A Histone-Like Protein Induces Plasmid DNA to Form Liquid Crystals in Vitro and Gene Compaction in Vivo
Abstract
:1. Introduction
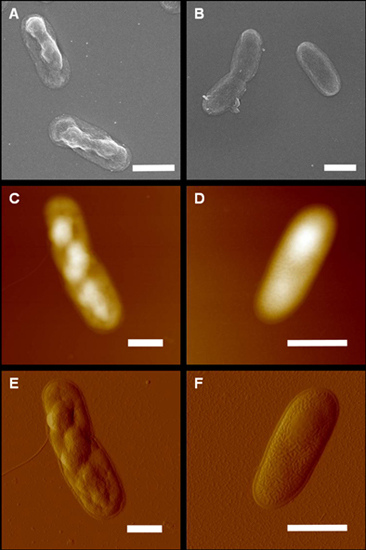
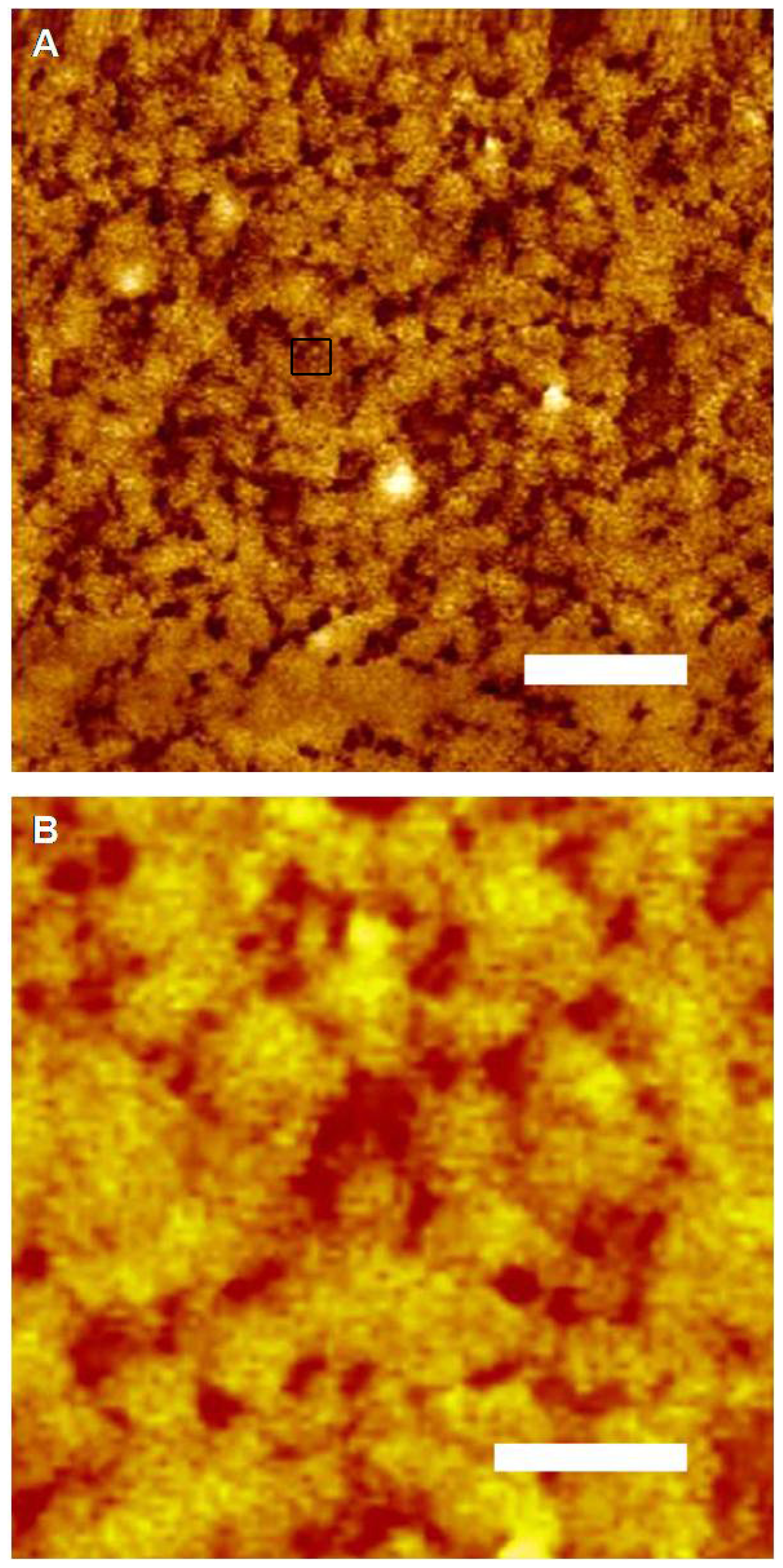
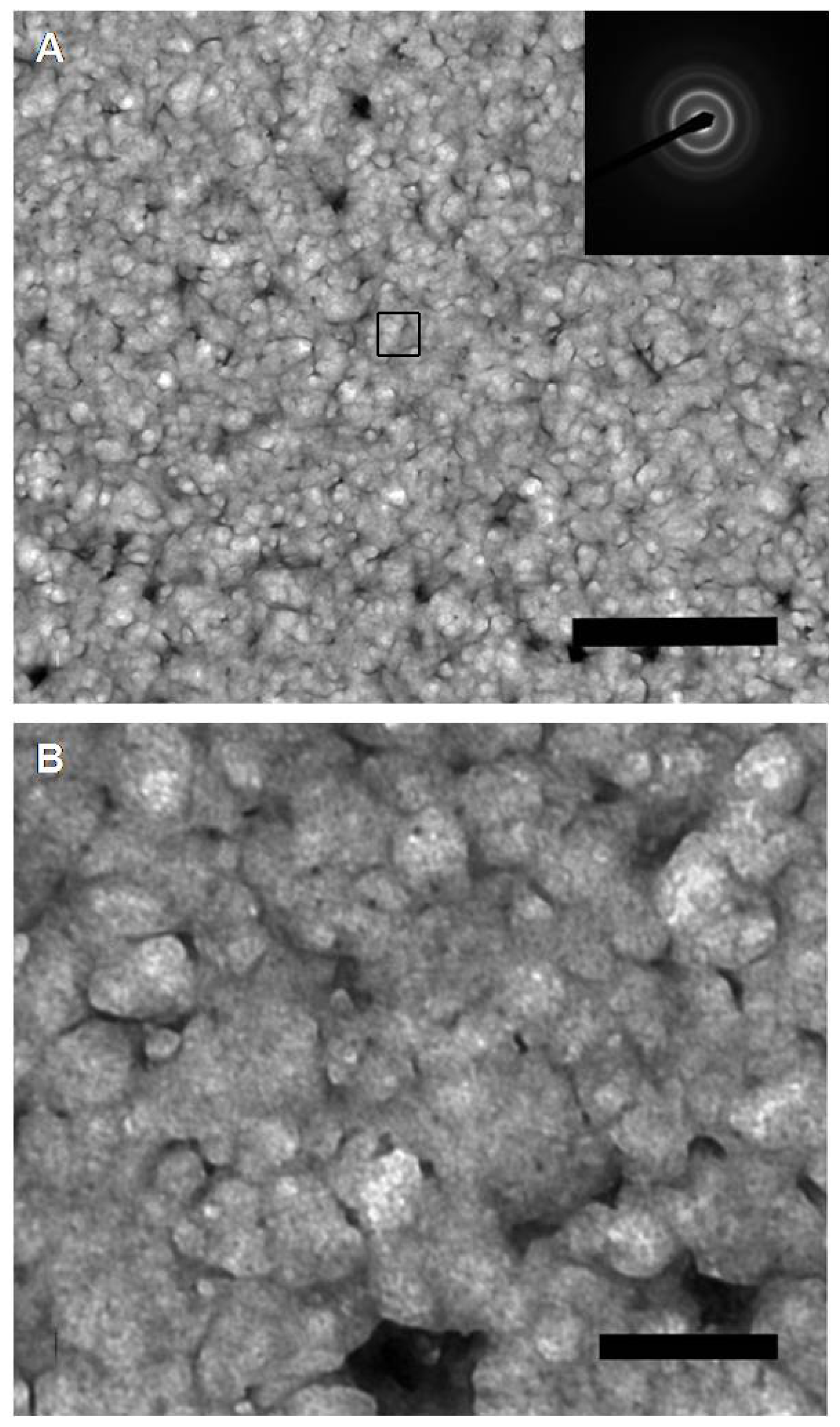
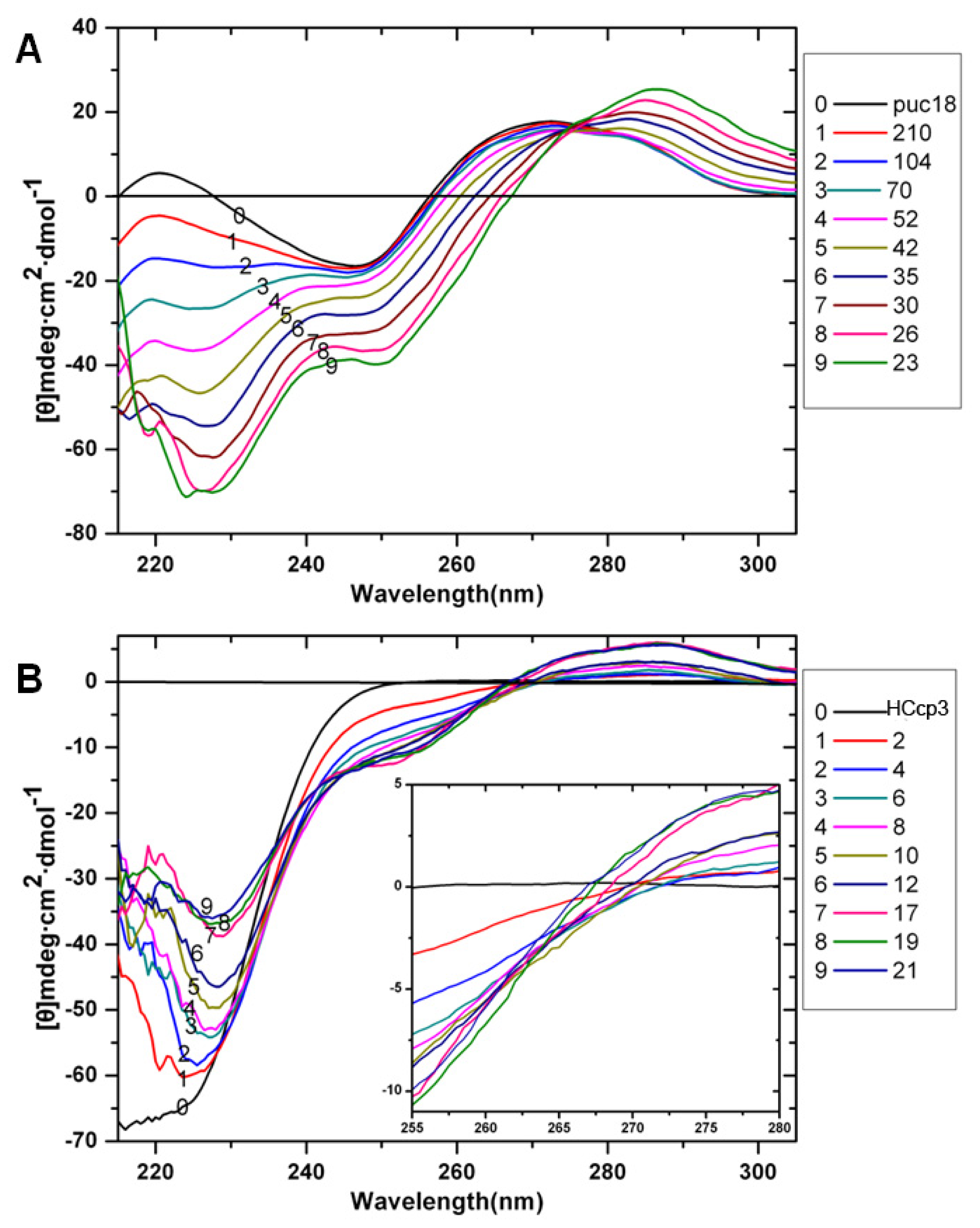
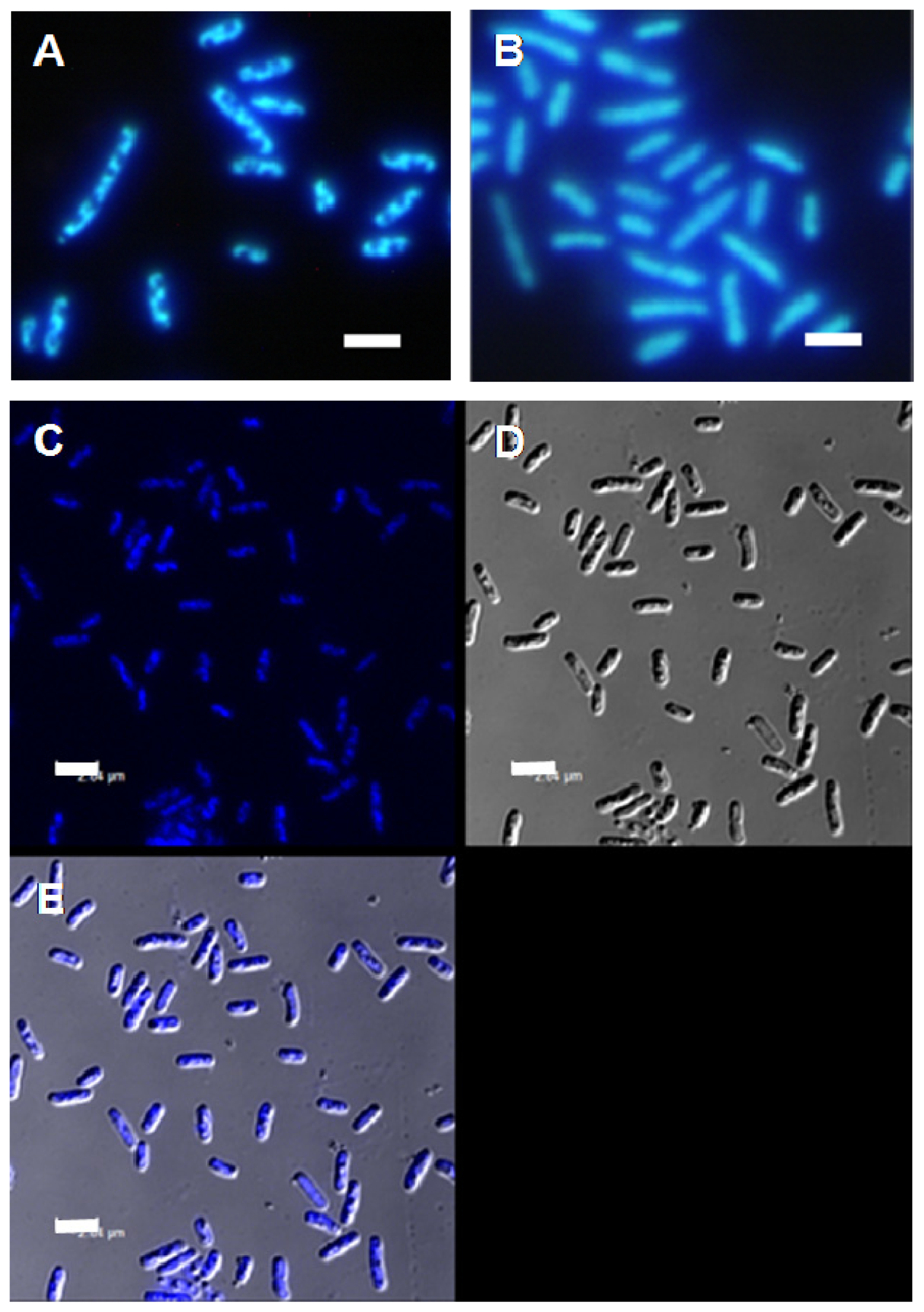
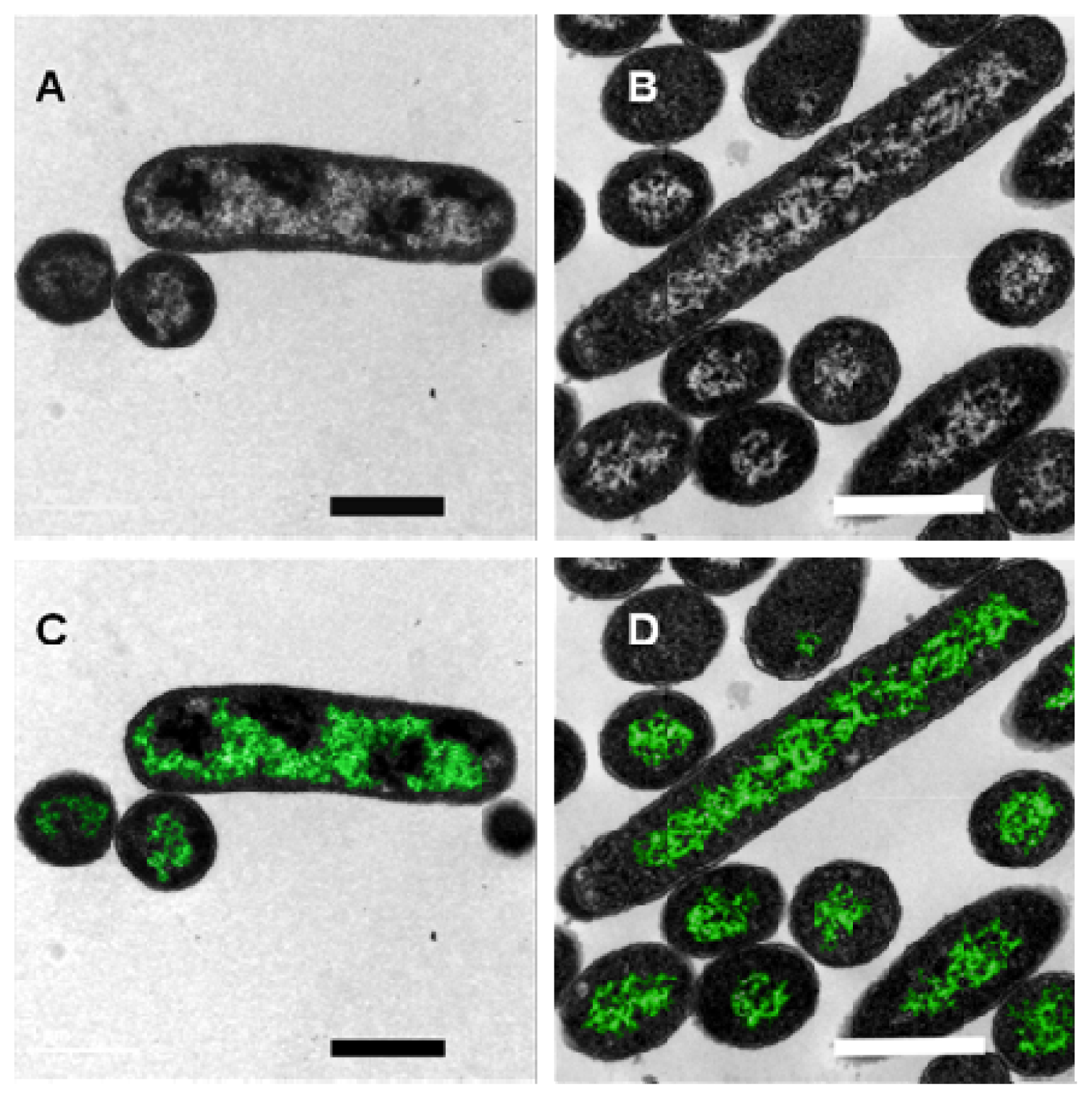
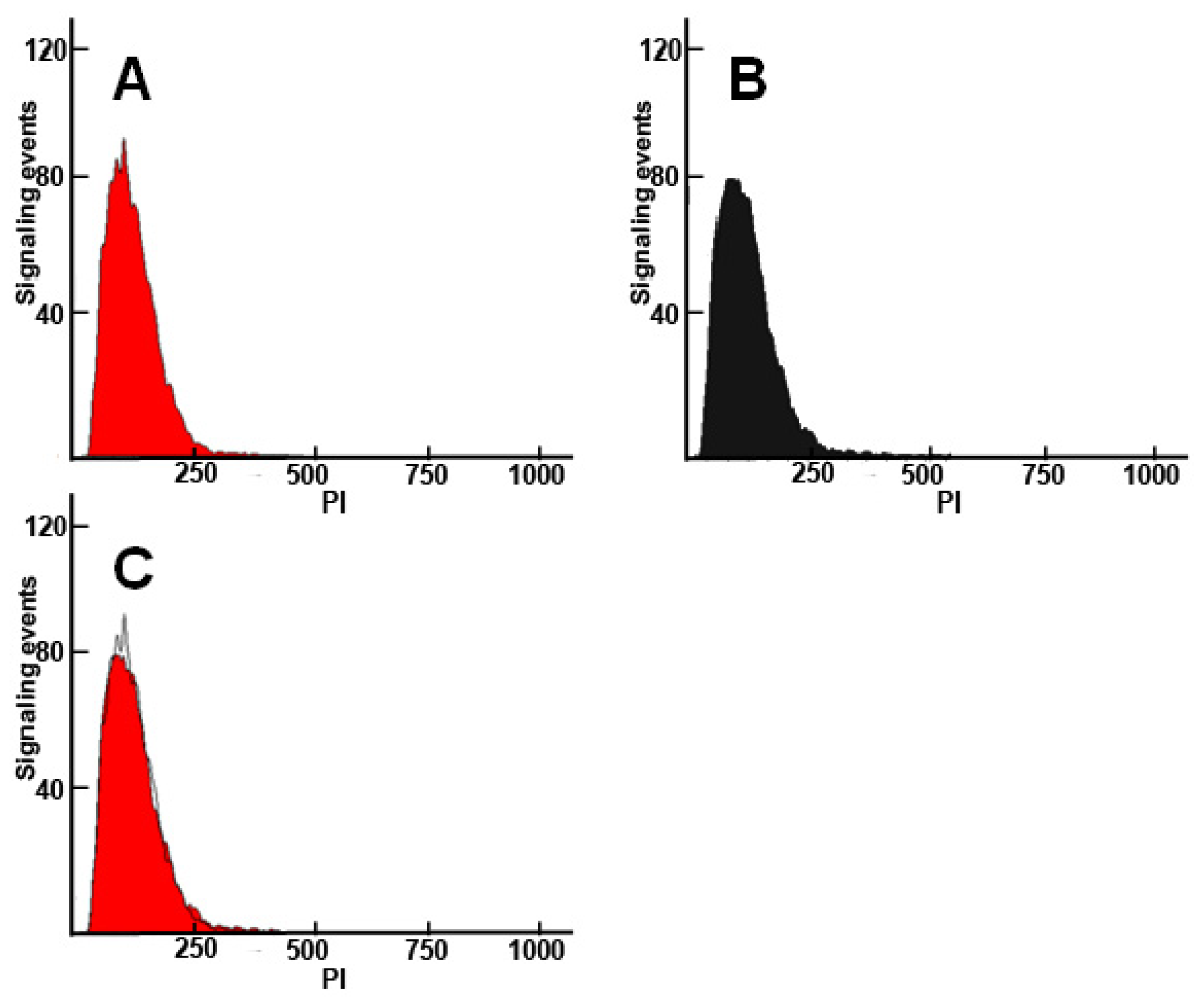
2. Results and Discussion
3. Materials and Methods
3.1. Over-Expression and Purification of Recombinant HCcp3
3.2. Preparation of pDNA
3.3. Atomic Force Microscopy
3.4. Fluorescence Microscopy
3.5. Electron Microscopy
3.6. Polarization Microscopy
3.7. Circular Dichroism
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bouligand, Y. Liquid crystals and biological morphogenesis: Ancient and new questions. C. R. Chim 2008, 11, 281–296. [Google Scholar]
- Iwabata, K.; Sugai, U.; Seki, Y.; Furue, H.; Sakaguchi, K. Applications of biomaterials to liquid crystals. Molecules 2013, 18, 4703–4717. [Google Scholar]
- Livolant, F.; Leforestier, A. Condensed phases of DNA: Structures and phase transitions. Prog. Polym. Sci 1996, 21, 1115–1164. [Google Scholar]
- Minsky, A.; Shimoni, E.; Frenkiel-Krispin, D. Stress, order and survival. Nat. Rev. Mol. Cell Biol 2002, 3, 50–60. [Google Scholar]
- Kato, T. Self-assembly of phase-segregated liquid crystal structures. Science 2002, 295, 2414–2418. [Google Scholar]
- Bloomfield, V.A. DNA condensation. Curr. Opin. Struct. Biol 1996, 6, 334–341. [Google Scholar]
- Strzelecka, T.E.; Rill, R.L. Solid-state 31P NMR studies of DNA liquid crystalline phases. The isotropic to cholesteric transition. J. Am. Chem. Soc 1987, 109, 4513–4518. [Google Scholar]
- Van der Maarel, J.R.C.; Zakharova, S.S.; Jesse, W.; Backendorf, C.; Egelhaaf, S.U.; Lapp, A. Supercoiled DNA; plectonemic structure and liquid crystal formation. J. Phys.-Condes. Matter 2003, 15, S183–S189. [Google Scholar]
- Zakharova, S.S.; Jesse, W.; Backendorf, C.; van der Maarel, J.R.C. Liquid crystal formation in supercoiled DNA solutions. Biophys. J 2002, 83, 1119–1129. [Google Scholar]
- Sundaresan, N.; Suresh, C.H.; Thomas, T.; Thomas, T.J.; Pillai, C.K.S. Liquid crystalline phase behavior of high molecular weight DNA: A comparative study of the influence of metal ions of different size, charge and binding mode. Biomacromolecules 2008, 9, 1860–1869. [Google Scholar]
- Le Ny, A.L.M.; Lee, C.T. Conformation and dynamics of DNA molecules during photoreversible condensation. Biophys. Chem 2009, 142, 76–83. [Google Scholar]
- Saito, T.; Iwaki, T.; Yoshikawa, K. DNA compaction induced by neutral polymer is retarded more effectively by divalent anion than monovalent anion. Chem. Phys. Lett 2008, 465, 40–44. [Google Scholar]
- Hou, S.; Yang, K.; Yao, Y.; Liu, Z.; Feng, X.Z.; Wang, R.; Yang, Y.L.; Wang, C. DNA condensation induced by a cationic polymer studied by atomic force microscopy and electrophoresis assay. Coll. Surf. B 2008, 62, 151–156. [Google Scholar]
- Mann, A.; Richa, R.; Ganguli, M. DNA condensation by poly-l-lysine at the single molecule level: Role of DNA concentration and polymer length. J. Control. Release 2008, 125, 252–262. [Google Scholar]
- Shah, D.S.; Sakthivel, T.; Toth, I.; Florence, A.T.; Wilderspin, A.F. DNA transfection and transfected cell viability using amphipathic asymmetric dendrimers. Int. J. Pharm 2000, 208, 41–48. [Google Scholar]
- Sun, X.L.; Zhang, N. Cationic polymer optimization for efficient gene delivery. Mini-Rev. Med. Chem 2010, 10, 108–125. [Google Scholar]
- Wang, X.L.; Zhang, X.H.; Cao, M.W.; Zheng, H.Z.; Xiao, B.; Wang, Y.L.; Li, M. Gemini surfactant-induced DNA condensation into a beadlike structure. J. Phys. Chem. B 2009, 113, 2328–2332. [Google Scholar]
- Angelov, B.; Angelova, A.; Filippov, S.; Karlsson, G.; Terrill, N.; Lesieur, S.; Stepanek, P. SAXS study of sterically stabilized lipid nanocarriers functionalized by DNA. J. Phys 2012, 351. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Mutafchieva, R.; Lesieur, S.; Couvreur, P. Self-assembled multicompartment liquid crystalline lipid carriers for protein, peptide, and nucleic acid drug delivery. Acc. Chem. Res 2011, 44, 147–156. [Google Scholar]
- Angelov, B.; Angelova, A.; Filippov, S.K.; Narayanan, T.; Drechsler, M.; Stepanek, P.; Couvreur, P.; Lesieur, S. DNA/fusogenic lipid nanocarrier assembly: Millisecond structural dynamics. J. Phys. Chem. Lett 2013, 4, 1959–1964. [Google Scholar]
- Angelov, B.; Angelova, A.; Filippov, S.K.; Karlsson, G.; Terrill, N.; Lesieur, S.; Stepanek, P. Topology and internal structure of PEGylated lipid nanocarriers for neuronal transfection: Synchrotron radiation SAXS and cryo-TEM studies. Soft Matter 2011, 7, 9714–9720. [Google Scholar]
- Todd, B.A.; Rau, D.C. Interplay of ion binding and attraction in DNA condensed by multivalent cations. Nucleic Acids Res 2008, 36, 501–510. [Google Scholar]
- DeRouchey, J.; Netz, R.R.; Radler, J.O. Structural investigations of DNA-polycation complexes. Eur. Phys. J. E 2005, 16, 17–28. [Google Scholar]
- Burak, Y.; Ariel, G.; Andelman, D. Onset of DNA aggregation in presence of monovalent and multivalent counterions. Biophys. J 2003, 85, 2100–2110. [Google Scholar]
- Pinto, M.F.V.; Moran, M.C.; Miguel, M.G.; Lindman, B.; Jurado, A.S.; Pais, A. Controlling the morphology in DNA condensation and precipitation. Biomacromolecules 2009, 10, 1319–1323. [Google Scholar]
- Minsky, A.; Ghirlando, R.; Reich, Z. Nucleosomes: A solution to a crowded intracellular environment? J. Theor. Biol 1997, 188, 379–385. [Google Scholar]
- Patel, M.M.; Anchordoquy, T.J. Contribution of hydrophobicity to thermodynamics of ligand-DNA binding and DNA collapse. Biophys. J 2005, 88, 2089–2103. [Google Scholar]
- Sun, S.; Wong, J.T.; Liu, M.; Dong, F. Counterion-mediated decompaction of liquid crystalline chromosomes. DNA Cell Biol 2012, 31, 1657–1664. [Google Scholar]
- Sun, S.; Wong, J.T.; Dong, F.; Liu, M. Histone-like protein HCcp3-induced liquid crystalline DNA condensation. Chem. Lett 2012, 41, 874–876. [Google Scholar]
- Wargo, M.J.; Rizzo, P.J. Characterization of Gymnodinium mikimotoi (Dinophyceae) nuclei and identification of the major histone-like protein, HGm. J. Phycol 2000, 36, 584–589. [Google Scholar]
- Chudnovsky, Y.; Li, J.F.; Rizzo, P.J.; Hastings, J.W.; Fagan, T.F. Cloning, expression, and characterization of a histone-like protein from the marine dinoflagellate Lingulodinium polyedrum (Dinophyceae). J. Phycol 2002, 38, 543–550. [Google Scholar]
- Chan, Y.H.; Wong, J.T.Y. Concentration-dependent organization of DNA by the dinoflagellate histone-like protein HCc3. Nucleic Acids Res 2007, 35, 2573–2583. [Google Scholar]
- Salarovira, M.; Geraud, M.L.; Caput, D.; Jacques, F.; Soyergobillard, M.O.; Vernet, G.; Herzog, M. Molecular-cloning and immunolocalization of 2 variants of the major basic nuclear-protein(HCc) from the histone-less eukaryote Crypthecodinium cohni (Pyrrhophyta). Chromosoma 1991, 100, 510–518. [Google Scholar]
- Wong, J.T.Y.; New, D.C.; Wong, J.C.W.; Hung, V.K.L. Histone-like proteins of the dinoflagellate Crypthecodinium cohnii have homologies to bacterial DNA-binding proteins. Eukaryot. Cell 2003, 2, 646–650. [Google Scholar]
- Balaz, M.; Li, B.C.; Steinkruger, J.D.; Ellestad, G.A.; Nakanishi, K.; Berova, N. Porphyrins conjugated to DNA as CD reporters of the salt-induced B to Z-DNA transition. Org. Biomol. Chem 2006, 4, 1865–1867. [Google Scholar]
- Arscott, P.G.; Ma, C.L.; Wenner, J.R.; Bloomfield, V.A. DNA condensation by cobalt hexaammine(III) in alcohol-water mixtures-dielectric constant and other solvent effects. Biopolymers 1995, 36, 345–364. [Google Scholar]
- Kassapidou, K.; Jesse, W.; van Dijk, J.A.P.P.; van der Maarel, J.R.C. Liquid crystal formation in DNA fragment solutions. Biopolymers 1998, 46, 31–37. [Google Scholar]
- Rudd, L.; Lee, D.J.; Kornyshev, A.A. The role of electrostatics in the B to A transition of DNA: From solution to assembly. J. Phys.-Condes. Matter 2007, 19. [Google Scholar] [CrossRef]
- Jose, D.; Porschke, D. The dynamics of the B-A transition of natural DNA double helices. J. Am. Chem. Soc 2005, 127, 16120–16128. [Google Scholar]
- Jones, S.; van Heyningen, P.; Berman, H.M.; Thornton, J.M. Protein-DNA interactions: A structural analysis. J. Mol. Biol 1999, 287, 877–896. [Google Scholar]
- Kankia, B.I.; Buckin, V.; Bloomfield, V.A. Hexamminecobalt(III)-induced condensation of calf thymus DNA: Circular dichroism and hydration measurements. Nucleic Acids Res 2001, 29, 2795–2801. [Google Scholar]
- Marty, R.; N’Soukpoe-Kossi, C.N.; Charbonneau, D.; Weinert, C.M.; Kreplak, L.; Tajmir-Riahi, H.A. Structural analysis of DNA complexation with cationic lipids. Nucleic Acids Res 2009, 37, 849–857. [Google Scholar]
- Marchetti, S.; Onori, G.; Cametti, C. Calorimetric and dynamic light-scattering investigation of cationic surfactant-DNA complexes. J. Phys. Chem. B 2006, 110, 24761–24765. [Google Scholar]
- Knee, K.M.; Dixit, S.B.; Aitken, C.E.; Ponomarev, S.; Beveridge, D.L.; Mukerji, I. Spectroscopic and molecular dynamics evidence for a sequential mechanism for the A-to-B transition in DNA. Biophys. J 2008, 95, 257–272. [Google Scholar]
- Hud, N.V.; Polak, M. DNA-cation interactions: The major and minor grooves are flexible ionophores. Curr. Opin. Struct. Biol 2001, 11, 293–301. [Google Scholar]
- Kornyshev, A.A.; Lee, D.J.; Leikin, S.; Wynveen, A. Structure and interactions of biological helices. Rev. Mod. Phys 2007, 79, 943–996. [Google Scholar]
- Brewer, L.R.; Corzett, M.; Balhorn, R. Protamine-induced condensation and decondensation of the same DNA molecule. Science 1999, 286, 120–123. [Google Scholar]
- Kamashev, D.; Balandina, A.; Mazur, A.K.; Arimondo, P.B.; Rouviere-Yaniv, J. HU binds and folds single-stranded DNA. Nucleic Acids Res 2008, 36, 1026–1036. [Google Scholar]
- Ram, E.; Naik, R.; Ganguli, M.; Habib, S. DNA organization by the apicoplast-targeted bacterial histone-like protein of Plasmodium falciparum. Nucleic Acids Res 2008, 36, 5061–5073. [Google Scholar]
- Sarkar, T.; Vitoc, I.; Mukerji, I.; Hud, N.V. Bacterial protein HU dictates the morphology of DNA condensates produced by crowding agents and polyamines. Nucleic Acids Res 2007, 35, 951–961. [Google Scholar]
- Reyes-Larnothe, R.; Wang, X.D.; Sherratt, D. Escherichia coli and its chromosome. Trends Microbiol 2008, 16, 238–245. [Google Scholar]
- Zimmerman, S.B.; Murphy, L.D. Macromolecular crowding and the mandatory condensation of DNA in bacteria. FEBS Lett 1996, 390, 245–248. [Google Scholar]
- Hancock, R. Packing of the polynucleosome chain in interphase chromosomes: Evidence for a contribution of crowding and entropic forces. Semin. Cell Dev. Biol 2007, 18, 668–675. [Google Scholar]
- Murphy, L.D.; Zimmerman, S.B. Condensation and cohesion of lambda DNA in cell extracts and other media: Implications for the structure and function of DNA in prokaryotes. Biophys. Chem 1995, 57, 71–92. [Google Scholar]
- Marenduzzo, D.; Finan, K.; Cook, P.R. The depletion attraction: An underappreciated force driving cellular organization. J. Cell Biol 2006, 175, 681–686. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sun, S.; Liu, M.; Dong, F.; Fan, S.; Yao, Y. A Histone-Like Protein Induces Plasmid DNA to Form Liquid Crystals in Vitro and Gene Compaction in Vivo. Int. J. Mol. Sci. 2013, 14, 23842-23857. https://doi.org/10.3390/ijms141223842
Sun S, Liu M, Dong F, Fan S, Yao Y. A Histone-Like Protein Induces Plasmid DNA to Form Liquid Crystals in Vitro and Gene Compaction in Vivo. International Journal of Molecular Sciences. 2013; 14(12):23842-23857. https://doi.org/10.3390/ijms141223842
Chicago/Turabian StyleSun, Shiyong, Mingxue Liu, Faqin Dong, Shenglan Fan, and Yanchen Yao. 2013. "A Histone-Like Protein Induces Plasmid DNA to Form Liquid Crystals in Vitro and Gene Compaction in Vivo" International Journal of Molecular Sciences 14, no. 12: 23842-23857. https://doi.org/10.3390/ijms141223842