A Combination of Pre- and Post-Exposure Ascorbic Acid Rescues Mice from Radiation-Induced Lethal Gastrointestinal Damage
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Mouse Survival after Abdominal Irradiation
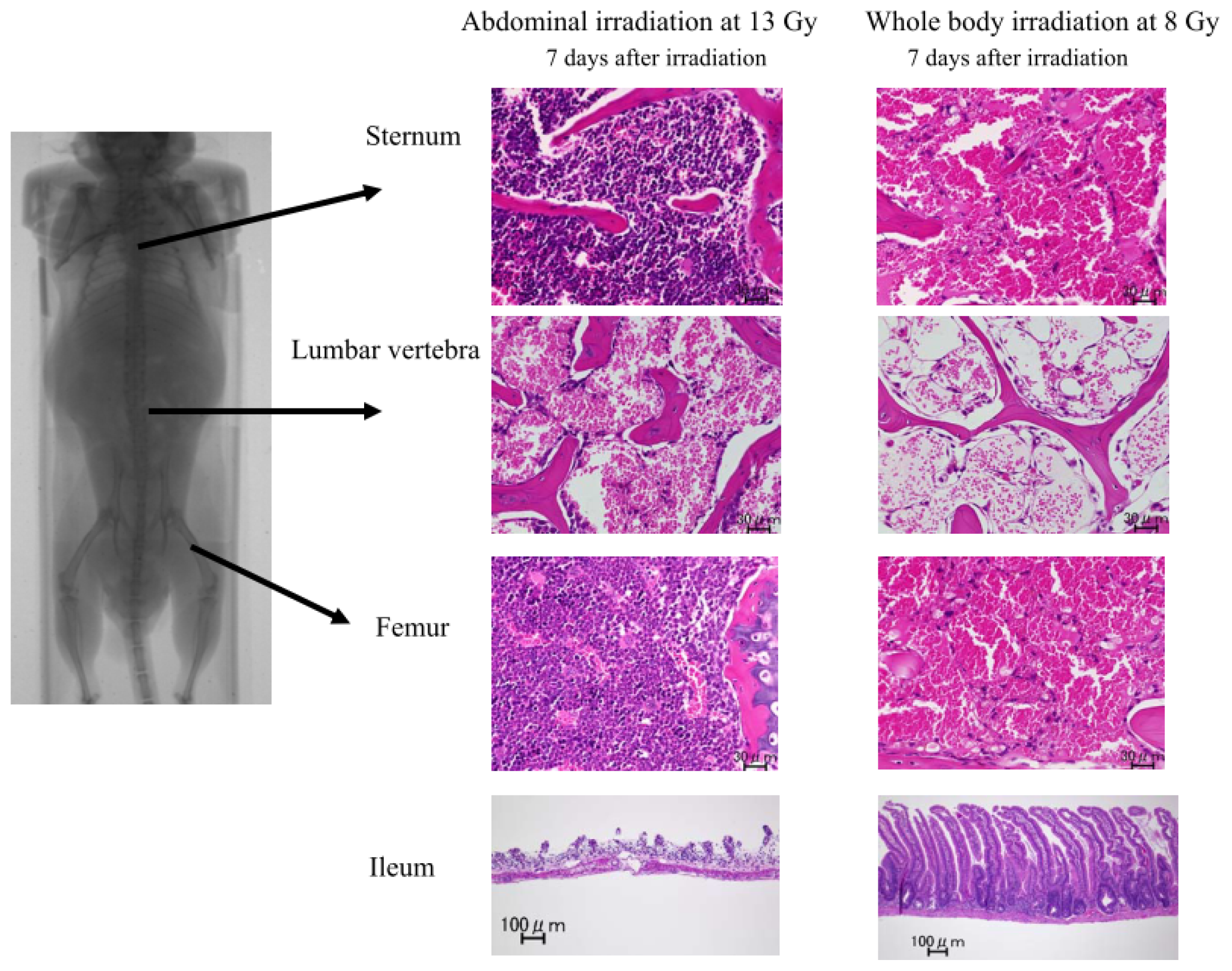
2.1.2. Damage to the Mouse Bone Marrow and Gastrointestinal Tract after Abdominal Irradiation
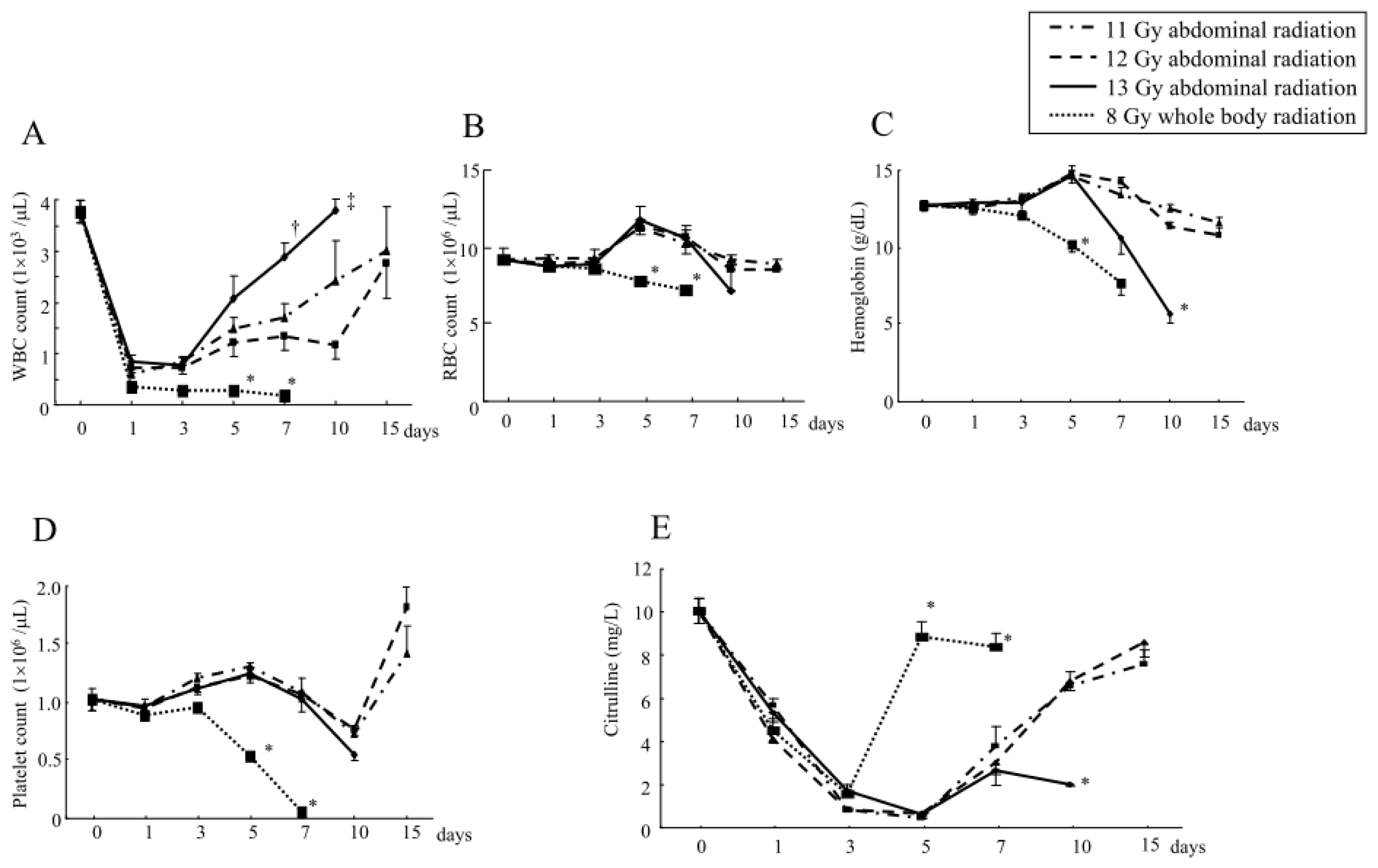
2.1.3. Changes in the Hematological Parameters and Plasma Citrulline Levels after Abdominal Irradiation
2.1.4. Treatment with Ascorbic Acid before and after Abdominal Irradiation
2.1.5. Intestinal Tissue and Plasma Levels of Ascorbic Acid in Mice Treated with Ascorbic Acid
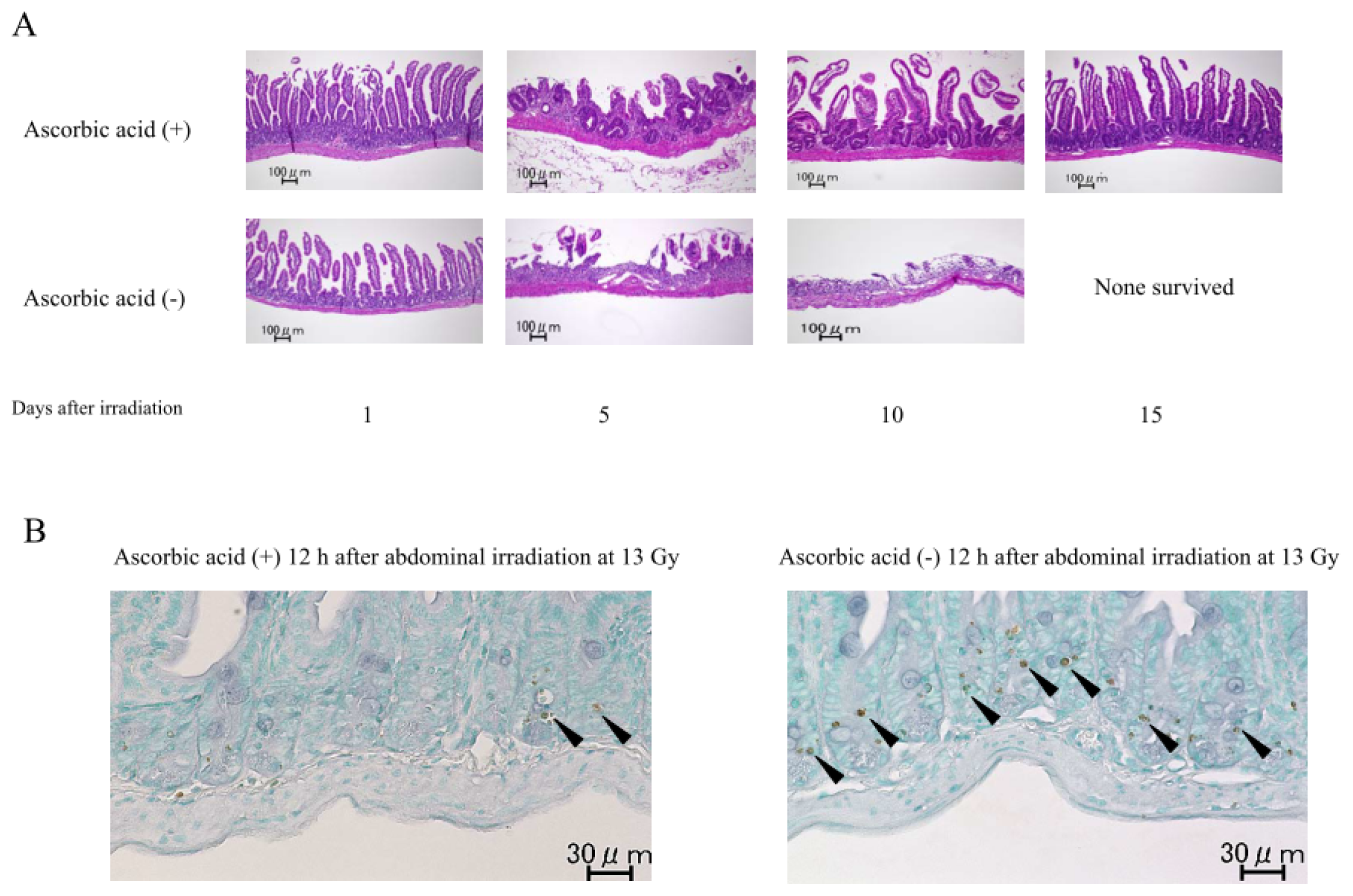
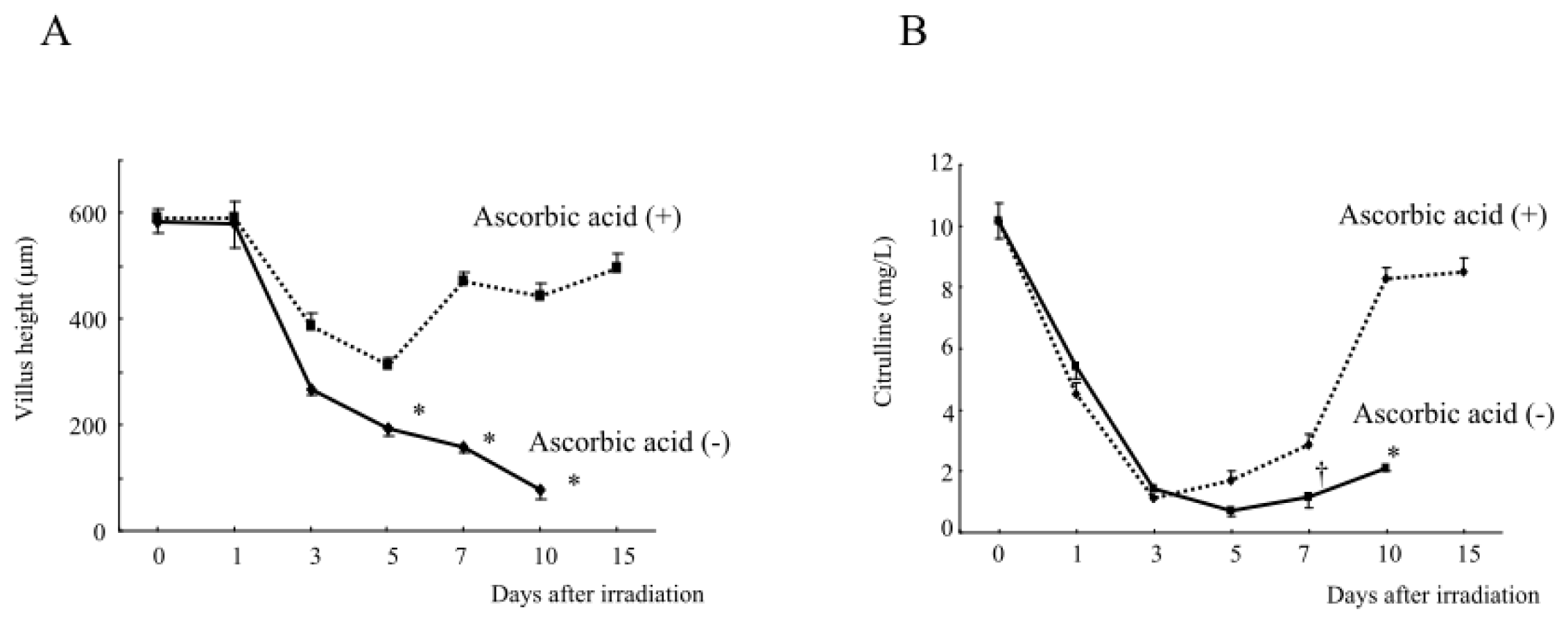
2.1.6. Intestinal Damage, Plasma Citrulline Levels, Plasma and Intestinal Tissue Levels of Free Radical Metabolites after Abdominal Radiation in Mice Treated with Ascorbic Acid
2.2. Discussion
3. Experimental Section
3.1. Mice and Reagents
3.2. Experimental Animals and Abdominal X-Ray Irradiation
3.3. Administration of Ascorbic Acid
3.4. Measurement of the Ascorbic Acid Levels in the Intestinal Tissue and Plasma
3.5. Pathological Examination of the Gastrointestinal Tract and Bone Marrow
3.6. Measurement of the Villus Height in the Intestine
3.7. TUNEL Staining of the Intestine
3.8. Measurements of the Hematological Parameters, Plasma Citrulline Levels, Plasma and Intestinal Tissue Levels of Free Radical Metabolites and Proinflammatory Cytokines
3.9. Statistical Analysis
4. Conclusions
Supplementary Information
ijms-14-19618-s001.pdf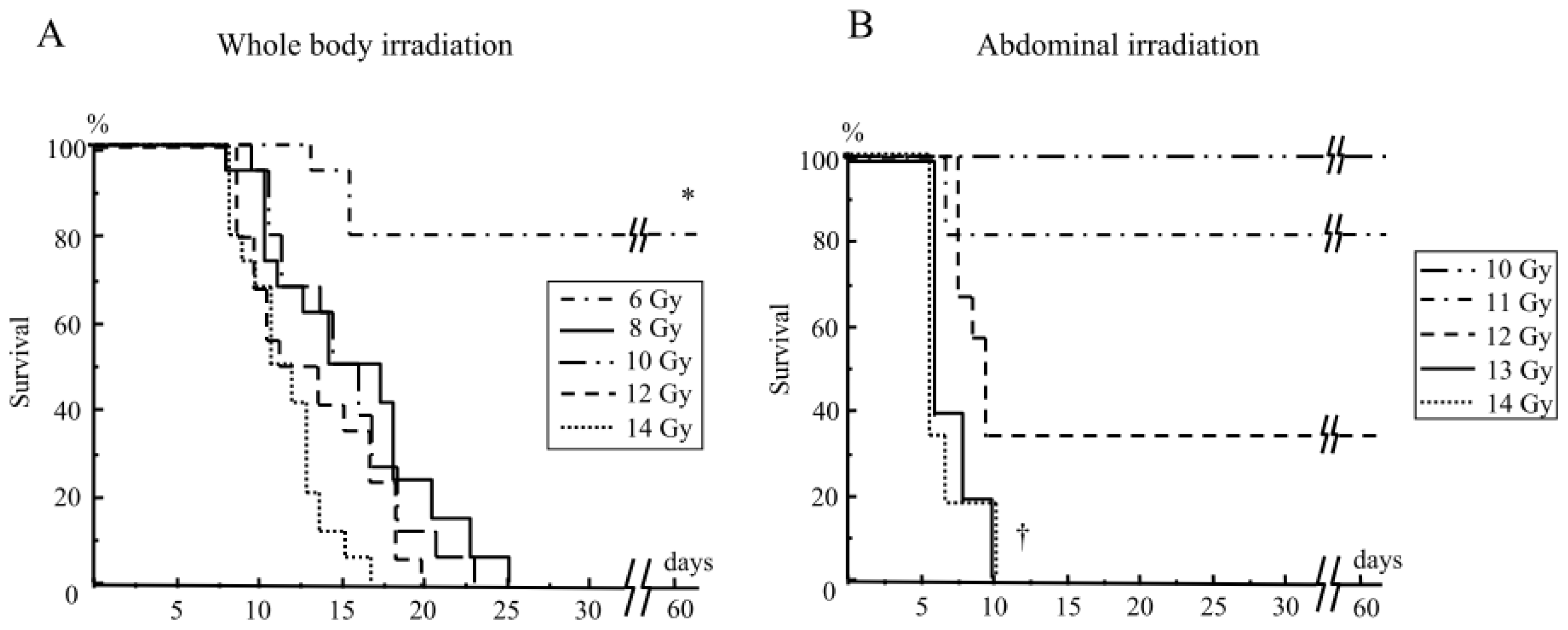



| Group | Ascorbic acid levels | |
|---|---|---|
| Tissue level of the small intestine (mg/L) | Plasma (mg/L) | |
| No treatment with ascorbic acid | 22.8 ± 2.0 | 5.2 ± 0.5 |
| Engulfment (8 h before) with ascorbic acid | 27.1 ± 1.4 | 5.2 ± 0.9 |
| Pretreatment (for 3 days) with ascorbic acid | 30.5 ± 1.6 † | 6.2 ± 0.7 |
| Pretreatment (for 3 days) + engulfment (8 h before) with ascorbic acid | 36.1 ± 1.7 * | 6.3 ± 0.6 |
| Free radical metabolites, cytokines | Group | Tissue levels of the small intestine | Plasma levels |
|---|---|---|---|
| Free radical metabolites (UCarr) | No treatment | 30 ± 1 | 234 ± 39 |
| Treatment with ascorbic acid | 24 ± 2 * | 235 ± 45 | |
| TNF (tissue levels: pg/mg protein) | No treatment | 57 ± 7 | 40 ± 18 |
| (Plasma levels :pg/mL) | Treatment with ascorbic acid | 41 ± 1 † | 17 ± 10 |
| IL-1β (tissue levels: pg/mg protein) | No treatment | 258 ± 155 | Not detected |
| (Plasma levels :pg/mL) | Treatment with ascorbic acid | 274 ± 103 | Not detected |
| IL-6 (tissue levels: pg/mg protein) | No treatment | 179 ± 79 | 185 ± 113 |
| (Plasma levels :pg/mL) | Treatment with ascorbic acid | 122 ± 106 | 111 ± 90 |
Acknowledgments
Conflicts of Interest
References
- Klymenko, S.V.; Belyi, D.A.; Ross, J.R.; Owzar, K.; Jiang, C.; Li, Z.; Minchenko, J.N.; Kovalenko, A.N.; Bebeshko, V.G.; Chao, N.J. Hematopoietic cell infusion for the treatment of nuclear disaster victims: New data from the Chernobyl accident. Int. J. Radiat. Biol 2011, 87, 846–850. [Google Scholar]
- Lange, C.; Brunswig-Spickenheier, B.; Cappallo-Obermann, H.; Eggert, K.; Gehling, U.M.; Rudolph, C.; Schlegelberger, B.; Cornils, K.; Zustin, J.; Spiess, A.N.; et al. Radiation rescue: Mesenchymal stromal cells protect from lethal irradiation. PLoS One 2011, 6, e14486. [Google Scholar]
- Mettler, F.A., Jr.; Voelz, G.L. Major radiation exposure—What to expect and how to respond. N. Engl. J. Med 2002, 346, 1554–1561. [Google Scholar]
- Yamamoto, T.; Kinoshita, M.; Shinomiya, N.; Hiroi, S.; Sugasawa, H.; Matsushita, Y.; Majima, T.; Saitoh, D.; Seki, S. Pretreatment with ascorbic acid prevents lethal gastrointestinal syndrome in mice receiving a massive amount of radiation. J. Radiat. Res. (Tokyo) 2010, 51, 145–156. [Google Scholar]
- Bolis, G.; Zanaboni, F.; Vanoli, P.; Russo, A.; Franchi, M.; Scarfone, G.; Pecorelli, S. The impact of whole-abdomen radiotherapy on survival in advanced ovarian cancer patients with minimal residual disease after chemotherapy. Gynecol. Oncol 1990, 39, 150–154. [Google Scholar]
- Hauer-Jensen, M.; Wang, J.; Boerma, M.; Fu, Q.; Denham, J.W. Radiation damage to the gastrointestinal tract: mechanisms, diagnosis, and management. Curr. Opin. Support. Palliat. Care 2007, 1, 23–29. [Google Scholar]
- Crenn, P.; Messing, B.; Cynober, L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin. Nutr 2008, 27, 328–339. [Google Scholar]
- Mathew, D.; Nair, C.K.; Jacob, J.A.; Biswas, N.; Mukherjee, T.; Kapoor, S.; Kagiya, T.V. Ascorbic acid monoglucoside as antioxidant and radioprotector. J. Radiat. Res. (Tokyo) 2007, 48, 369–376. [Google Scholar]
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1978, 75, 4538–4542. [Google Scholar]
- Creagan, E.T.; Moertel, C.G.; O’Fallon, J.R.; Schutt, A.J.; O’Connell, M.J.; Rubin, J.; Frytak, S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N. Engl. J. Med 1979, 301, 687–690. [Google Scholar]
- Nishikimi, M.; Fukuyama, R.; Minoshima, S.; Shimizu, N.; Yagi, K. Cloning and chromosomal mapping of the human nonfunctional gene for l-gulono-gamma-lactone oxidase, the enzyme for l-ascorbic acid biosynthesis missing in man. J. Biol. Chem 1944, 269, 13685–13688. [Google Scholar]
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1976, 73, 3685–3689. [Google Scholar]
- Cole, A. The Study of Radiosensitive Structures with Low Voltage Electron Beams. In Cellular Radiation Biology; Williams and Wilkins: Baltimore, MD, USA, 1965; pp. 267–271. [Google Scholar]
- Roots, R.; Okada, S. Estimation of life times and diffusion distances of radicals involved in X-ray-induced DNA strand breaks of killing of mammalian cells. Radiat. Res 1975, 64, 306–320. [Google Scholar]
- Berenyi, E.; Szendro, Z.; Rozsahegyl, P.; Bogner, P.; Sulyok, E. Postnatal changes in water content and proton magnetic resonance relaxation times in newborn rabbit tissues. Pediatr. Res 1996, 39, 1091–1098. [Google Scholar]
- Koyama, S.; Kodama, S.; Suzuki, K.; Matsumoto, T.; Miyazaki, T.; Watanabe, M. Radiation-induced long-lived radicals which cause mutation and transformation. Mutat. Res 1998, 421, 45–54. [Google Scholar]
- Yoshimura, T.; Matsuno, K.; Miyazaki, T.; Suzuki, K.; Watanabe, M. Electron spin resonance studies of free radicals in gamma-irradiated golden hamster embryo cells: Radical formation at 77 and 295 K, and radioprotective effects of vitamin C at 295 K. Radiat. Res 1993, 136, 361–365. [Google Scholar]
- Van der Meeren, A.; Monti, P.; Vandamme, M.; Squiban, C.; Wysocki, J.; Griffiths, N. Abdominal radiation exposure elicits inflammatory responses and abscopal effects in the lungs of mice. Radiat. Res 2005, 163, 144–152. [Google Scholar]
- Ong, Z.Y.; Gibson, R.J.; Bowen, J.M.; Stringer, A.M.; Darby, J.M.; Logan, R.M.; Yeoh, A.S.J.; Keefe, D.M. Pro-inflammatory cytokines play a key role in the development of radiotherapy-induced gastrointestinal mucositis. Radiat. Oncol 2010, 5, 22. [Google Scholar]
- Gridley, D.S.; Makinde, A.Y.; Luo, X.; Rizvi, A.; Crapo, J.D.; Dewhirst, M.W.; Moeller, B.J.; Pearlstein, R.D.; Slater, J.M. Radiation and a metalloporphyrin radioprotectant in a mouse prostate tumor model. Anticancer Res 2007, 27, 3101–3109. [Google Scholar]
- Jia, D.; Koonce, N.A.; Griffin, R.J.; Jackson, C.; Corry, P.M. Prevention and mitigation of acute death of mice after abdominal irradiation by the antioxidant N-acetyl-cysteine (NAC). Radiat. Res 2010, 173, 579–589. [Google Scholar]
- Heard, K.J. Acetylcysteine for acetaminophen poisoning. N. Engl. J. Med 2008, 359, 285–292. [Google Scholar]
- Hanly, L.; Rieder, M.J.; Huang, S.S.; Vasylyeva, T.V.; Shah, R.K.; Regueira, O.; Koren, G. N-acetylcysteine rescue protocol for nephrotoxicity in children caused by ifosfamide. J. Popul. Ther. Clin. Pharmacol 2013, 20, e132–e145. [Google Scholar]
- Stey, C.; Steurer, J.; Bachmann, S.; Medici, T.C.; Tramer, M.R. The effect of oral N-acetylcysteine in chronic bronchitis: A quantitative systematic review. Eur. Respir. J 2000, 16, 253–262. [Google Scholar]
- Kerr, F.; Dawson, A.; Whyte, I.M.; Buckley, N.; Murray, L.; Graudins, A.; Chan, B.; Trudinger, B. The Australasian clinical toxicology investigators collaboration randomized trial of different loading infusion rates of N-acetylcysteine. Ann. Emerg. Med 2005, 45, 402–408. [Google Scholar]
- Rabbani, Z.N.; Salahuddin, F.K.; Yarmolenko, P.; Batinic-Haberle, I.; Thrasher, B.A.; Gauter-Fleckenstein, B.; Dewhirst, M.W.; Anscher, M.S.; Vujaskovic, Z. Low molecular weight catalytic metalloporphyrin antioxidant AEOL 10150 protects lungs from fractionated radiation. Free Radic. Res 2007, 41, 1273–1282. [Google Scholar]
- Orrell, R.W. AEOL-10150 (Aeolus). Curr. Opin. Investig. Drugs 2006, 7, 70–80. [Google Scholar]
- Lutgens, L.; Lambin, P. Biomarkers for radiation-induced small bowel epithelial damage: Sn emerging role for plasma Citrulline. World. J. Gastroenterol 2007, 13, 3033–3042. [Google Scholar]
- Lu, S.H.; Ohshima, H.; Fu, H.M.; Tian, Y.; Li, F.M.; Blettner, M.; Wahrendorf, J.; Bartsch, H. Urinary excretion of N-nitrosamino acids and nitrate by inhabitants of high- and low-risk areas for esophageal cancer in Northern China: Endogenous formation of nitrosoproline and its inhibition by vitamin C. Cancer Res 1986, 46, 1485–1491. [Google Scholar]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar]
- Okunieff, P. Interactions between ascorbic acid and the radiation of bone marrow, skin, and tumor. Am. J. Clin. Nutr 1991, 54, 1281S–1283S. [Google Scholar]
- Shinozaki, K.; Hosokawa, Y.; Hazawa, M.; Kashiwakura, I.; Okumura, K.; Kaku, T.; Nakayama, E. Ascorbic acid enhances radiation-induced apoptosis in an HL60 human leukemia cell line. J. Radiat. Res 2011, 52, 229–237. [Google Scholar]
- Goo, M.J.; Park, J.K.; Hong, I.H.; Kim, A.Y.; Lee, E.M.; Lee, E.J.; Hwang, M.; Jeong, K.S. Increased susceptibility of radiation-induced intestinal apoptosis in SMP30 KO mice. Int. J. Mol. Sci 2013, 14, 11084–11095. [Google Scholar]
- Nomura, S.; Maeda, K.; Noda, E.; Inoue, T.; Fukunaga, S.; Nagahara, H.; Hirakawa, K. Clinical significance of the expression of connexin26 in colorectal cancer. J. Exp. Clin. Cancer Res 2010, 29, 79. [Google Scholar]
- Yasuda, M.; Kato, S.; Yamanaka, N.; Iimori, M.; Utsumi, D.; Kitahara, Y.; Iwata, K.; Matsuno, K.; Amagase, K.; Yabe-Nishimura, C.; et al. Potential role of the NADPH oxidase NOX1 in the pathogenesis of 5-fluorouracil-induced intestinal mucositis in mice. Am. J. Physiol. Gastrointest. Liver Physiol 2012, 302, G1133–G1142. [Google Scholar]
- Cesarone, M.R.; Belcaro, G.; Carratelli, M.; Cornelli, U.; de Sanctis, M.T.; Incandela, L.; Barsotti, A.; Terranova, R.; Nicolaides, A. A simple test to monitor oxidative stress. Int. Angiol 1999, 18, 127–130. [Google Scholar]
- Buonocore, G.; Perrone, S.; Longini, M.; Terzuoli, L.; Bracci, R. Total hydroperoxide and advanced oxidation protein products in preterm hypoxic babies. Pediatr. Res 2000, 47, 221–224. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ito, Y.; Kinoshita, M.; Yamamoto, T.; Sato, T.; Obara, T.; Saitoh, D.; Seki, S.; Takahashi, Y. A Combination of Pre- and Post-Exposure Ascorbic Acid Rescues Mice from Radiation-Induced Lethal Gastrointestinal Damage. Int. J. Mol. Sci. 2013, 14, 19618-19635. https://doi.org/10.3390/ijms141019618
Ito Y, Kinoshita M, Yamamoto T, Sato T, Obara T, Saitoh D, Seki S, Takahashi Y. A Combination of Pre- and Post-Exposure Ascorbic Acid Rescues Mice from Radiation-Induced Lethal Gastrointestinal Damage. International Journal of Molecular Sciences. 2013; 14(10):19618-19635. https://doi.org/10.3390/ijms141019618
Chicago/Turabian StyleIto, Yasutoshi, Manabu Kinoshita, Tetsuo Yamamoto, Tomohito Sato, Takeyuki Obara, Daizoh Saitoh, Shuhji Seki, and Yukihiro Takahashi. 2013. "A Combination of Pre- and Post-Exposure Ascorbic Acid Rescues Mice from Radiation-Induced Lethal Gastrointestinal Damage" International Journal of Molecular Sciences 14, no. 10: 19618-19635. https://doi.org/10.3390/ijms141019618
APA StyleIto, Y., Kinoshita, M., Yamamoto, T., Sato, T., Obara, T., Saitoh, D., Seki, S., & Takahashi, Y. (2013). A Combination of Pre- and Post-Exposure Ascorbic Acid Rescues Mice from Radiation-Induced Lethal Gastrointestinal Damage. International Journal of Molecular Sciences, 14(10), 19618-19635. https://doi.org/10.3390/ijms141019618




