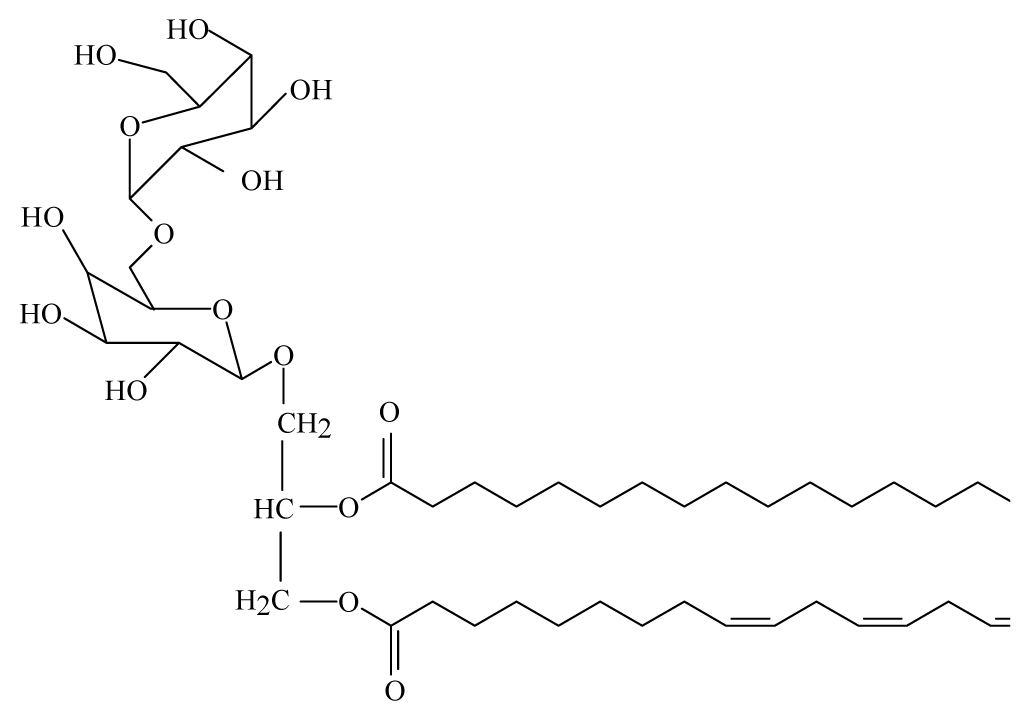 Galactolipid 2 | B. malayi: In vivo and in vitro antifilarial activity | [44] |
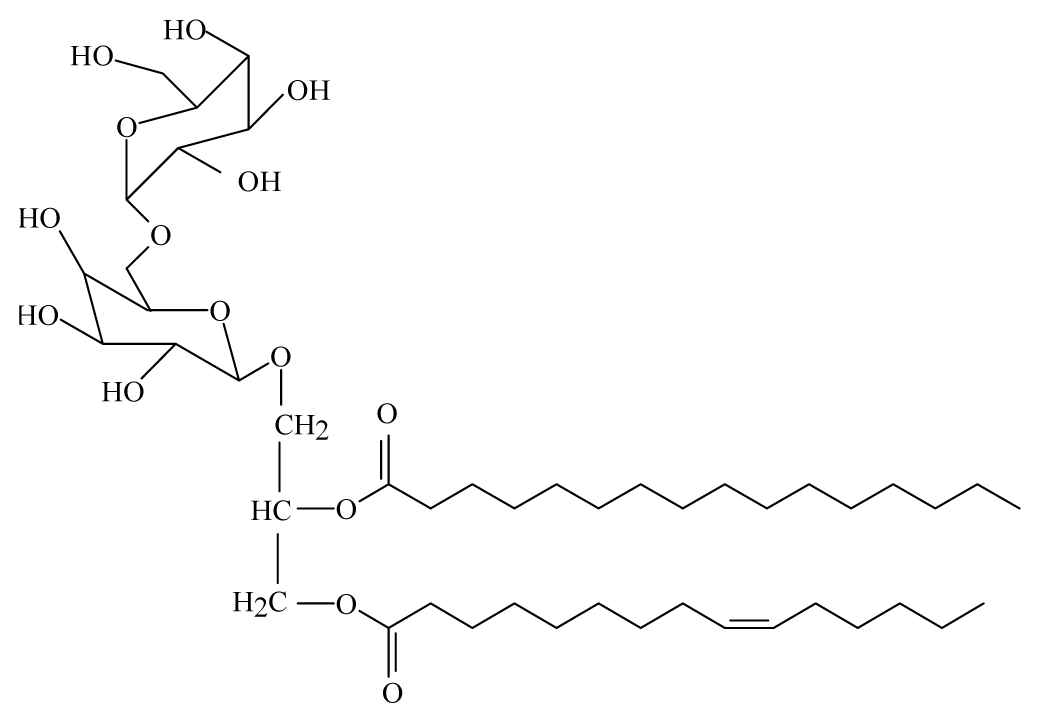 Galactolipid 3 | ||||||
| Corinder: Coriandrum sativum | Apiaceae | 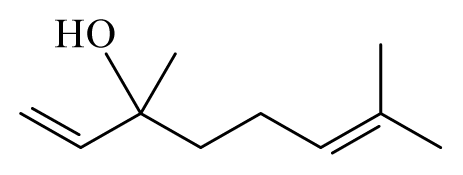 Linalool | [45] | |||
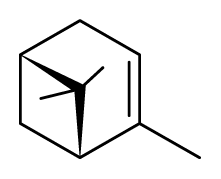 α-Pinene | ||||||
| Cassia: Cassia | Fabaceae | 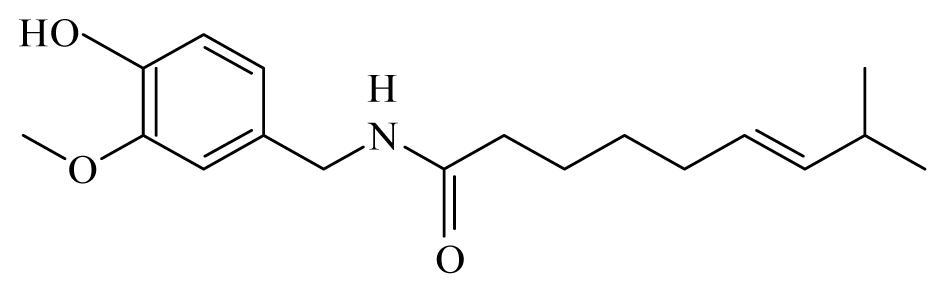 Capsaicin | [45] | |||
| Turmeric: Curcuma longa | Zingiberaceae | 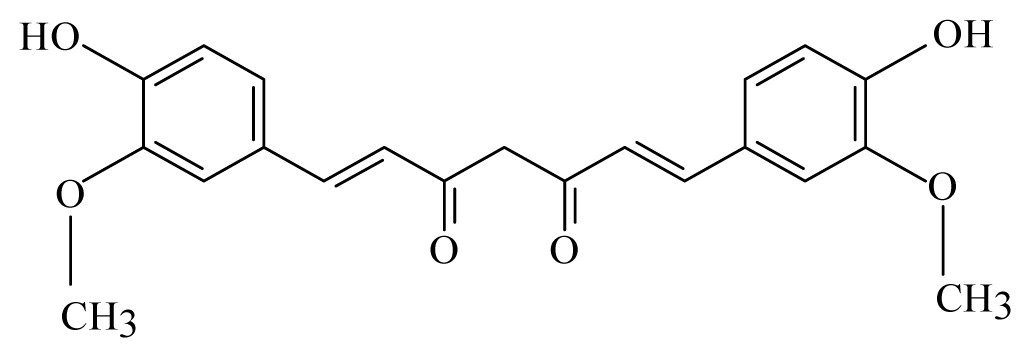 Curcumin | ||||
| Allspice: Pimenta dioica | Myrtaceae |  Piperine | ||||
 β-Caryophyllene | ||||||
| Cinnamon: Cinnamomum | Lauraceae | 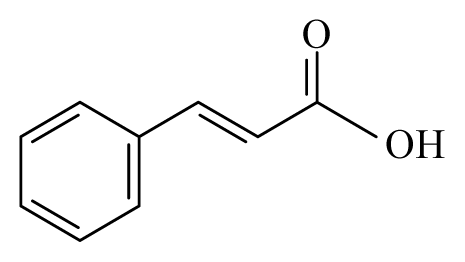 Cinnamic acid β-Caryophyllene | B. malayi: GST inhibitory activity in vitro | [45] | ||
| Strychnous: Strychnos | Loganiaceae | 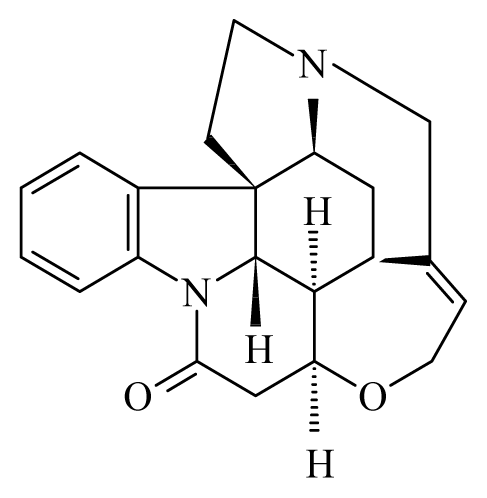 Strychnine | ||||
| Lemongrass: Cymbopogon | Poaceae |  Citronellol | ||||
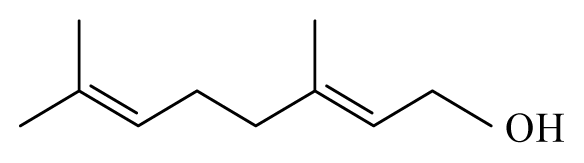 Geraniol | ||||||
| Garlic: Allium sativum | Amaryllidaceae | 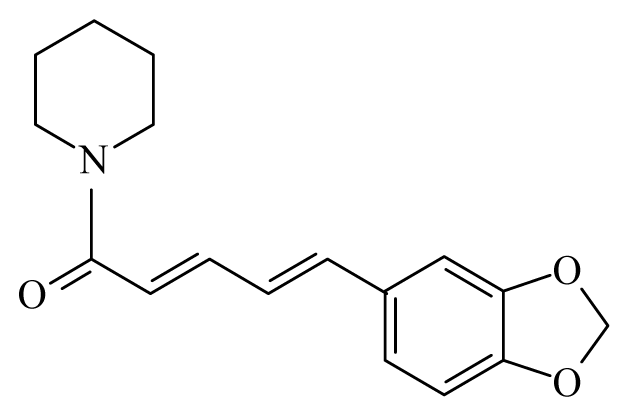 Piperine | ||||
| Litsea: Litsea | Lauraceae | 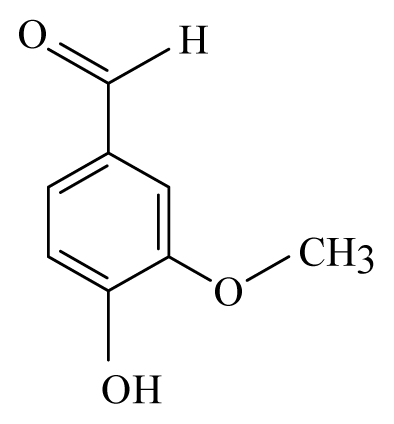 Vanillin | [45] | |||
| Vanilla: Vanilla | Orchidaceae | 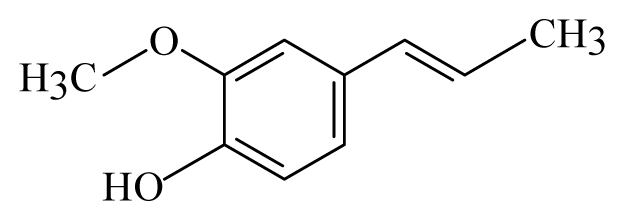 Isoeugenol | ||||
| Withania somnifera | Solanaceae Roots | 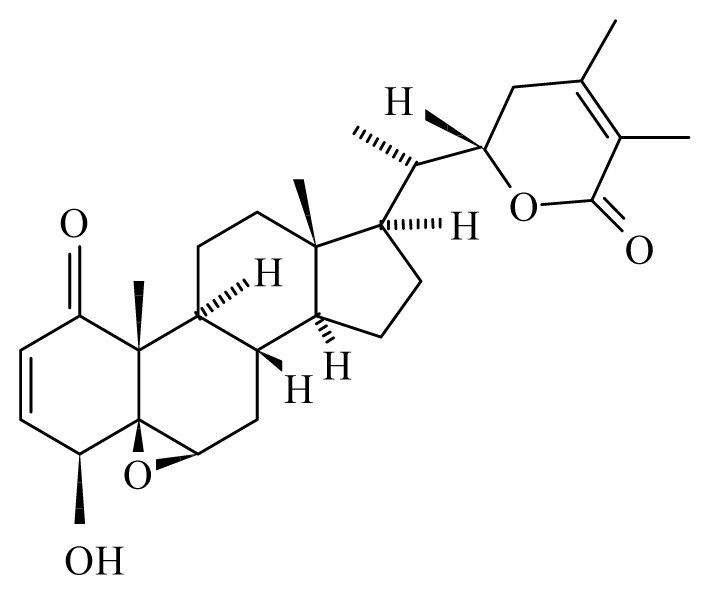 Withanolide | Protection to the rodent host M. coucha against infection of filarial parasite B. malayi | [46,47] | ||
| Nigella sativa | Ranunculaceae | Seeds | B. malayi: Immunomodulatory and therapeutic properties in mices | [48] | ||
| Compounds/Substances | Origin | Schistosomicidal activities | Assays | References | |||
|---|---|---|---|---|---|---|---|
| in vitro | in vivo | Observations | Toxicity | Clinical | |||
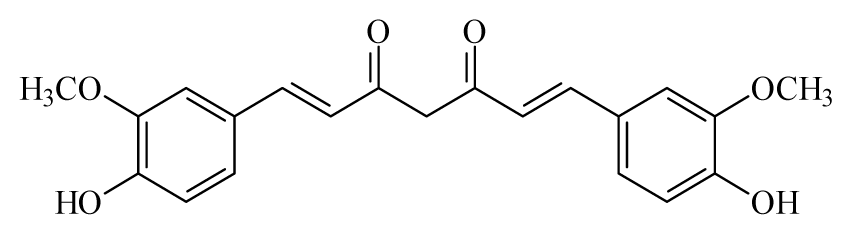 Curcumin | Curcuma longa | S. mansoni adult—50 μM (100% mortality in male and female) | 400mg/Kg in mouse 1—43.5% mortality in male and 4.6% in female of S. mansoni) | Reduction in the oviposition Induced separation of males and females Reduction in the motor activity | nd | nd | [99,113] |
| Essencial oil (sesquiterpenes 57.20% and monoterpenes 42.13%) | Plectranthus neochilus | S. mansoni adult—LC50-value 89.65 mg/mL at 24 h LC50-value 58.18 mg/mL at 120 h | nd | Reduction in the oviposition Induced separation of males and females Reduction in the motor activity | Non-toxic in V79 cells2 (concentrations lower than 200 μg/mL) | nd | [101] |
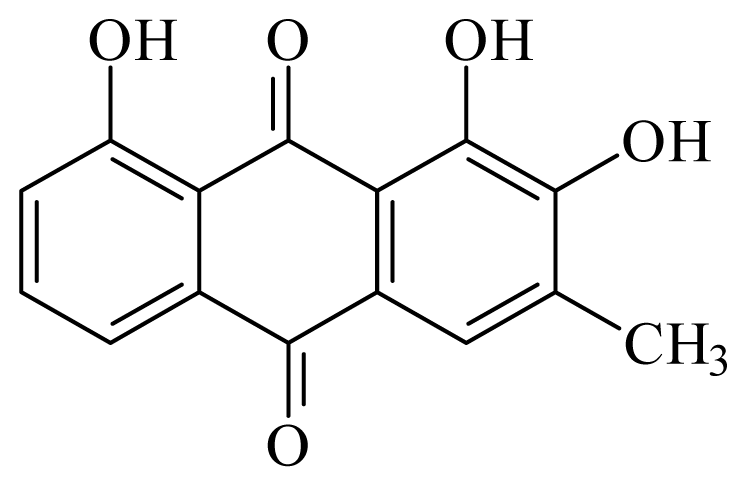 2-hydroxychrysophanol | Hemerocallis fulva | S. mansoni adult—50 μg/mL (35% mortality in male and female) Schistosomula—25 μg/mL (80% mortality) | nd | nd | nd | nd | [114] |
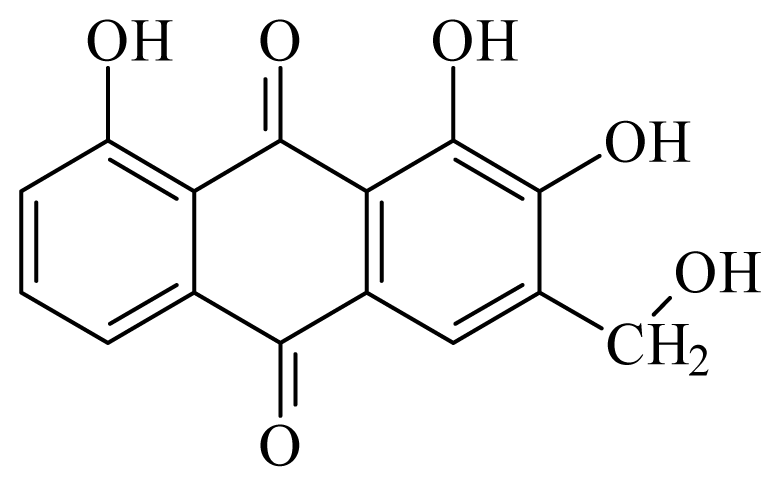 Kwanzoquinone E | Hemerocallis fulva | S. mansoni adult—50 μg/mL (55% mortality in male and female) Schistosomula—25 μg/mL (100% mortality) | nd | nd | nd | nd | [114] |
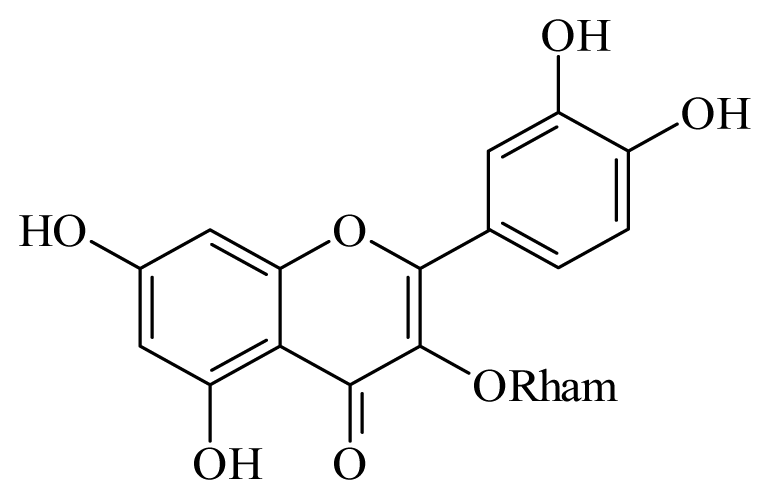 Quercetin 3-O-β-D-rhamnoside | Schefflera vinosa | S. mansoni adult—100 μM (25% mortality in male and female) | nd | Reduction in the Motor activity | nd | nd | [98] |
| Methanol leaves extract | Cleome droserifolia | Nd | Doses of 0.31 g kg−1 for 21 days—32.46% reduction of S. mansoni in mice 3 | nd | nd | nd | [115] |
| Aqueous leaves extract | Clerodendrum umbellatum | Nd | 80 mg/kg in mice 4—100% mortality in S. mansoni | Reduction in the oviposition (75.49% released eggs in faeces) | nd | nd | [116] |
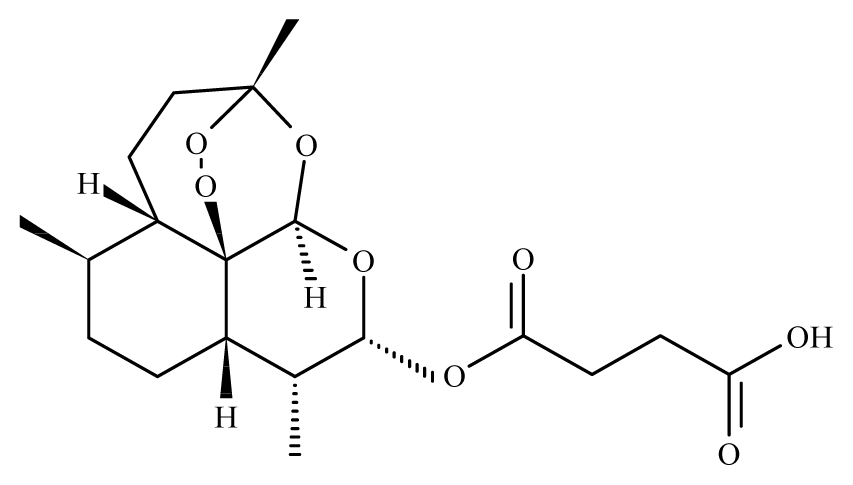 Artesunate | Artemisia annua | S. mekongi adult—40 μg/mL (100% mortality in male and female) S. mansoni adult—40 μg/mL (80% mortality in male and female) | 150 to 300 mg/kg in mice—67 and 77% mortality in male and female) | Reduction in the motor activity Reduction in the oviposition Tegumental disruption | nd | nd | [117,118] |
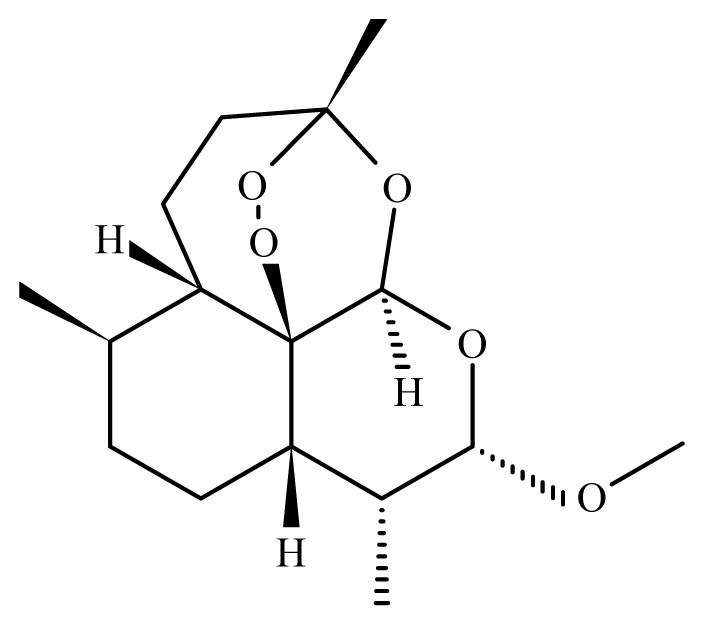 Artemether | Artemisia annua | Nd | 50 mg/Kg in mice 5—reduction of S. mansoni female 100 mg/Kg in mice—61.5% mortality in S. mansoni female 150 to 300 mg/kg in mice—88 and 97% mortality in S. mansoni male and female 10 mg/Kg in rabbit 6—97% mortality in S. japonicum male and female 10 mg/Kg in dog 6—99.3% mortality in S. japonicum male and female | Reduced liver and spleen weight of treated animals Reduction in the motor activity Tegumental disruption Alteration of the reproductive organs, ovarian volume reduction and depigmentation of the intestinal parasites portion | nd | 30 mg/kg (2 oral doses) | [109,110, 118–120] |
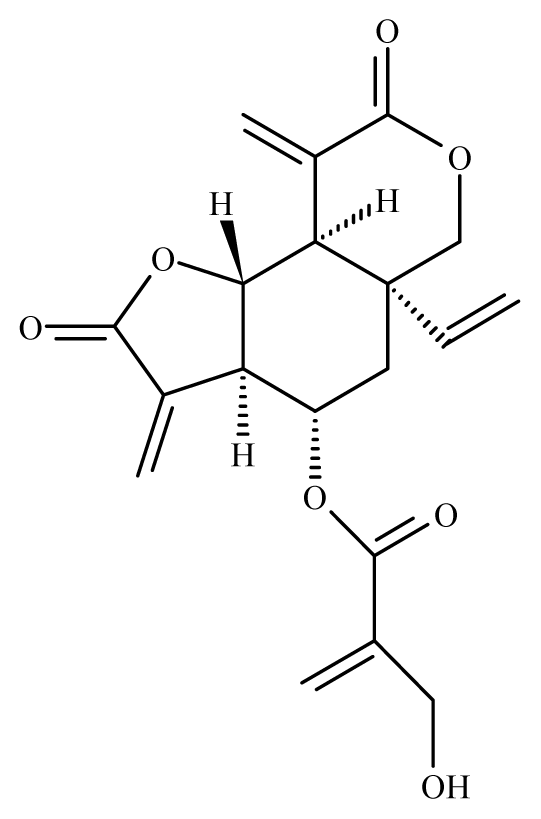 Vernodalin | Vernonia amygdalina | S. japonicum adult —20 μg/mL (100% immobilization and oviposition) | 2.5 mg/kg in mice – no mortality S. japonicum | Inhibition of the oviposition Inhibition of the motor activity | Toxic in KB, P-388 L-1210 cells7 at 120 mg/Kg | nd | [104] |
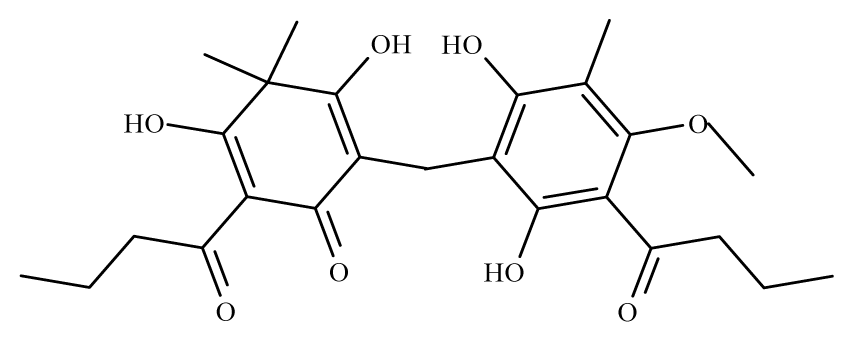 Aspidin | Dryopteris spp. | S. mansoni adult—25 μM (100% mortality in male and female) | nd | Reduction in the motor activity Tegumental alterations | nd | nd | [100] |
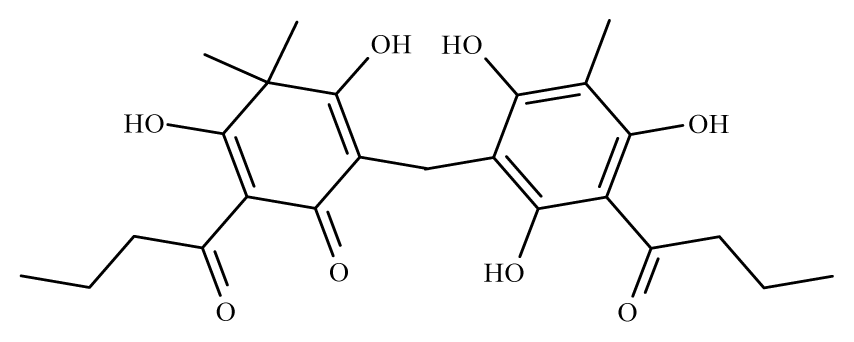 Favaspidic acid | Dryopteris spp. | S. mansoni adult—50 μM (100% mortality in male and female) | nd | Reduction in motor the activity Tegumental alterations | nd | nd | [100] |
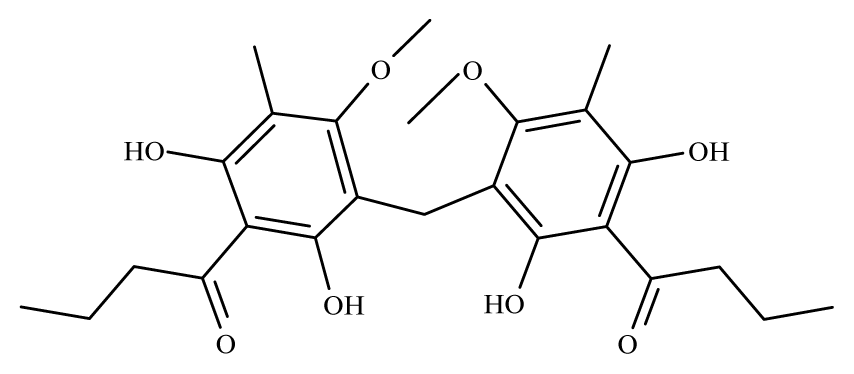 Methylene-bis-aspidinol | Dryopteris spp. | S. mansoni adult—100 μM (100% mortality in male and female) | nd | Reduction in the motor activity | nd | nd | [100] |
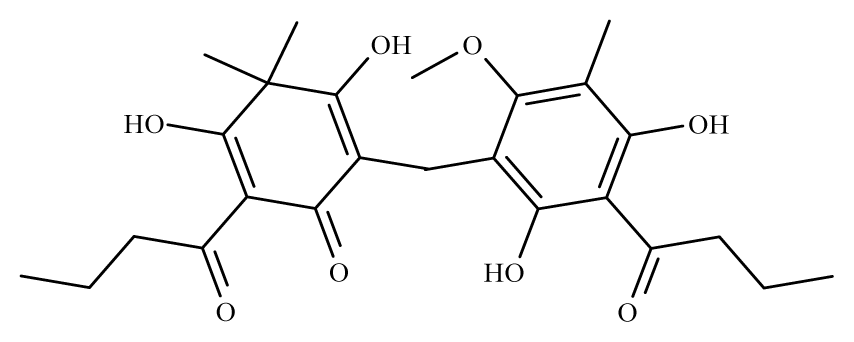 Desaspidin | Dryopteris spp. | S. mansoni adult—25 μM (100% mortality in male and female) | nd | Reduction in the motor activity | nd | nd | [100] |
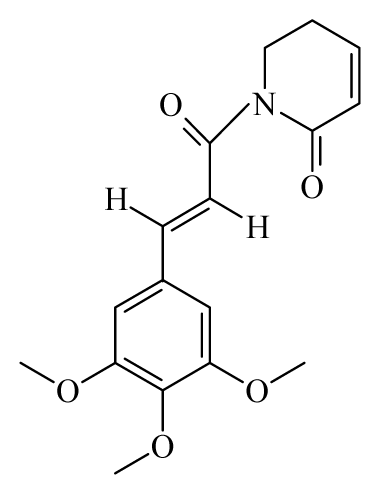 Piplartine | Piper tuberculatum | S. mansoni adult—15.8 μM (100% mortality in male and female) Schistosomula—5 μM (100% mortality) | nd | Reduction in the motor activity Reduction in the oviposition | Non-toxic in Vero cell 8 at 31.5 μM | nd | [92,103] |
| Methanol leaves extract | Baccharis trimera | S. mansoni—130 μg/mL (100% mortality in male, female and schistosomula | nd | Reduction in the motor activity Tegumental alterations | Non-toxic in human keratinocytes cell line at 250 μg/mL | nd | [121] |
| Avocado/soybean unsaponifiables | Persea americana | Nd | 300 mg/kg—3 oral doses in mice—30% S. mansoni mortality | Reduction in the motor activity Tegumental alterations Reduction in the oviposition | nd | nd | [122] |
 Allicin | Allium sativum | S. mansoni adult—20 mg/mL—no mortality | nd | 5mg/mL tegumental alterations 10mg/mL changes in tubercles, loss or changes in the spines; 15 and 20mg/mL tegumental disruption (vesicle and ulceration) | nd | nd | [123] |
| Compounds/Substances | Origin | Trypanocidal activities | Assays | References | |||
|---|---|---|---|---|---|---|---|
| in vitro | in vivo | Observations | Toxicity | Clinical | |||
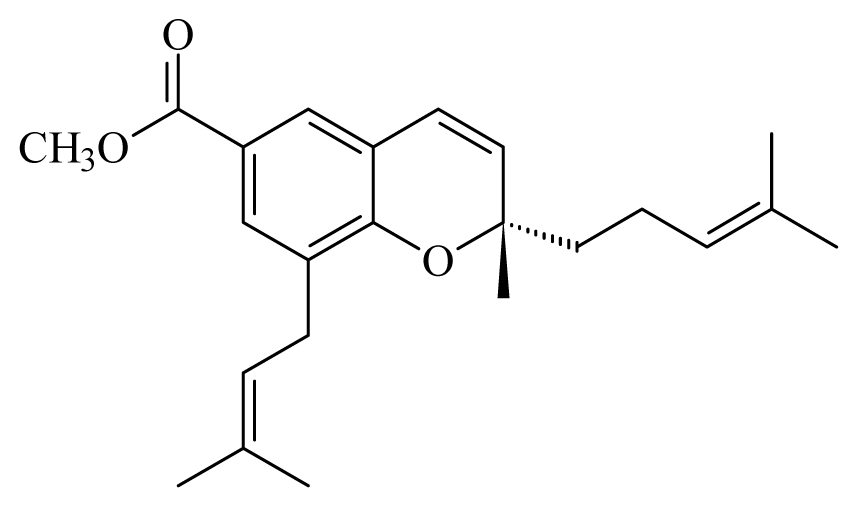 [(2S)-methyl-2-methyl-8-(3-methylbut-2′-enyl)-2-(4-methylpent-3-enyl)-2Hchromene-6-carboxylate] | Piper1 | T. cruzi epimastigote—IC50-value 2.82 μM | nd | nd | nd | nd | [143] |
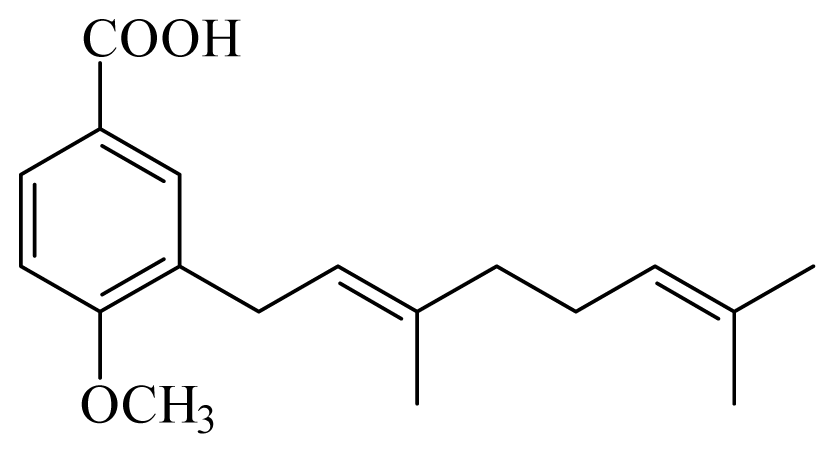 3-(3,7-dimethyl-2,6-octadienyl)-4-methoxy-benzoic acid | Piper aduncum | L. braziliensis promastigote—IC50-value 6.5 μg/mL | nd | nd | nd | nd | [144] |
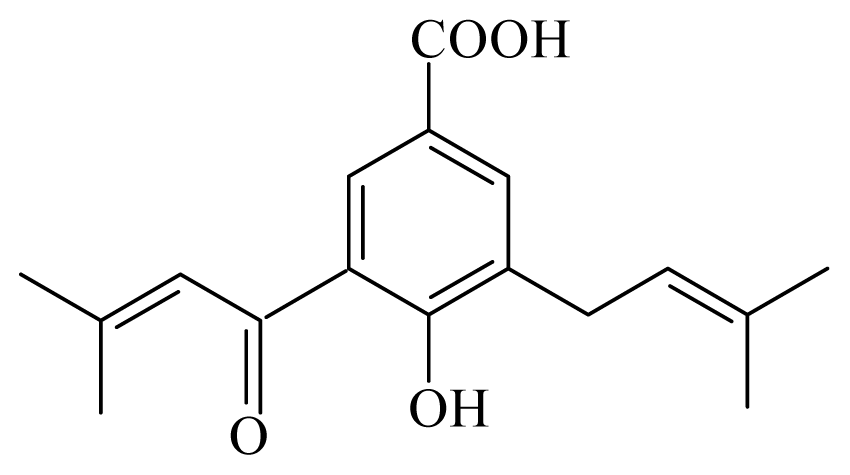 4-hydroxy-3-(3-methyl-1-oxo-2-butenyl)-5-(3-methyl-2-butenyl)benzoic acid | Piper aduncum | T. cruzi epimastigote—IC50 16.5 μg/mL | nd | nd | nd | nd | [144] |
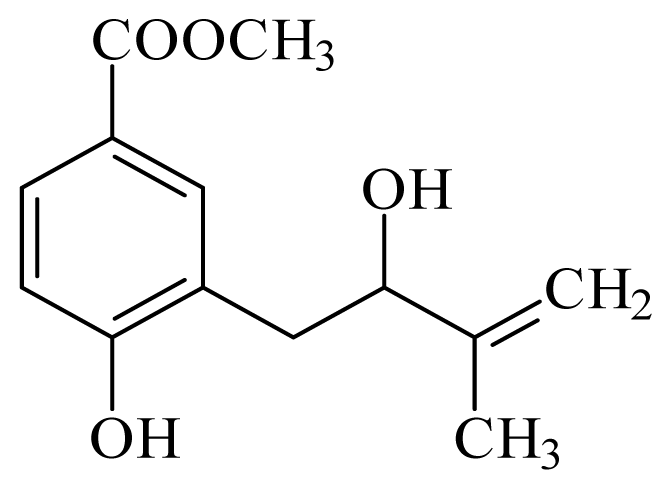 methyl 4-hydroxy-3-(2-hydroxy-3-methyl-3-butenyl)benzoate | Piper glabratum | T. cruzi epimastigote—IC50-value 15.6 μg/mL | nd | nd | nd | nd | [145] |
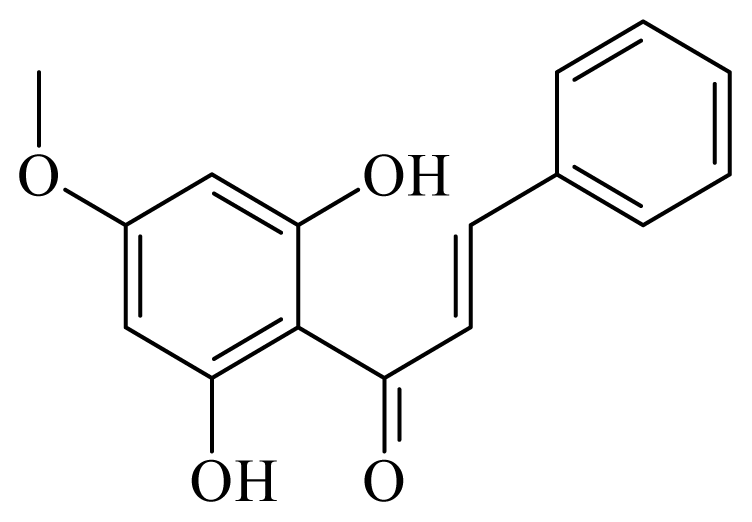 2,6-dihydroxi-4-metoxichalcone | Piper aduncum | L. amazonensis promastigote—IC50-value 0.5 μg/mL amastigotes—IC50-value 24 μg/mL | nd | nd | nd | nd | [146] |
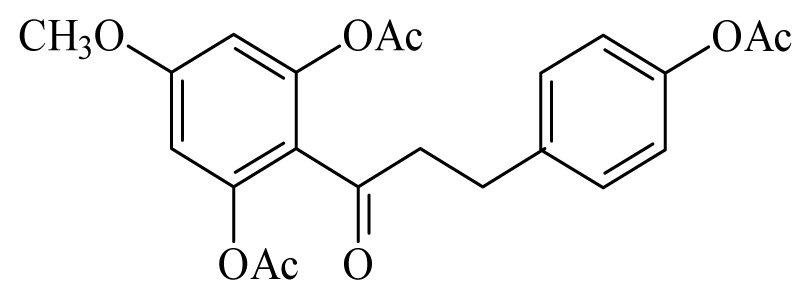 dihydrochalcone | Piper elongatum | L. braziliensis promastigote—IC50-value 2.98 μg/mL | nd | nd | nd | nd | [147] |
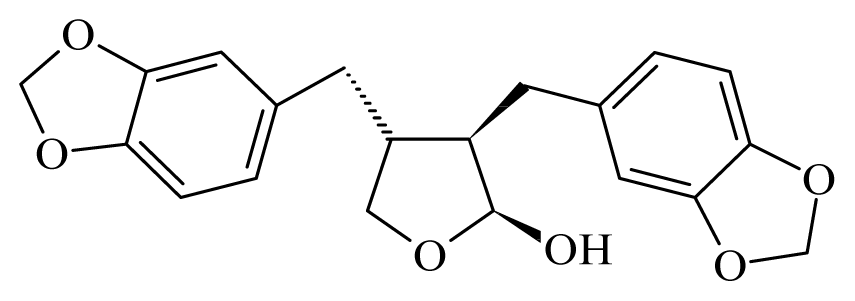 Cubebin | Piper cueba | L. donovani promastigotes—IC50-value 28 μg/mL | 100.0 mg/kg, i.p. in hamster infected with L. donovani | nd | nd | nd | [148] |
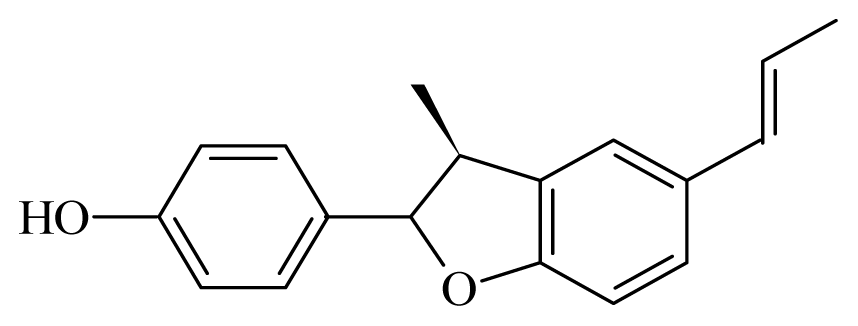 Conocarpan | Piper regnellii | T. cruzi epimastigote—IC50-value 8.0 μg/mL | nd | nd | nd | nd | [149] |
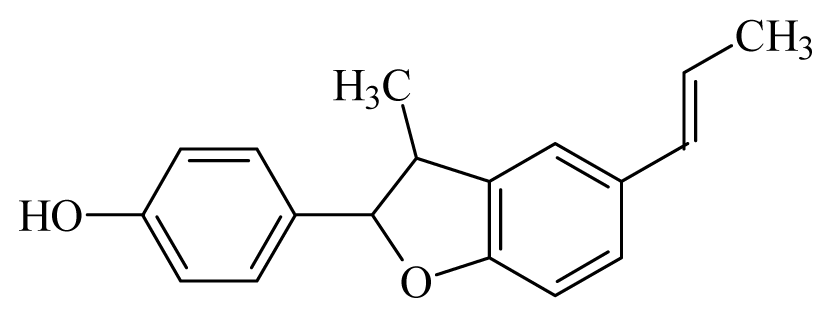 Eupomatenoid | Piper regnellii | T. cruzi epimastigote—IC50-value 7.0 μg/mL | nd | nd | Non-toxic in Vero cells (CC50 250 μg/mL) | nd | [149] |
 Grandisin | Piper solmsianum | T. cruzi trypomastigote—IC50-value 8.74 μg/mL | nd | nd | nd | nd | [150] |
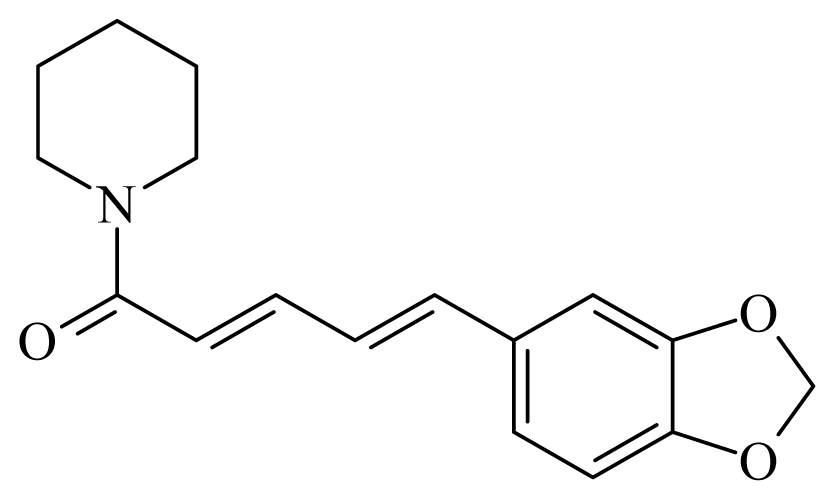 Piperine | Piper | T. cruzi epimastigote—IC50-value 7.36 μM amastigote—IC50-value 4.91 μM | nd | nd | nd | nd | [151] |
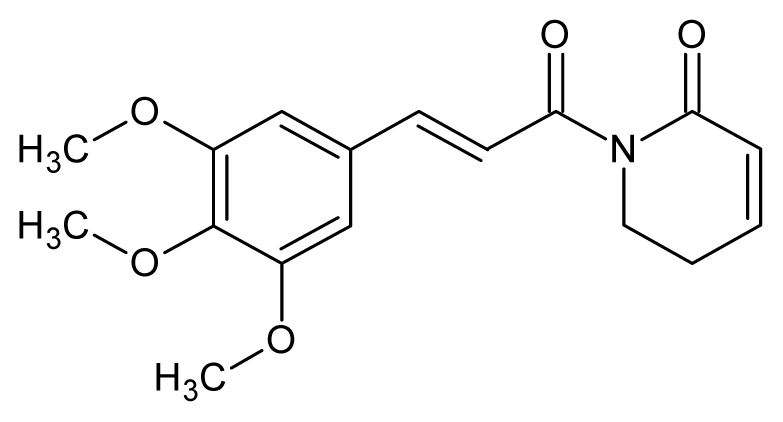 Piplartine | Piper tuberculatum Piper retrofractum | T. cruzi epimastigote—IC50-value 10.5 μM L. donovani promastigotes—IC50-value 7.5 μg/mL | 30 mg/kg ip. in hamster infected with L. donovani | nd | nd | nd | [148,152] |
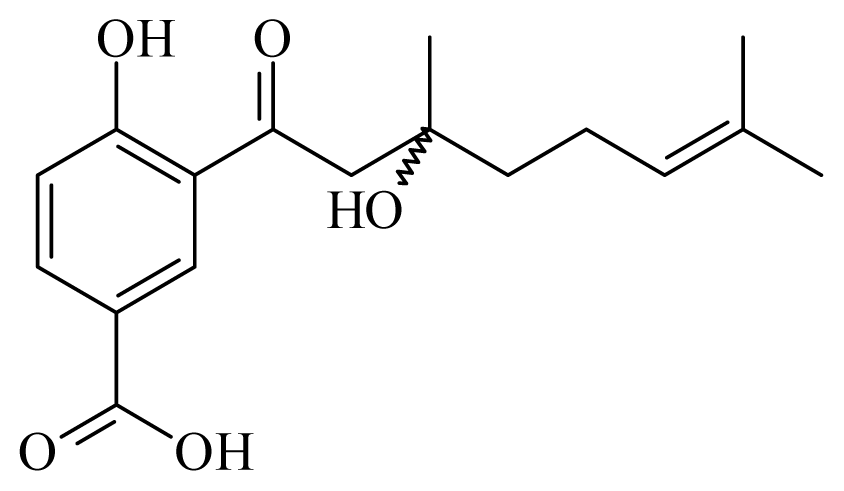 prenylated hydroquinone [1,4-dihydroxy-2-(3′,7′-dimethyl-1′-oxo-2′-E,6′-octadienyl)benzene | Piper crassinervium | T. cruzi epimastigote—IC50-value 6.10 μg/mL | nd | nd | nd | nd | [153] |
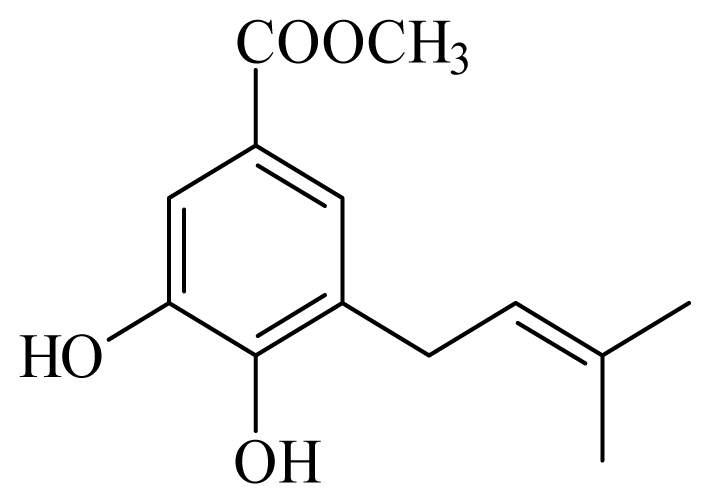 methyl 3,4-dihydroxy-5-(3′-methyl-2′-butenyl)benzoate | Piper glabratum | L. braziliensis, L. amazonensis and L. donovani—IC50-value 13.8–18.5 μg/mL) | nd | nd | nd | nd | [145] |
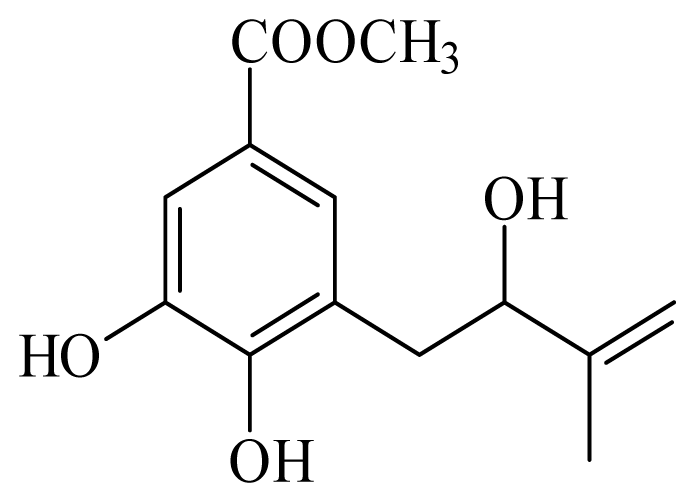 methyl 3,4-dihydroxy-5-(2-hydroxy-3-methylbutenyl)benzoate | Piper glabratum | T cruzi epimestigote—IC50-value 16.4 μg/mL | nd | nd | nd | nd | [145] |
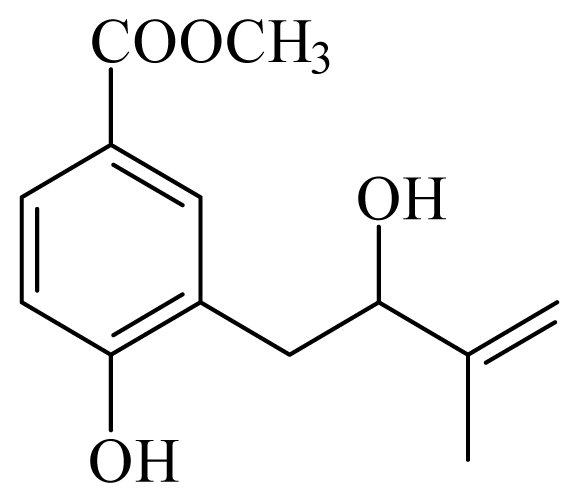 methyl 4-hydroxy-3-(2-hydroxy-3-methyl-3-butenyl)benzoate | Piper glabratum | T cruzi epimestigote—IC50-value 15.6 μg/mL | nd | nd | nd | nd | [145] |
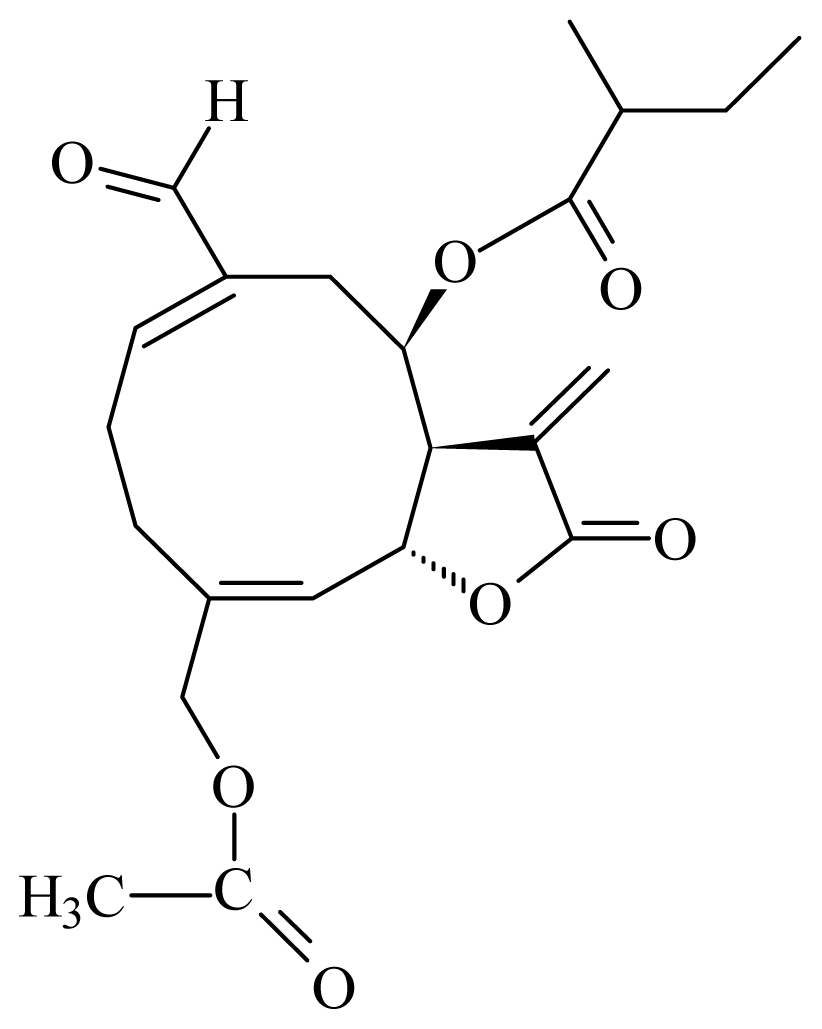 (15-acetoxy-8_-[(2-methylbutyryloxy)]-14-oxo-4,5-cis-acanthospermolide) | Acanthospermum hispidum | T. brucei brucei—IC50-value 2.45 μM L. mexicana Mexicana —IC50-value 0.94 μM | nd | nd | nd | nd | [154] |
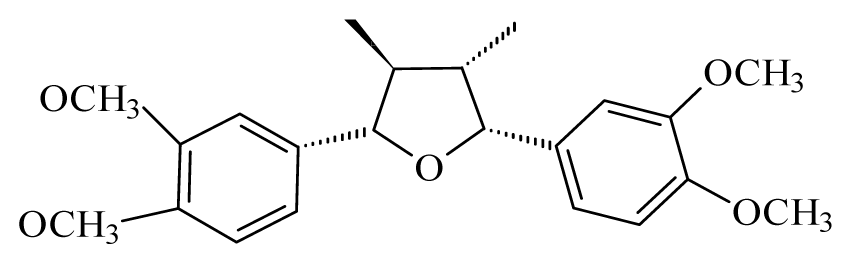 Veraguensin | Nectandra megapotamica | L.donovani promastigotes—IC50-value 18 μg/mL and IC90-value 36 μg/mL | nd | nd | Non-toxic in Vero cells5 in 10 μg/ml | nd | [91] |
| Methanol extract from stem bark | Acacia nilotica | na | 200 mg/kg body weight in mice infected with T. brucei brucei | Clear the parasites from circulation within 6 days of treatment | na | na | [136] |
| Methanol stem bark extract | Bombax buonopozense | nd | 300 mg/kg body weight in mice infected with T. brucei brucei | Clear the parasites from circulation within 7 days of treatment | nd | nd | [136] |
| Dichloromethane bark extract | Warburgia salutaris | T. brucei brucei—IC50 -value 10.68 μg/mL | nd | nd | nd | nd | [155] |
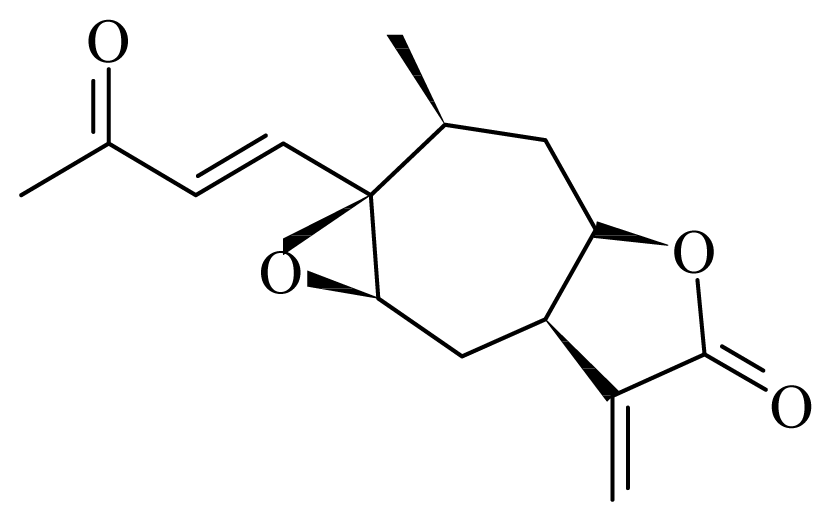 8-epixanthatin 1β,5β-epoxide | Xanthium brasilicum Vell | T. brucei—IC50 0.09 μg/mL T. cruzi—IC50-value 2.95 μg/L L. donovani—IC50 0.16 μg/mL | nd | nd | nd | nd | [142] |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ndjonka, D.; Rapado, L.N.; Silber, A.M.; Liebau, E.; Wrenger, C. Natural Products as a Source for Treating Neglected Parasitic Diseases. Int. J. Mol. Sci. 2013, 14, 3395-3439. https://doi.org/10.3390/ijms14023395
Ndjonka D, Rapado LN, Silber AM, Liebau E, Wrenger C. Natural Products as a Source for Treating Neglected Parasitic Diseases. International Journal of Molecular Sciences. 2013; 14(2):3395-3439. https://doi.org/10.3390/ijms14023395
Chicago/Turabian StyleNdjonka, Dieudonné, Ludmila Nakamura Rapado, Ariel M. Silber, Eva Liebau, and Carsten Wrenger. 2013. "Natural Products as a Source for Treating Neglected Parasitic Diseases" International Journal of Molecular Sciences 14, no. 2: 3395-3439. https://doi.org/10.3390/ijms14023395





