Annexin-Phospholipid Interactions. Functional Implications
Abstract
:1. Introduction
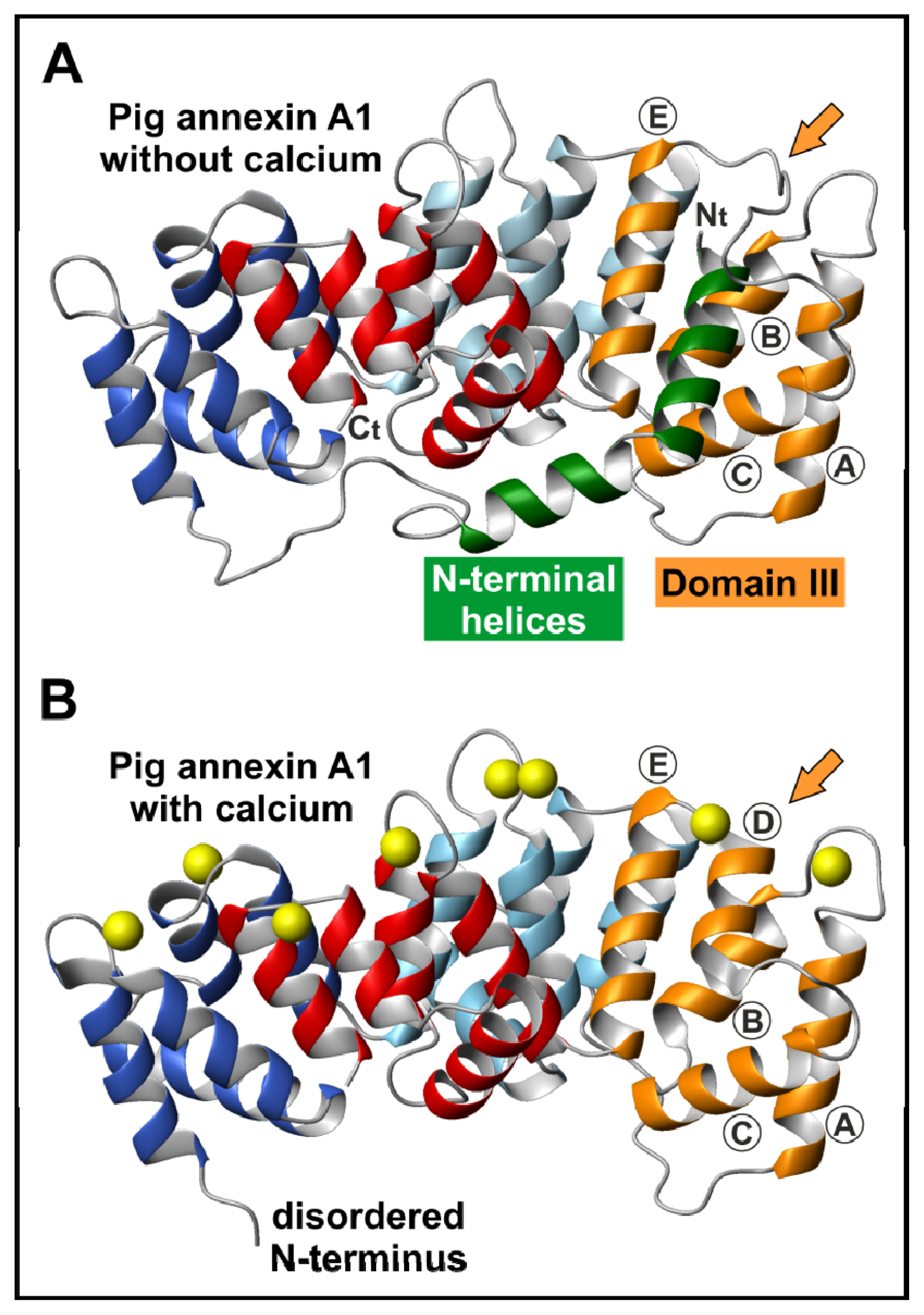
2. Annexin Structure
2.1. The Annexin Core
2.2. The N-Terminal Domain
3. Annexin Binding to Phospholipid Membranes
3.1. Calcium-Dependent Phospholipid Binding
3.2. Calcium-Independent Lipid Binding
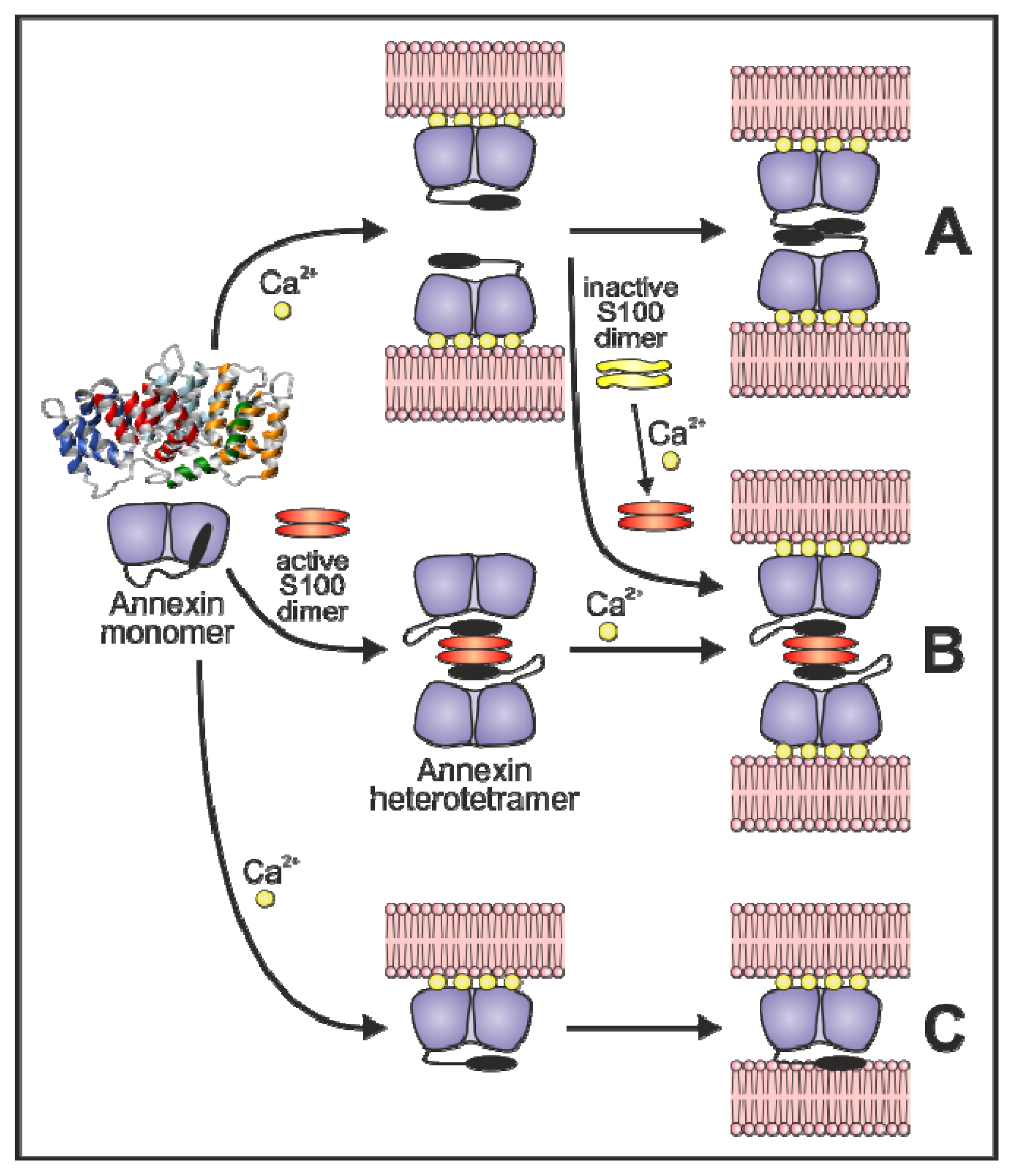
3.3. Annexin-Induced Vesicle Aggregation
4. Functional Implications of Phospholipid Binding
4.1. Intracellular Functions
4.1.1. Annexin Interactions with the Cytoskeleton
4.1.2. Annexins as Membrane Scaffolds
4.1.3. Annexins in Vesicle Traffic
4.1.4. Annexins and Intracellular Signaling
4.1.5. Annexins and Membrane Repair
4.1.6. Annexins as Calcium Channels and Ion Channel Regulators
4.2. Extracellular Annexin Activities
4.2.1. Interaction with Virus and Extracellular Matrix Components
4.2.2. Annexins and Inflammation
4.2.3. Annexins in Coagulation and Fibrinolysis
5. Annexin A5: A Tool in Research and Diagnostic
6. Conclusions
Acknowledgements
Conflict of Interest
References
- Gerke, V.; Moss, S.E. Annexins: From structure to function. Physiol. Rev 2002, 82, 331–371. [Google Scholar]
- Raynal, P.; Pollard, H.B. Annexins: The problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim. Biophys. Acta 1994, 1197, 63–93. [Google Scholar]
- Clark, G.B.; Morgan, R.O.; Fernandez, M.P.; Roux, S.J. Evolutionary adaptation of plant annexins has diversified their molecular structures, interactions and functional roles. New Phytol 2012, 196, 695–712. [Google Scholar]
- Swairjo, M.A.; Seaton, B.A. Annexin structure and membrane interactions: A molecular perspective. Annu. Rev. Biophys. Biomol. Struct 1994, 23, 193–213. [Google Scholar]
- Morgan, R.O.; Jenkins, N.A.; Gilbert, D.J.; Copeland, N.G.; Balsara, B.R.; Testa, J.R.; Fernandez, M.P. Novel human and mouse annexin A10 are linked to the genome duplications during early chordate evolution. Genomics 1999, 60, 40–49. [Google Scholar]
- Huber, R.; Römisch, J.; Paques, E.P. The crystal and molecular structure of human annexin V, an anticoagulant protein that binds to calcium and membranes. EMBO J 1990, 9, 3867–3874. [Google Scholar]
- Lewit-Bentley, A.; Morera, S.; Huber, R.; Bodo, G. The effect of metal binding on the structure of annexin V and implications for membrane binding. Eur. J. Biochem 1992, 210, 73–77. [Google Scholar]
- Hofmann, A.; Benz, J.; Liemann, S.; Huber, R. Voltage dependent binding of annexin V, annexin VI and annexin VII-core to acidic phospholipid membranes. Biochim. Biophys. Acta 1997, 1330, 254–264. [Google Scholar]
- Liemann, S.; Benz, J.; Burger, A.; Voges, D.; Hofmann, A.; Huber, R.; Gottig, P. Structural and functional characterisation of the voltage sensor in the ion channel human annexin V. J. Mol. Biol 1996, 258, 555–561. [Google Scholar]
- Matsuda, R.; Kaneko, N.; Horikawa, Y. Presence and comparison of Ca2+ transport activity of annexins I, II, V, and VI in large unilamellar vesicles. Biochem. Biophys. Res. Commun 1997, 237, 499–503. [Google Scholar]
- Koradi, R.; Billeter, M.; Wuthrich, K. MOLMOL: A program for display and analysis of macromolecular structures. J. Mol. Graph. 1996, 14. [Google Scholar]
- Swairjo, M.A.; Concha, N.O.; Kaetzel, M.A.; Dedman, J.R.; Seaton, B.A. Ca2+-bridging mechanism and phospholipid head group recognition in the membrane-binding protein annexin V. Nat. Struct. Biol 1995, 2, 968–974. [Google Scholar]
- Turnay, J.; Guzmán-Aránguez, A.; Lecona, E.; Pérez-Ramos, P.; Fernández-Lizarbe, S.; Olmo, N.; Lizarbe, M.A. Influence of the N-terminal domain of annexins in their functional properties. Recent Res. Dev. Biochem 2003, 4, 53–78. [Google Scholar]
- Turnay, J.; Lecona, E.; Guzmán-Aránguez, A.; Pérez-Ramos, P.; Fernández-Lizarbe, S.; Olmo, N.; Lizarbe, M.A. Annexins: Structural characteristics of the N-terminus and influence on the overall structure of the protein. Recent Res. Dev. Biochem 2003, 4, 79–95. [Google Scholar]
- Bewley, M.C.; Boustead, C.M.; Walker, J.H.; Waller, D.A.; Huber, R. Structure of chicken annexin V at 2.25-A resolution. Biochemistry 1993, 32, 3923–3929. [Google Scholar]
- Huber, R.; Berendes, R.; Burger, A.; Luecke, H.; Karshikov, A. Annexin V-crystal structure and its implications on function. Behring Inst. Mitt. 1992, 107–125. [Google Scholar]
- Sopkova, J.; Renouard, M.; Lewit-Bentley, A. The crystal structure of a new high-calcium form of annexin V. J. Mol. Biol 1993, 234, 816–825. [Google Scholar]
- Voges, D.; Berendes, R.; Burger, A.; Demange, P.; Baumeister, W.; Huber, R. Three-dimensional structure of membrane-bound annexin V. A correlative electron microscopy-X-ray crystallography study. J. Mol. Biol 1994, 238, 199–213. [Google Scholar]
- Arboledas, D.; Olmo, N.; Lizarbe, M.A.; Turnay, J. Role of the N-terminus in the structure and stability of chicken annexin V. FEBS Lett 1997, 416, 217–220. [Google Scholar]
- Kaetzel, M.A.; Mo, Y.D.; Mealy, T.R.; Campos, B.; Bergsma-Schutter, W.; Brisson, A.; Dedman, J.R.; Seaton, B.A. Phosphorylation mutants elucidate the mechanism of annexin IV-mediated membrane aggregation. Biochemistry 2001, 40, 4192–4199. [Google Scholar]
- Porte, F.; de Santa Barbara, P.; Phalipou, S.; Liautard, J.P.; Widada, J.S. Change in the N-terminal domain conformation of annexin I that correlates with liposome aggregation is impaired by Ser-27 to Glu mutation that mimics phosphorylation. Biochim. Biophys. Acta 1996, 1293, 177–184. [Google Scholar]
- Rosengarth, A.; Gerke, V.; Luecke, H. X-ray structure of full-length annexin 1 and implications for membrane aggregation. J. Mol. Biol 2001, 306, 489–498. [Google Scholar]
- De la Fuente, M.; Ossa, C.G. Binding to phosphatidyl serine membranes causes a conformational change in the concave face of annexin I. Biophys. J 1997, 72, 383–387. [Google Scholar]
- Rosengarth, A.; Luecke, H. A calcium-driven conformational switch of the N-terminal and core domains of annexin A1. J. Mol. Biol 2003, 326, 1317–1325. [Google Scholar]
- Lecona, E.; Turnay, J.; Olmo, N.; Guzman-Aranguez, A.; Morgan, R.O.; Fernandez, M.P.; Lizarbe, M.A. Structural and functional characterization of recombinant mouse annexin A11: Influence of calcium binding. Biochem. J 2003, 373, 437–449. [Google Scholar]
- Huber, R.; Schneider, M.; Mayr, I.; Romisch, J.; Paques, E.P. The calcium binding sites in human annexin V by crystal structure analysis at 2.0 A resolution. Implications for membrane binding and calcium channel activity. FEBS Lett 1990, 275, 15–21. [Google Scholar]
- Evans, T.C., Jr; Nelsestuen, G.L. Calcium and membrane-binding properties of monomeric andmultimeric annexin II. Biochemistry 1994, 33, 13231–13238. [Google Scholar]
- Patel, D.R.; Isas, J.M.; Ladokhin, A.S.; Jao, C.C.; Kim, Y.E.; Kirsch, T.; Langen, R.; Haigler, H.T. The conserved core domains of annexins A1, A2, A5, and B12 can be divided into two groups with different Ca2+-dependent membrane-binding properties. Biochemistry 2005, 44, 2833–2844. [Google Scholar]
- Patel, D.R.; Jao, C.C.; Mailliard, W.S.; Isas, J.M.; Langen, R.; Haigler, H.T. Calcium-dependent binding of annexin 12 to phospholipid bilayers: Stoichiometry and implications. Biochemistry 2001, 40, 7054–7060. [Google Scholar]
- Jin, M.; Smith, C.; Hsieh, H.Y.; Gibson, D.F.; Tait, J.F. Essential role of B-helix calcium binding sites in annexin V-membrane binding. J. Biol. Chem 2004, 279, 40351–40357. [Google Scholar]
- Jost, M.; Weber, K.; Gerke, V. Annexin II contains two types of Ca2+-binding sites. Biochem. J 1994, 298, 553–559. [Google Scholar]
- Montaville, P.; Neumann, J.M.; Russo-Marie, F.; Ochsenbein, F.; Sanson, A. A new consensus sequence for phosphatidylserine recognition by annexins. J. Biol. Chem 2002, 277, 24684–24693. [Google Scholar]
- Ayala-Sanmartin, J.; Vincent, M.; Sopkova, J.; Gallay, J. Modulation by Ca2+ and by membrane binding of the dynamics of domain III of annexin 2 (p36) and the annexin 2-p11 complex (p90): Implications for their biochemical properties. Biochemistry 2000, 39, 15179–15189. [Google Scholar]
- Schlaepfer, D.D.; Mehlman, T.; Burgess, W.H.; Haigler, H.T. Structural and functional characterization of endonexin II, a calcium- and phospholipid-binding protein. Proc. Natl. Acad. Sci. USA 1987, 84, 6078–6082. [Google Scholar]
- Turnay, J.; Olmo, N.; Gasset, M.; Iloro, I.; Arrondo, J.L.; Lizarbe, M.A. Calcium-dependent conformational rearrangements and protein stability in chicken annexin A5. Biophys. J 2002, 83, 2280–2291. [Google Scholar]
- Merzel, F.; Hodoscek, M.; Janezic, D.; Sanson, A. New force field for calcium binding sites in annexin-membrane complexes. J. Comput. Chem 2006, 27, 446–452. [Google Scholar]
- Turnay, J.; Lecona, E.; Fernández-Lizarbe, S.; Guzmán-Aránguez, A.; Fernández, M.P.; Olmo, N.; Lizarbe, M.A. Structure-function relationship in annexin A13, the founder member of the vertebrate family of annexins. Biochem. J 2005, 389, 899–911. [Google Scholar]
- Gerke, V.; Creutz, C.E.; Moss, S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol 2005, 6, 449–461. [Google Scholar]
- Goebeler, V.; Ruhe, D.; Gerke, V.; Rescher, U. Annexin A8 displays unique phospholipid and F-actin binding properties. FEBS Lett 2006, 580, 2430–2434. [Google Scholar]
- Rescher, U.; Ruhe, D.; Ludwig, C.; Zobiack, N.; Gerke, V. Annexin 2 is a phosphatidylinositol (4,5)-bisphosphate binding protein recruited to actin assembly sites at cellular membranes. J. Cell Sci 2004, 117, 3473–3480. [Google Scholar]
- Hayes, M.J.; Shao, D.M.; Grieve, A.; Levine, T.; Bailly, M.; Moss, S.E. Annexin A2 at the interface between F-actin and membranes enriched in phosphatidylinositol 4,5-bisphosphate. Biochim. Biophys. Acta 2009, 1793, 1086–1095. [Google Scholar]
- Morgan, R.O.; Martin-Almedina, S.; Iglesias, J.M.; Gonzalez-Florez, M.I.; Fernandez, M.P. Evolutionary perspective on annexin calcium-binding domains. Biochim. Biophys. Acta 2004, 1742, 133–140. [Google Scholar]
- Goebeler, V.; Ruhe, D.; Gerke, V.; Rescher, U. Atypical properties displayed by annexin A9, a novel member of the annexin family of Ca2+ and lipid binding proteins. FEBS Lett 2003, 546, 359–364. [Google Scholar]
- Boczonadi, V.; Maatta, A. Annexin A9 is a periplakin interacting partner in membrane-targeted cytoskeletal linker protein complexes. FEBS Lett 2012, 586, 3090–3096. [Google Scholar]
- Nakayama, H.; Fukuda, S.; Inoue, H.; Nishida-Fukuda, H.; Shirakata, Y.; Hashimoto, K.; Higashiyama, S. Cell surface annexins regulate ADAM-mediated ectodomain shedding of proamphiregulin. Mol. Biol. Cell 2012, 23, 1964–1975. [Google Scholar]
- Beermann ofm cap, Br. B.; Hinz, H.J.; Hofmann, A.; Huber, R. Acid induced equilibrium unfolding of annexin V wild type shows two intermediate states. FEBS Lett. 1998, 423, 265–269. [Google Scholar]
- Sopkova, J.; Vincent, M.; Takahashi, M.; Lewit-Bentley, A.; Gallay, J. Conformational flexibility of domain III of annexin V studied by fluorescence of tryptophan 187 and circular dichroism: The effect of pH. Biochemistry 1998, 37, 11962–11970. [Google Scholar]
- Van der Goot, F.G.; Gonzalez-Manas, J.M.; Lakey, J.H.; Pattus, F. A “molten-globule” membrane-insertion intermediate of the pore-forming domain of colicin A. Nature 1991, 354, 408–410. [Google Scholar]
- Golczak, M.; Kirilenko, A.; Bandorowicz-Pikula, J.; Desbat, B.; Pikula, S. Structure of human annexin a6 at the air-water interface and in a membrane-bound state. Biophys. J 2004, 87, 1215–1226. [Google Scholar]
- Köhler, G.; Hering, U.; Zschornig, O.; Arnold, K. Annexin V interaction with phosphatidylserine-containing vesicles at low and neutral pH. Biochemistry 1997, 36, 8189–8194. [Google Scholar]
- Hoekstra, D.; Buist-Arkema, R.; Klappe, K.; Reutelingsperger, C.P. Interaction of annexins with membranes: The N-terminus as a governing parameter as revealed with a chimeric annexin. Biochemistry 1993, 32, 14194–14202. [Google Scholar]
- Zschornig, O.; Opitz, F.; Muller, M. Annexin A4 binding to anionic phospholipid vesicles modulated by pH and calcium. Eur. Biophys. J 2007, 36, 415–424. [Google Scholar]
- Golczak, M.; Kicinska, A.; Bandorowicz-Pikula, J.; Buchet, R.; Szewczyk, A.; Pikula, S. Acidic pH-induced folding of annexin VI is a prerequisite for its insertion into lipid bilayers and formation of ion channels by the protein molecules. FASEB J 2001, 15, 1083–1085. [Google Scholar]
- Rosengarth, A.; Wintergalen, A.; Galla, H.J.; Hinz, H.J.; Gerke, V. Ca2+-independent interaction of annexin I with phospholipid monolayers. FEBS Lett 1998, 438, 279–284. [Google Scholar]
- Isas, J.M.; Cartailler, J.P.; Sokolov, Y.; Patel, D.R.; Langen, R.; Luecke, H.; Hall, J.E.; Haigler, H.T. Annexins V and XII insert into bilayers at mildly acidic pH and form ion channels. Biochemistry 2000, 39, 3015–3022. [Google Scholar]
- Isas, J.M.; Kim, Y.E.; Jao, C.C.; Hegde, P.B.; Haigler, H.T.; Langen, R. Calcium- and membrane-induced changes in the structure and dynamics of three helical hairpins in annexin B12. Biochemistry 2005, 44, 16435–16444. [Google Scholar]
- Isas, J.M.; Langen, R.; Haigler, H.T.; Hubbell, W.L. Structure and dynamics of a helical hairpin and loop region in annexin 12: A site-directed spin labeling study. Biochemistry 2002, 41, 1464–1473. [Google Scholar]
- Isas, J.M.; Patel, D.R.; Jao, C.; Jayasinghe, S.; Cartailler, J.P.; Haigler, H.T.; Langen, R. Global structural changes in annexin 12. The roles of phospholipid, Ca2+, and pH. J. Biol. Chem 2003, 278, 30227–30234. [Google Scholar]
- Kim, Y.E.; Isas, J.M.; Haigler, H.T.; Langen, R. A helical hairpin region of soluble annexin B12 refolds and forms a continuous transmembrane helix at mildly acidic pH. J. Biol. Chem 2005, 280, 32398–32404. [Google Scholar]
- Langen, R.; Isas, J.M.; Hubbell, W.L.; Haigler, H.T. A transmembrane form of annexin XII detected by site-directed spin labeling. Proc. Natl. Acad. Sci. USA 1998, 95, 14060–14065. [Google Scholar]
- Hegde, B.G.; Isas, J.M.; Zampighi, G.; Haigler, H.T.; Langen, R. A novel calcium-independent peripheral membrane-bound form of annexin B12. Biochemistry 2006, 45, 934–942. [Google Scholar]
- Morgan, R.O.; Pilar Fernandez, M. Distinct annexin subfamilies in plants and protists diverged prior to animal annexins and from a common ancestor. J. Mol. Evol 1997, 44, 178–188. [Google Scholar]
- Wice, B.M.; Gordon, J.I. A strategy for isolation of cDNAs encoding proteins affecting human intestinal epithelial cell growth and differentiation: Characterization of a novel gut-specific N-myristoylated annexin. J. Cell Biol 1992, 116, 405–422. [Google Scholar]
- Fiedler, K.; Lafont, F.; Parton, R.G.; Simons, K. Annexin XIIIb: A novel epithelial specific annexin is implicated in vesicular traffic to the apical plasma membrane. J. Cell Biol 1995, 128, 1043–1053. [Google Scholar]
- Blackwood, R.A.; Ernst, J.D. Characterization of Ca2+-dependent phospholipid binding, vesicle aggregation and membrane-fusion by annexins. Biochem. J 1990, 266, 195–200. [Google Scholar]
- Turnay, J.; Guzmán-Aránguez, A.; Lecona, E.; Barrasa, J.I.; Olmo, N.; Lizarbe, M.A. Key role of the N-terminus of chicken annexin A5 in vesicle aggregation. Protein Sci 2009, 18, 1095–1106. [Google Scholar]
- Rothhut, B. Participation of annexins in protein phosphorylation. Cell. Mol. Life Sci 1997, 53, 522–526. [Google Scholar]
- Dorovkov, M.V.; Kostyukova, A.S.; Ryazanov, A.G. Phosphorylation of annexin A1 by TRPM7 kinase: A switch regulating the induction of an alpha-helix. Biochemistry 2011, 50, 2187–2193. [Google Scholar]
- Bitto, E.; Cho, W. Structural determinant of the vesicle aggregation activity of annexin I. Biochemistry 1999, 38, 14094–14100. [Google Scholar]
- Naidu, D.G.; Raha, A.; Chen, X.L.; Spitzer, A.R.; Chander, A. Partial truncation of the NH2-terminus affects physical characteristics and membrane binding, aggregation, and fusion properties of annexin A7. Biochim. Biophys. Acta 2005, 1734, 152–168. [Google Scholar]
- Illien, F.; Piao, H.R.; Coue, M.; di Marco, C.; Ayala-Sanmartin, J. Lipid organization regulates annexin A2 Ca2+-sensitivity for membrane bridging and its modulator effects on membrane fluidity. Biochim. Biophys. Acta 2012, 1818, 2892–2900. [Google Scholar]
- Dempsey, A.C.; Walsh, M.P.; Shaw, G.S. Unmasking the annexin I interaction from the structure of Apo-S100A11. Structure 2003, 11, 887–897. [Google Scholar]
- Illien, F.; Finet, S.; Lambert, O.; Ayala-Sanmartin, J. Different molecular arrangements of the tetrameric annexin 2 modulate the size and dynamics of membrane aggregation. Biochim. Biophys. Acta 2010, 1798, 1790–1796. [Google Scholar]
- Ayala-Sanmartin, J.; Zibouche, M.; Illien, F.; Vincent, M.; Gallay, J. Insight into the location and dynamics of the annexin A2 N-terminal domain during Ca2+-induced membrane bridging. Biochim. Biophys. Acta 2008, 1778, 472–482. [Google Scholar]
- Hu, N.J.; Bradshaw, J.; Lauter, H.; Buckingham, J.; Solito, E.; Hofmann, A. Membrane-induced folding and structure of membrane-bound annexin A1 N-terminal peptides: Implications for annexin-induced membrane aggregation. Biophys. J 2008, 94, 1773–1781. [Google Scholar]
- Zibouche, M.; Vincent, M.; Illien, F.; Gallay, J.; Ayala-Sanmartin, J. The N-terminal domain of annexin 2 serves as a secondary binding site during membrane bridging. J. Biol. Chem 2008, 283, 22121–22127. [Google Scholar]
- Shesham, R.D.; Bartolotti, L.J.; Li, Y. Molecular dynamics simulation studies on Ca2+-induced conformational changes of annexin I. Protein Eng. Des. Sel 2008, 21, 115–120. [Google Scholar]
- Avila-Sakar, A.J.; Creutz, C.E.; Kretsinger, R.H. Crystal structure of bovine annexin VI in a calcium-bound state. Biochim. Biophys. Acta 1998, 1387, 103–116. [Google Scholar]
- Freye-Minks, C.; Kretsinger, R.H.; Creutz, C.E. Structural and dynamic changes in human annexin VI induced by a phosphorylation-mimicking mutation, T356D. Biochemistry 2003, 42, 620–630. [Google Scholar]
- Avila-Sakar, A.J.; Kretsinger, R.H.; Creutz, C.E. Membrane-bound 3D structures reveal the intrinsic flexibility of annexin VI. J. Struct. Biol 2000, 130, 54–62. [Google Scholar]
- Benz, J.; Bergner, A.; Hofmann, A.; Demange, P.; Gottig, P.; Liemann, S.; Huber, R.; Voges, D. The structure of recombinant human annexin VI in crystals and membrane-bound. J. Mol. Biol 1996, 260, 638–643. [Google Scholar]
- Buzhynskyy, N.; Golczak, M.; Lai-Kee-Him, J.; Lambert, O.; Tessier, B.; Gounou, C.; Berat, R.; Simon, A.; Granier, T.; Chevalier, J.M.; et al. Annexin-A6 presents two modes of association with phospholipid membranes. A combined QCM-D, AFM and cryo-TEM study. J. Struct. Biol. 2009, 168, 107–116. [Google Scholar]
- Chapman, L.P.; Epton, M.J.; Buckingham, J.C.; Morris, J.F.; Christian, H.C. Evidence for a role of the adenosine 5′-triphosphate-binding cassette transporter A1 in the externalization of annexin I from pituitary folliculo-stellate cells. Endocrinology 2003, 144, 1062–1073. [Google Scholar]
- Wein, S.; Fauroux, M.; Laffitte, J.; de Nadai, P.; Guaini, C.; Pons, F.; Comera, C. Mediation of annexin 1 secretion by a probenecid-sensitive ABC-transporter in rat inflamed mucosa. Biochem. Pharmacol 2004, 67, 1195–1202. [Google Scholar]
- Wang, X.; Campos, B.; Kaetzel, M.A.; Dedman, J.R. Secretion of annexin V from cultured cells requires a signal peptide. Placenta 2001, 22, 837–845. [Google Scholar]
- Hayes, M.J.; Rescher, U.; Gerke, V.; Moss, S.E. Annexin-actin interactions. Traffic 2004, 5, 571–576. [Google Scholar]
- Morel, E.; Parton, R.G.; Gruenberg, J. Annexin A2-dependent polymerization of actin mediates endosome biogenesis. Dev. Cell 2009, 16, 445–457. [Google Scholar]
- Alvarez-Martinez, M.T.; Porte, F.; Liautard, J.P.; Sri Widada, J. Effects of profilin-annexin I association on some properties of both profilin and annexin I: Modification of the inhibitory activity of profilin on actin polymerization and inhibition of the self-association of annexin I and its interactions with liposomes. Biochim. Biophys. Acta 1997, 1339, 331–340. [Google Scholar]
- Alvarez-Martinez, M.T.; Mani, J.C.; Porte, F.; Faivre-Sarrailh, C.; Liautard, J.P.; Sri Widada, J. Characterization of the interaction between annexin I and profilin. Eur. J. Biochem 1996, 238, 777–784. [Google Scholar]
- McArthur, S.; Yazid, S.; Christian, H.; Sirha, R.; Flower, R.; Buckingham, J.; Solito, E. Annexin A1 regulates hormone exocytosis through a mechanism involving actin reorganization. FASEB J 2009, 23, 4000–4010. [Google Scholar]
- Tzima, E.; Trotter, P.J.; Orchard, M.A.; Walker, J.H. Annexin V relocates to the platelet cytoskeleton upon activation and binds to a specific isoform of actin. Eur. J. Biochem 2000, 267, 4720–4730. [Google Scholar]
- Babiychuk, E.B.; Palstra, R.J.; Schaller, J.; Kampfer, U.; Draeger, A. Annexin VI participates in the formation of a reversible, membrane-cytoskeleton complex in smooth muscle cells. J. Biol. Chem 1999, 274, 35191–35195. [Google Scholar]
- Farnaes, L.; Ditzel, H.J. Dissecting the cellular functions of annexin XI using recombinant human annexin XI-specific autoantibodies cloned by phage display. J. Biol. Chem 2003, 278, 33120–33126. [Google Scholar]
- Tomas, A.; Futter, C.; Moss, S.E. Annexin 11 is required for midbody formation and completion of the terminal phase of cytokinesis. J. Cell Biol 2004, 165, 813–822. [Google Scholar]
- Van Genderen, H.O.; Kenis, H.; Hofstra, L.; Narula, J.; Reutelingsperger, C.P. Extracellular annexin A5: Functions of phosphatidylserine-binding and two-dimensional crystallization. Biochim. Biophys. Acta 2008, 1783, 953–963. [Google Scholar]
- Faiss, S.; Kastl, K.; Janshoff, A.; Steinem, C. Formation of irreversibly bound annexin A1 protein domains on POPC/POPS solid supported membranes. Biochim. Biophys. Acta 2008, 1778, 1601–1610. [Google Scholar]
- Pollard, H.B.; Pazoles, C.J.; Creutz, C.E.; Zinder, O. Role of intracellular proteins in the regulation of calcium action and transmitter release during exocytosis. Monogr. Neural Sci 1980, 7, 106–116. [Google Scholar]
- Chasserot-Golaz, S.; Vitale, N.; Umbrecht-Jenck, E.; Knight, D.; Gerke, V.; Bader, M.F. Annexin 2 promotes the formation of lipid microdomains required for calcium-regulated exocytosis of dense-core vesicles. Mol. Biol. Cell 2005, 16, 1108–1119. [Google Scholar]
- Futter, C.E.; White, I.J. Annexins and endocytosis. Traffic 2007, 8, 951–958. [Google Scholar]
- White, I.J.; Bailey, L.M.; Aghakhani, M.R.; Moss, S.E.; Futter, C.E. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J 2006, 25, 1–12. [Google Scholar]
- Grewal, T.; Enrich, C. Annexins—Modulators of EGF receptor signalling and trafficking. Cell. Signal 2009, 21, 847–858. [Google Scholar]
- Cubells, L.; Vila de Muga, S.; Tebar, F.; Wood, P.; Evans, R.; Ingelmo-Torres, M.; Calvo, M.; Gaus, K.; Pol, A.; Grewal, T.; et al. Annexin A6-induced alterations in cholesterol transport and caveolin export from the Golgi complex. Traffic 2007, 8, 1568–1589. [Google Scholar]
- Enrich, C.; Rentero, C.; de Muga, S.V.; Reverter, M.; Mulay, V.; Wood, P.; Koese, M.; Grewal, T. Annexin A6-Linking Ca(2+) signaling with cholesterol transport. Biochim. Biophys. Acta 2011, 1813, 935–947. [Google Scholar]
- Grewal, T.; Koese, M.; Rentero, C.; Enrich, C. Annexin A6-regulator of the EGFR/Ras signalling pathway and cholesterol homeostasis. Int. J. Biochem. Cell Biol 2010, 42, 580–584. [Google Scholar]
- Pittis, M.G.; Muzzolin, L.; Giulianini, P.G.; Garcia, R.C. Mycobacteria-containing phagosomes associate less annexins I, VI, VII and XI, but not II, concomitantly with a diminished phagolysosomal fusion. Eur. J. Cell Biol 2003, 82, 9–17. [Google Scholar]
- Babiychuk, E.B.; Draeger, A. Annexins in cell membrane dynamics. Ca2+-regulated association of lipid microdomains. J. Cell Biol 2000, 150, 1113–1124. [Google Scholar]
- Babiychuk, E.B.; Draeger, A. Biochemical characterization of detergent-resistant membranes: A systematic approach. Biochem. J 2006, 397, 407–416. [Google Scholar]
- Bandorowicz-Pikula, J.; Wos, M.; Pikula, S. Do annexins participate in lipid messenger mediated intracellular signaling? A question revisited. Mol. Membr. Biol 2012, 29, 229–242. [Google Scholar]
- Schug, Z.T.; Frezza, C.; Galbraith, L.C.; Gottlieb, E. The music of lipids: How lipid composition orchestrates cellular behaviour. Acta Oncol 2012, 51, 301–310. [Google Scholar]
- Monastyrskaya, K.; Babiychuk, E.B.; Draeger, A. The annexins: Spatial and temporal coordination of signaling events during cellular stress. Cell. Mol. Life Sci 2009, 66, 2623–2642. [Google Scholar]
- Hannun, Y.A.; Obeid, L.M. The Ceramide-centric universe of lipid-mediated cell regulation: Stress encounters of the lipid kind. J. Biol. Chem 2002, 277, 25847–25850. [Google Scholar]
- Babiychuk, E.B.; Monastyrskaya, K.; Draeger, A. Fluorescent annexin A1 reveals dynamics of ceramide platforms in living cells. Traffic 2008, 9, 1757–1775. [Google Scholar]
- Koese, M.; Rentero, C.; Kota, B.P.; Hoque, M.; Cairns, R.; Wood, P.; Vila de Muga, S.; Reverter, M.; Alvarez-Guaita, A.; Monastyrskaya, K.; et al. Annexin A6 is a scaffold for PKCα to promote EGFR inactivation. Oncogene 2012. [Google Scholar] [CrossRef]
- Dubois, T.; Mira, J.P.; Feliers, D.; Solito, E.; Russo-Marie, F.; Oudinet, J.P. Annexin V inhibits protein kinase C activity via a mechanism of phospholipid sequestration. Biochem. J 1998, 330, 1277–1282. [Google Scholar]
- Vila de Muga, S.; Timpson, P.; Cubells, L.; Evans, R.; Hayes, T.E.; Rentero, C.; Hegemann, A.; Reverter, M.; Leschner, J.; Pol, A.; et al. Annexin A6 inhibits Ras signalling in breast cancer cells. Oncogene 2009, 28, 363–377. [Google Scholar]
- Shetty, P.K.; Thamake, S.I.; Biswas, S.; Johansson, S.L.; Vishwanatha, J.K. Reciprocal regulation of annexin A2 and EGFR with Her-2 in Her-2 negative and herceptin-resistant breast cancer. PLoS One 2012, 7, e44299. [Google Scholar]
- Zhao, P.; Zhang, W.; Wang, S.J.; Yu, X.L.; Tang, J.; Huang, W.; Li, Y.; Cui, H.Y.; Guo, Y.S.; Tavernier, J.; et al. HAb18G/CD147 promotes cell motility by regulating annexin II-activated RhoA and Rac1 signaling pathways in hepatocellular carcinoma cells. Hepatology 2011, 54, 2012–2024. [Google Scholar]
- Draeger, A.; Monastyrskaya, K.; Babiychuk, E.B. Plasma membrane repair and cellular damage control: The annexin survival kit. Biochem. Pharmacol 2011, 81, 703–712. [Google Scholar]
- Creutz, C.E.; Hira, J.K.; Gee, V.E.; Eaton, J.M. Protection of the membrane permeability barrier by annexins. Biochemistry 2012, 51, 9966–9983. [Google Scholar]
- Lennon, N.J.; Kho, A.; Bacskai, B.J.; Perlmutter, S.L.; Hyman, B.T.; Brown, R.H., Jr. Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J. Biol. Chem. 2003, 278, 50466–50473. [Google Scholar]
- McNeil, A.K.; Rescher, U.; Gerke, V.; McNeil, P.L. Requirement for annexin A1 in plasma membrane repair. J. Biol. Chem 2006, 281, 35202–35207. [Google Scholar]
- Bouter, A.; Gounou, C.; Berat, R.; Tan, S.; Gallois, B.; Granier, T.; d’Estaintot, B.L.; Poschl, E.; Brachvogel, B.; Brisson, A.R. Annexin-A5 assembled into two-dimensional arrays promotes cell membrane repair. Nat. Commun 2011, 2, 270. [Google Scholar] [Green Version]
- D’Acquisto, F.; Perretti, M.; Flower, R.J. Annexin-A1: A pivotal regulator of the innate and adaptive immune systems. Br. J. Pharmacol 2008, 155, 152–169. [Google Scholar]
- Ling, Q.; Jacovina, A.T.; Deora, A.; Febbraio, M.; Simantov, R.; Silverstein, R.L.; Hempstead, B.; Mark, W.H.; Hajjar, K.A. Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. J. Clin. Invest 2004, 113, 38–48. [Google Scholar]
- Rand, J.H.; Wu, X.X. Antibody-mediated interference with annexins in the antiphospholipid syndrome. Thromb. Res 2004, 114, 383–389. [Google Scholar]
- Cornely, R.; Rentero, C.; Enrich, C.; Grewal, T.; Gaus, K. Annexin A6 is an organizer of membrane microdomains to regulate receptor localization and signalling. IUBMB Life 2011, 63, 1009–1017. [Google Scholar]
- Dempsey, B.R.; Rezvanpour, A.; Lee, T.W.; Barber, K.R.; Junop, M.S.; Shaw, G.S. Structure of an asymmetric ternary protein complex provides insight for membrane interaction. Structure 2012, 20, 1737–1745. [Google Scholar]
- Kourie, J.I.; Shorthouse, A.A. Properties of cytotoxic peptide-formed ion channels. Am. J. Physiol. Cell Physiol 2000, 278, C1063–C1087. [Google Scholar]
- Luecke, H.; Chang, B.T.; Mailliard, W.S.; Schlaepfer, D.D.; Haigler, H.T. Crystal structure of the annexin XII hexamer and implications for bilayer insertion. Nature 1995, 378, 512–515. [Google Scholar]
- Hertogs, K.; Leenders, W.P.; Depla, E.; de Bruin, W.C.; Meheus, L.; Raymackers, J.; Moshage, H.; Yap, S.H. Endonexin II, present on human liver plasma membranes, is a specific binding protein of small hepatitis B virus (HBV) envelope protein. Virology 1993, 197, 549–557. [Google Scholar]
- Huang, R.T.; Lichtenberg, B.; Rick, O. Involvement of annexin V in the entry of influenza viruses and role of phospholipids in infection. FEBS Lett 1996, 392, 59–62. [Google Scholar]
- Backes, P.; Quinkert, D.; Reiss, S.; Binder, M.; Zayas, M.; Rescher, U.; Gerke, V.; Bartenschlager, R.; Lohmann, V. Role of annexin A2 in the production of infectious hepatitis C virus particles. J. Virol 2010, 84, 5775–5789. [Google Scholar]
- Saxena, V.; Lai, C.K.; Chao, T.C.; Jeng, K.S.; Lai, M.M. Annexin A2 is involved in the formation of hepatitis C virus replication complex on the lipid raft. J. Virol 2012, 86, 4139–4150. [Google Scholar]
- Yang, S.L.; Chou, Y.T.; Wu, C.N.; Ho, M.S. Annexin II binds to capsid protein VP1 of enterovirus 71 and enhances viral infectivity. J. Virol 2011, 85, 11809–11820. [Google Scholar]
- Harrist, A.V.; Ryzhova, E.V.; Harvey, T.; Gonzalez-Scarano, F. Anx2 interacts with HIV-1 Gag at phosphatidylinositol (4,5) bisphosphate-containing lipid rafts and increases viral production in 293T cells. PLoS One 2009, 4, e5020. [Google Scholar]
- Li, M.; Aliotta, J.M.; Asara, J.M.; Tucker, L.; Quesenberry, P.; Lally, M.; Ramratnam, B. Quantitative proteomic analysis of exosomes from HIV-1-infected lymphocytic cells. Proteomics 2012, 12, 2203–2211. [Google Scholar]
- Derry, M.C.; Sutherland, M.R.; Restall, C.M.; Waisman, D.M.; Pryzdial, E.L. Annexin 2-mediated enhancement of cytomegalovirus infection opposes inhibition by annexin 1 or annexin 5. J. Gen. Virol 2007, 88, 19–27. [Google Scholar]
- Ma, H.; Kien, F.; Maniere, M.; Zhang, Y.; Lagarde, N.; Tse, K.S.; Poon, L.L.; Nal, B. Human annexin A6 interacts with influenza a virus protein M2 and negatively modulates infection. J. Virol 2012, 86, 1789–1801. [Google Scholar]
- Turnay, J.; Pfannmuller, E.; Lizarbe, M.A.; Bertling, W.M.; von der Mark, K. Collagen binding activity of recombinant and N-terminally modified annexin V (anchorin CII). J. Cell. Biochem 1995, 58, 208–220. [Google Scholar]
- Reid, D.L.; Aydelotte, M.B.; Mollenhauer, J. Cell attachment, collagen binding, and receptor analysis on bovine articular chondrocytes. J. Orthop. Res 2000, 18, 364–373. [Google Scholar]
- Kim, H.J.; Kirsch, T. Collagen/annexin V interactions regulate chondrocyte mineralization. J. Biol. Chem 2008, 283, 10310–10317. [Google Scholar]
- Chung, C.Y.; Erickson, H.P. Cell surface annexin II is a high affinity receptor for the alternatively spliced segment of tenascin-C. J. Cell Biol 1994, 126, 539–548. [Google Scholar]
- Sakwe, A.M.; Koumangoye, R.; Guillory, B.; Ochieng, J. Annexin A6 contributes to the invasiveness of breast carcinoma cells by influencing the organization and localization of functional focal adhesions. Exp. Cell Res 2011, 317, 823–837. [Google Scholar]
- Solito, E.; Kamal, A.; Russo-Marie, F.; Buckingham, J.C.; Marullo, S.; Perretti, M. A novel calcium-dependent proapoptotic effect of annexin 1 on human neutrophils. FASEB J 2003, 17, 1544–1546. [Google Scholar]
- Arur, S.; Uche, U.E.; Rezaul, K.; Fong, M.; Scranton, V.; Cowan, A.E.; Mohler, W.; Han, D.K. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev. Cell 2003, 4, 587–598. [Google Scholar]
- Buckland, A.G.; Wilton, D.C. Inhibition of secreted phospholipases A2 by annexin V. Competition for anionic phospholipid interfaces allows an assessment of the relative interfacial affinities of secreted phospholipases A2. Biochim. Biophys. Acta 1998, 1391, 367–376. [Google Scholar]
- Kenis, H.; van Genderen, H.; Deckers, N.M.; Lux, P.A.; Hofstra, L.; Narula, J.; Reutelingsperger, C.P. Annexin A5 inhibits engulfment through internalization of PS-expressing cell membrane patches. Exp. Cell Res 2006, 312, 719–726. [Google Scholar]
- Cubells, L.; Vila de Muga, S.; Tebar, F.; Bonventre, J.V.; Balsinde, J.; Pol, A.; Grewal, T.; Enrich, C. Annexin A6-induced inhibition of cytoplasmic phospholipase A2 is linked to caveolin-1 export from the Golgi. J. Biol. Chem 2008, 283, 10174–10183. [Google Scholar]
- Satoh, A.; Suzuki, K.; Takayama, E.; Kojima, K.; Hidaka, T.; Kawakami, M.; Matsumoto, I.; Ohsuzu, F. Detection of anti-annexin IV and V antibodies in patients with antiphospholipid syndrome and systemic lupus erythematosus. J. Rheumatol 1999, 26, 1715–1720. [Google Scholar]
- Rand, J.H. Antiphospholipid antibody-mediated disruption of the annexin-V antithrombotic shield: A thrombogenic mechanism for the antiphospholipid syndrome. J. Autoimmun 2000, 15, 107–111. [Google Scholar]
- Flood, E.C.; Hajjar, K.A. The annexin A2 system and vascular homeostasis. Vascul. Pharmacol 2011, 54, 59–67. [Google Scholar]
- Madureira, P.A.; Surette, A.P.; Phipps, K.D.; Taboski, M.A.; Miller, V.A.; Waisman, D.M. The role of the annexin A2 heterotetramer in vascular fibrinolysis. Blood 2011, 118, 4789–4797. [Google Scholar]
- Contreras, F.X.; Sanchez-Magraner, L.; Alonso, A.; Goni, F.M. Transbilayer (flip-flop) lipid motion and lipid scrambling in membranes. FEBS Lett 2010, 584, 1779–1786. [Google Scholar]
- Kiessling, V.; Wan, C.; Tamm, L.K. Domain coupling in asymmetric lipid bilayers. Biochim. Biophys. Acta 2009, 1788, 64–71. [Google Scholar]
- Clark, M.R. Flippin’ lipids. Nat. Immunol 2011, 12, 373–375. [Google Scholar]
- Estaquier, J.; Vallette, F.; Vayssiere, J.L.; Mignotte, B. The mitochondrial pathways of apoptosis. Adv. Exp. Med. Biol 2012, 942, 157–183. [Google Scholar]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol 2008, 9, 231–241. [Google Scholar]
- Erwig, L.P.; Henson, P.M. Clearance of apoptotic cells by phagocytes. Cell Death Differ 2008, 15, 243–250. [Google Scholar]
- Ravichandran, K.S. Find-me and eat-me signals in apoptotic cell clearance: Progress and conundrums. J. Exp. Med 2010, 207, 1807–1817. [Google Scholar]
- Balasubramanian, K.; Schroit, A.J. Aminophospholipid asymmetry: A matter of life and death. Annu. Rev. Physiol 2003, 65, 701–734. [Google Scholar]
- Boersma, H.H.; Kietselaer, B.L.; Stolk, L.M.; Bennaghmouch, A.; Hofstra, L.; Narula, J.; Heidendal, G.A.; Reutelingsperger, C.P. Past, present, and future of annexin A5: From protein discovery to clinical applications. J. Nucl. Med 2005, 46, 2035–2050. [Google Scholar]
- Demchenko, A.P. Beyond annexin V: Fluorescence response of cellular membranes to apoptosis. Cytotechnology 2012. [Google Scholar] [CrossRef]
- Hanshaw, R.G.; Smith, B.D. New reagents for phosphatidylserine recognition and detection of apoptosis. Bioorg. Med. Chem 2005, 13, 5035–5042. [Google Scholar]
- Van Engeland, M.; Nieland, L.J.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar]
- Brumatti, G.; Sheridan, C.; Martin, S.J. Expression and purification of recombinant annexin V for the detection of membrane alterations on apoptotic cells. Methods 2008, 44, 235–240. [Google Scholar]
- Schellenberger, E.A.; Weissleder, R.; Josephson, L. Optimal modification of annexin V with fluorescent dyes. Chembiochem 2004, 5, 271–274. [Google Scholar]
- Bauwens, M.; De Saint-Hubert, M.; Devos, E.; Deckers, N.; Reutelingsperger, C.; Mortelmans, L.; Himmelreich, U.; Mottaghy, F.M.; Verbruggen, A. Site-specific 68Ga-labeled Annexin A5 as a PET imaging agent for apoptosis. Nucl. Med. Biol 2011, 38, 381–392. [Google Scholar]
- De Saint-Hubert, M.; Prinsen, K.; Mortelmans, L.; Verbruggen, A.; Mottaghy, F.M. Molecular imaging of cell death. Methods 2009, 48, 178–187. [Google Scholar]
- Blankenberg, F.G.; Katsikis, P.D.; Tait, J.F.; Davis, R.E.; Naumovski, L.; Ohtsuki, K.; Kopiwoda, S.; Abrams, M.J.; Darkes, M.; Robbins, R.C.; et al. In vivo detection and imaging of phosphatidylserine expression during programmed cell death. Proc. Natl. Acad. Sci. USA 1998, 95, 6349–6354. [Google Scholar]
- Koumangoye, R.B.; Sakwe, A.M.; Goodwin, J.S.; Patel, T.; Ochieng, J. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS One 2011, 6, e24234. [Google Scholar]
- Vedeler, A.; Hollas, H.; Grindheim, A.K.; Raddum, A.M. Multiple roles of annexin A2 in post-transcriptional regulation of gene expression. Curr. Protein Pept. Sci 2012, 13, 401–412. [Google Scholar]
- Xiao, D.; Ohlendorf, J.; Chen, Y.; Taylor, D.D.; Rai, S.N.; Waigel, S.; Zacharias, W.; Hao, H.; McMasters, K.M. Identifying mRNA, microRNA and protein profiles of melanoma exosomes. PLoS One 2012, 7, e46874. [Google Scholar]
- Yuyama, K.; Sun, H.; Mitsutake, S.; Igarashi, Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J. Biol. Chem 2012, 287, 10977–10989. [Google Scholar]



© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lizarbe, M.A.; Barrasa, J.I.; Olmo, N.; Gavilanes, F.; Turnay, J. Annexin-Phospholipid Interactions. Functional Implications. Int. J. Mol. Sci. 2013, 14, 2652-2683. https://doi.org/10.3390/ijms14022652
Lizarbe MA, Barrasa JI, Olmo N, Gavilanes F, Turnay J. Annexin-Phospholipid Interactions. Functional Implications. International Journal of Molecular Sciences. 2013; 14(2):2652-2683. https://doi.org/10.3390/ijms14022652
Chicago/Turabian StyleLizarbe, María Antonia, Juan I. Barrasa, Nieves Olmo, Francisco Gavilanes, and Javier Turnay. 2013. "Annexin-Phospholipid Interactions. Functional Implications" International Journal of Molecular Sciences 14, no. 2: 2652-2683. https://doi.org/10.3390/ijms14022652




