Combining the Physical Adsorption Approach and the Covalent Attachment Method to Prepare a Bifunctional Bioreactor
Abstract
:1. Introduction
2. Results and Discussion
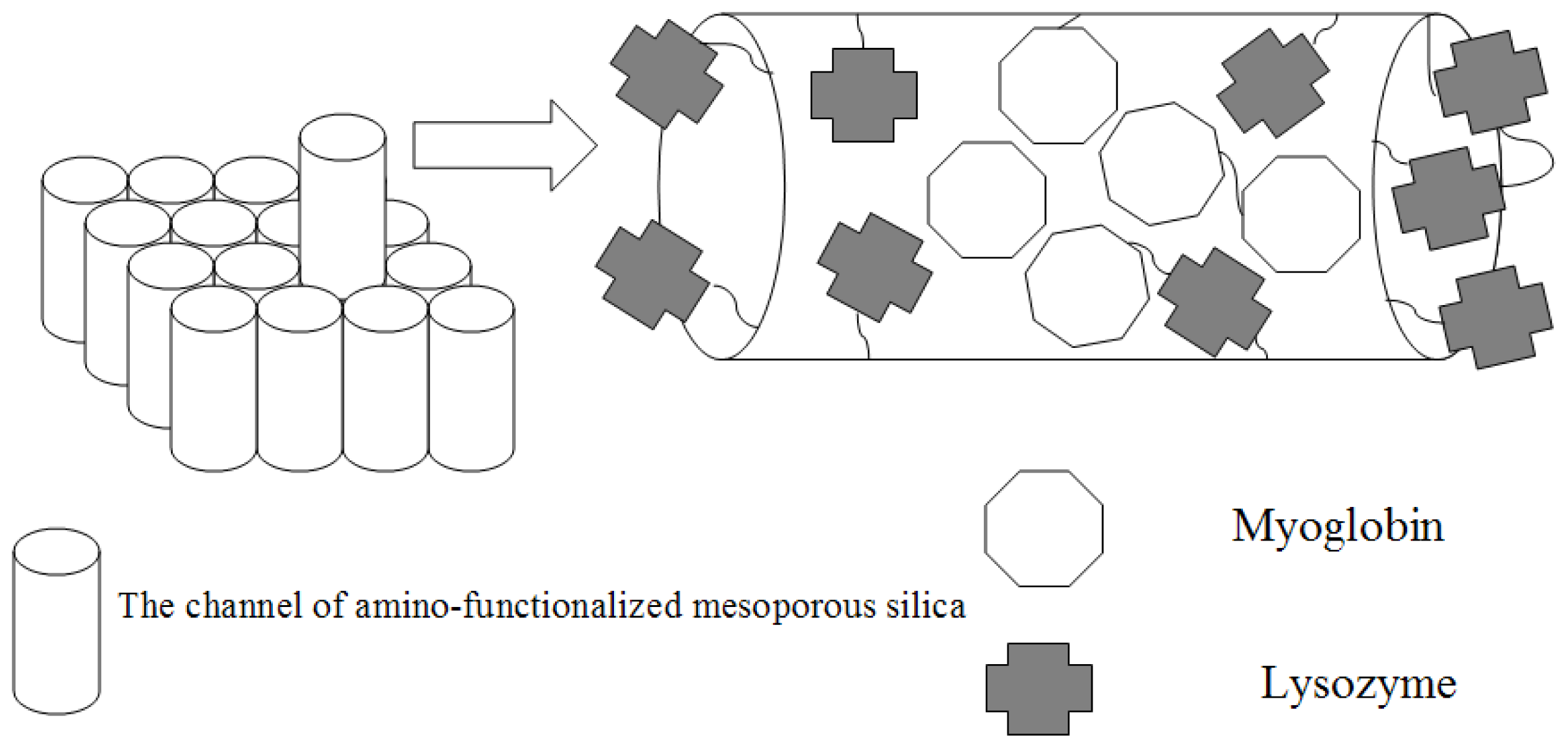
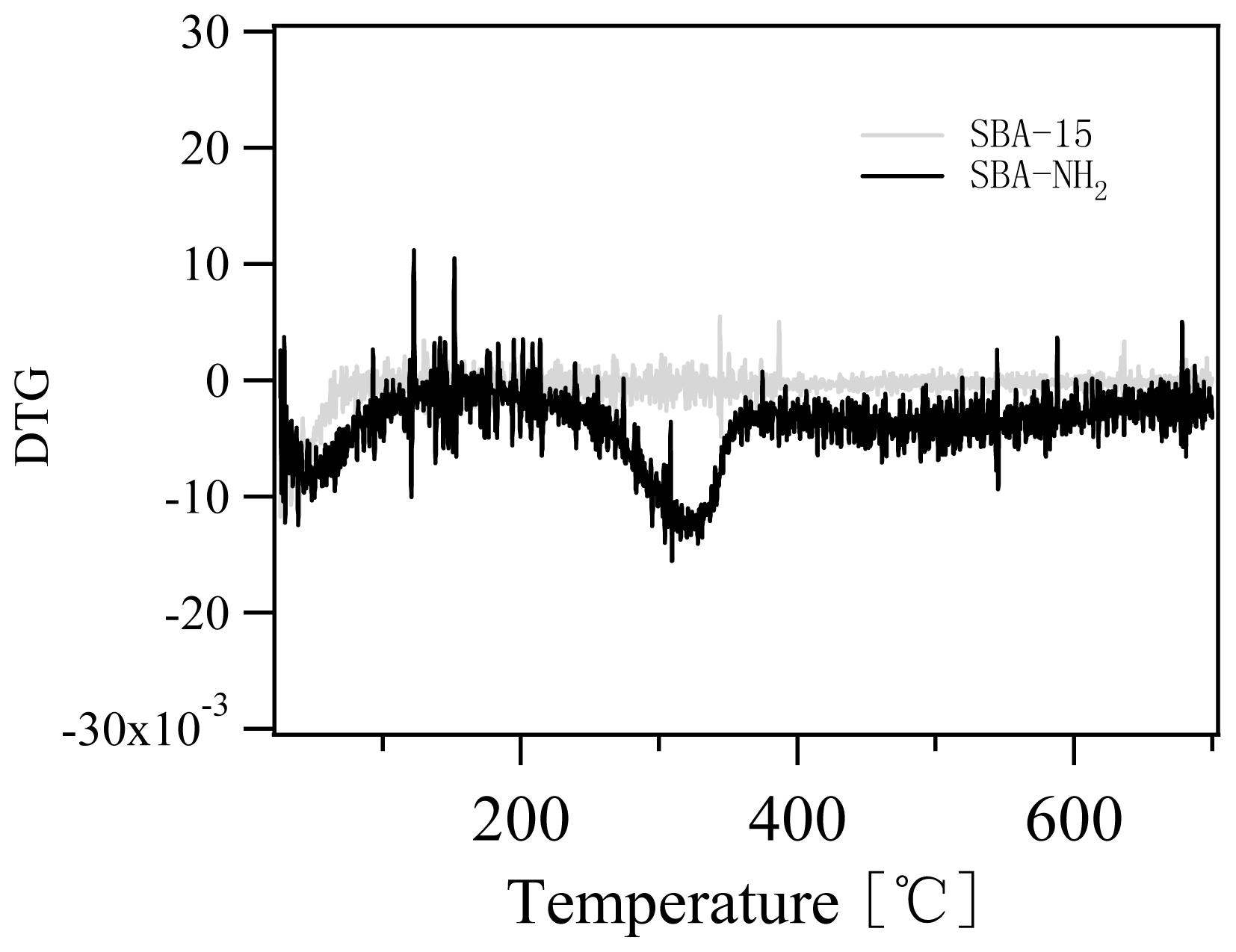
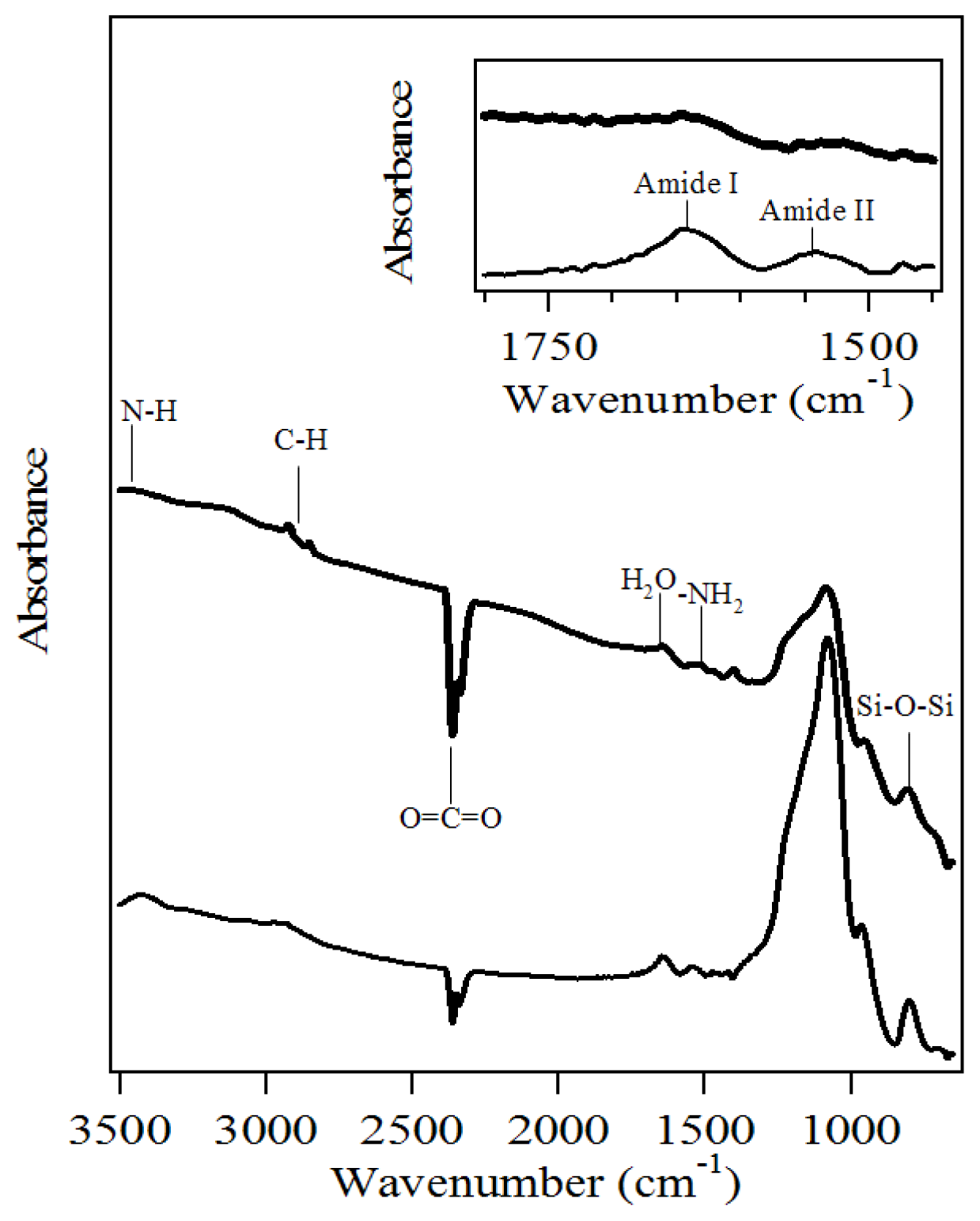
2.1. Characterization of Amino-Functionalized Mesoporous Silica
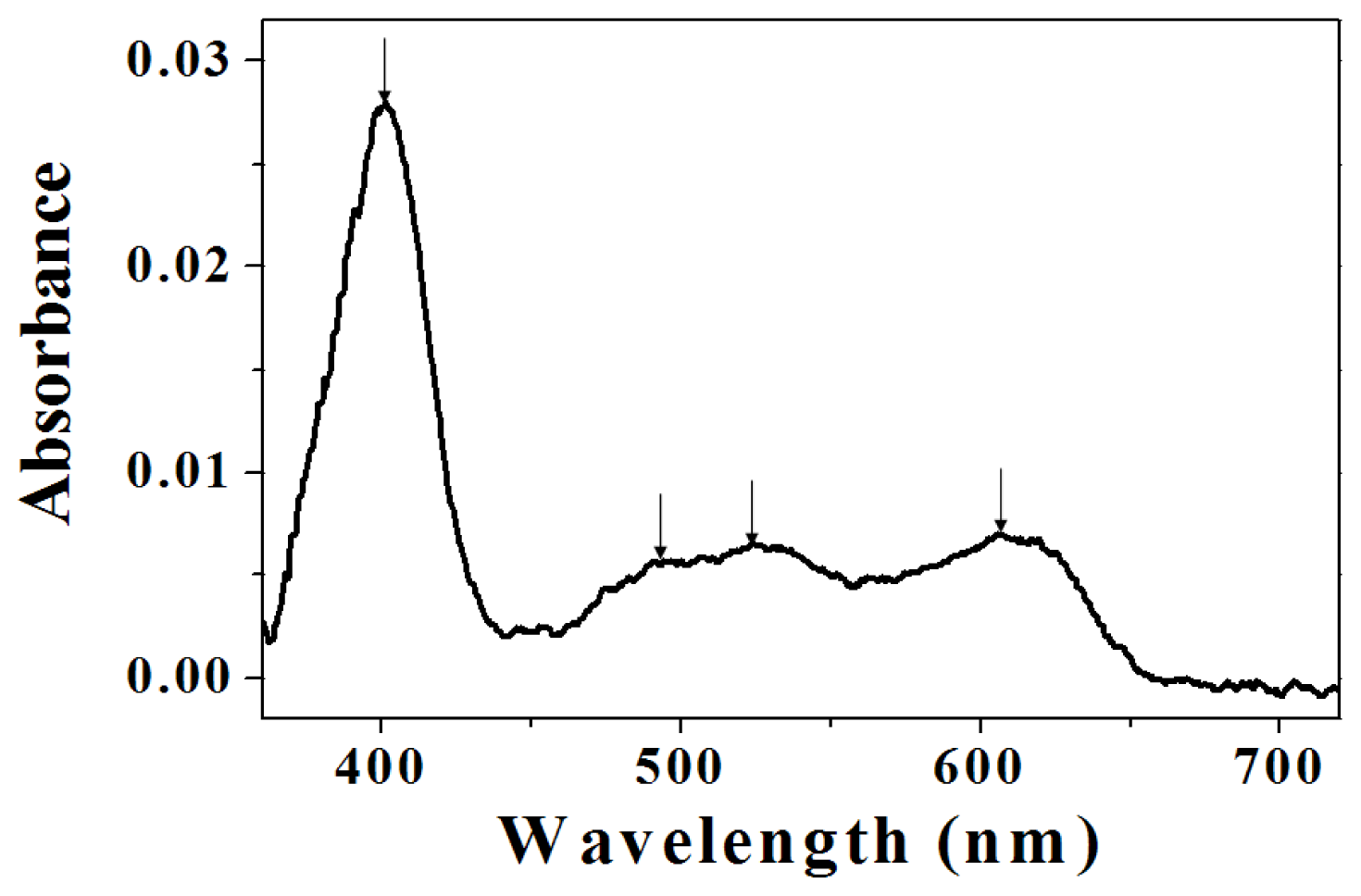
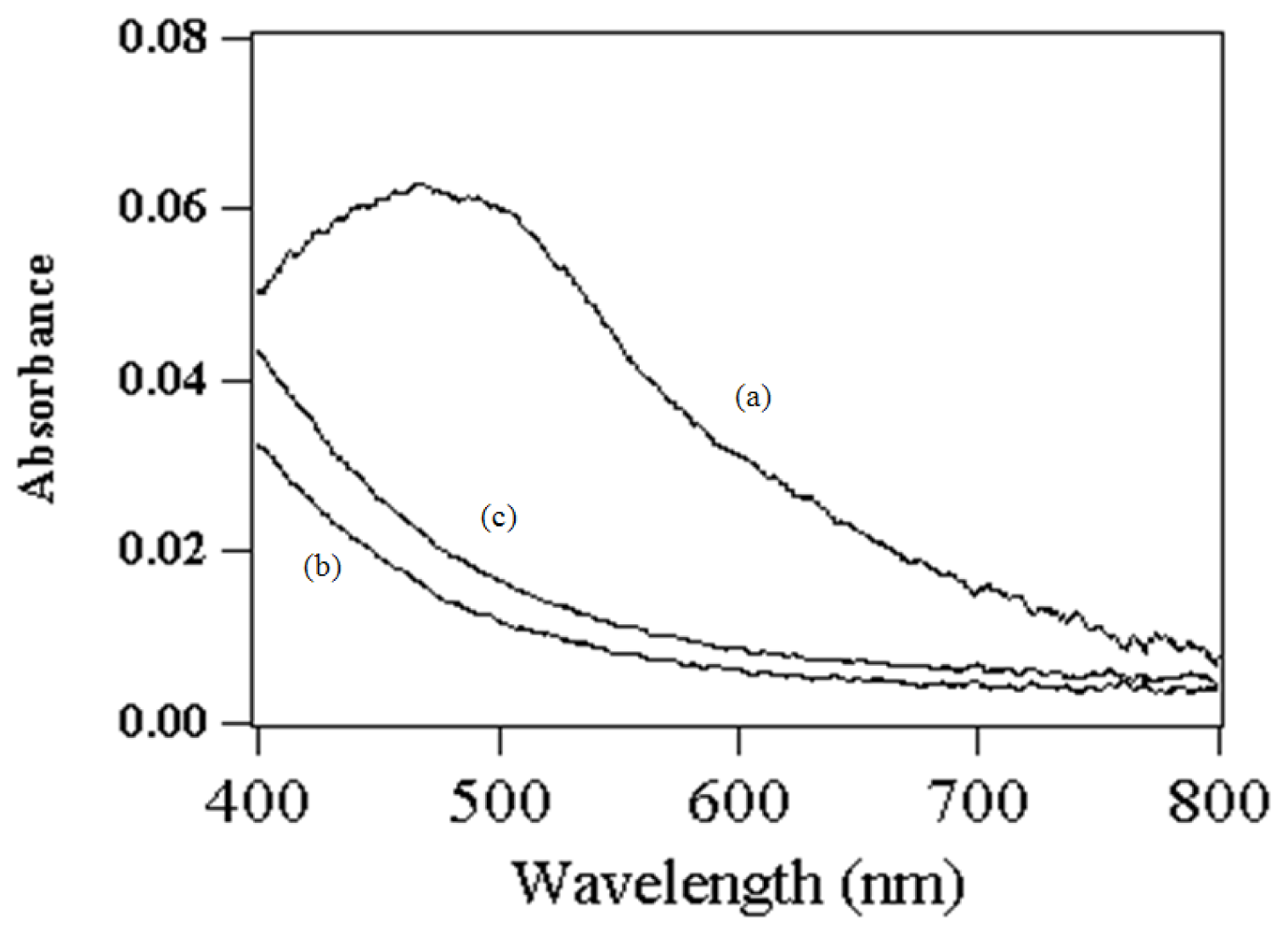
2.2. Assignment of UV/VIS and FTIR Spectra of Amino-Functionalized Mesoporous Silica after Adsorption Myoglobin
2.3. Evaluation of Peroxidative Activity of Myoglobin
2.4. Antibacterial Property of Lysozyme
3. Experimental Section
3.1. Materials
3.2. Experimental Procedures
3.2.1. Synthesis of Aminopropyl-Functionalized SBA-15 Mesoporous Silica
3.2.2. Characterization of the Amino-Functionalized Mesoporous Silica Materials
3.2.3. Adsorption of Myoglobin
3.2.4. Characterization of Amino-Functionalized Mesoporous Silica Materials after Adsorption
3.2.5. Measurement of Peroxidase Activity after Adsorption
3.2.6. Glutaraldehyde Coupling Procedure
3.2.7. Enzymatic Activity Assay
3.2.7.1. Assay of Myoglobin
3.2.7.2. Assay of Lysozyme
4. Conclusions
Acknowledgments
References
- Pan, D.; Chen, J.; Yao, S.; Tao, W.; Nie, L. An amperometric glucose biosensor based on glucose oxidase immobilized in electropolymerized poly (o-aminophenol) and carbon nanotubes composite film on a gold electrode. Anal. Sci 2005, 21, 367–371. [Google Scholar]
- Sakai-Kato, K.; Kato, M.; Ishihara, K.; Toyo’oka, T. An enzyme-immobilization method for integration of biofunctions on a microchip using a water-soluble amphiphilic phospholipid polymer having a reacting group. Lab Chip 2003, 4, 4–6. [Google Scholar]
- You, C.; Xu, X.; Tian, B.; Kong, J.; Zhao, D.; Liu, B. Electrochemistry and biosensing of glucose oxidase based on mesoporous carbons with different spatially ordered dimensions. Talanta 2009, 78, 705–710. [Google Scholar]
- Takahashi, H.; Li, B.; Sasaki, T.; Miyazaki, C.; Kajino, T.; Inagaki, S. Catalytic activity in organic solvents and stability of immobilized enzymes depend on the pore size and surface characteristics of mesoporous silica. Chem. Mater 2000, 12, 3301–3305. [Google Scholar]
- Kapoli, P.; Axarli, I.A.; Platis, D.; Fragoulaki, M.; Paine, M.; Hemingway, J.; Vontas, J.; Labrou, N.E. Engineering sensitive glutathione transferase for the detection of xenobiotics. Biosens. Bioelectr 2008, 24, 498–503. [Google Scholar]
- Avnir, D.; Braun, S.; Lev, O.; Ottolenghi, M. Enzymes and other proteins entrapped in sol-gel materials. Chem. Mater 1994, 6, 1605–1614. [Google Scholar]
- Diaz, J.F.; Balkus, K.J. Enzyme immobilization in MCM-41 molecular sieve. J. Mol. Catal. B 1996, 2, 115–126. [Google Scholar]
- Yang, X.Y.; Li, Z.Q.; Liu, B.; Klein-hofmann, A.; Tian, G.; Feng, Y.F.; Ding, Y.; Su, D.S.; Xiao, F.S. “Fish-in-net” encapsulation of enzymes in macroporous cages for stable, reusable, and active heterogeneous biocatalysts. Adv. Mater 2006, 18, 410–414. [Google Scholar]
- Wu, Z.; Dong, M.; Lu, M.; Li, Z. Encapsulation of β-galactosidase from Aspergillus oryzae based on “fish-in-net” approach with molecular imprinting technique. J. Mol. Catal. B 2010, 63, 75–80. [Google Scholar]
- Liu, J.; Guan, J.; Lu, M.; Kan, Q.; Li, Z. Hemoglobin immobilized with modified “fish-in-net” approach for the catalytic removal of aniline. J. Hazard. Mater 2012, 217–218, 156–163. [Google Scholar]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar]
- Fan, J.; Shui, W.; Yang, P.; Wang, X.; Xu, Y.; Wang, H.; Chen, X.; Zhao, D. Mesoporous silica nanoreactors for highly efficient proteolysis. Chem. Eur. J 2005, 11, 5391–5396. [Google Scholar]
- Burkett, S.L.; Sims, S.D.; Mann, S. Synthesis of hybrid inorganic-organic mesoporous silica by co-condensation of siloxane and organosiloxane precursors. Chem. Commun 1996, (11), 1367–1368. [Google Scholar]
- Richer, R. Direct synthesis of functionalized mesoporous silica by non-ionic alkylpolyethyleneoxide surfactant assembly. Chem. Commun 1998, (16), 1775–1777. [Google Scholar]
- Macquarrie, D.J.; Jackson, D.B. Aminopropylated MCMs as base catalysts: A comparison with aminopropylated silica. Chem. Commun 1997, (18), 1781–1782. [Google Scholar]
- Wang, X.; Lin, K.S.K.; Chan, J.C.C.; Cheng, S. Direct synthesis and catalytic applications of ordered large pore aminopropyl-functionalized SBA-15 mesoporous materials. J. Phys. Chem. B 2005, 109, 1763–1769. [Google Scholar]
- Yiu, H.H.P.; Wright, P.A. Enzymes supported on ordered mesoporous solids: A special case of an inorganic-organic hybrid. J. Mater. Chem 2005, 15, 3690–3700. [Google Scholar]
- Deere, J.; Magner, E.; Wall, J.G.; Hodnett, B.K. Mechanistic and structural features of protein adsorption onto mesoporous silicates. J. Phys. Chem. B 2002, 106, 7340–7347. [Google Scholar]
- Bos, M.A.; Shervani, Z.; Anusiem, A.C.I.; Giesbers, M.; Norde, W.; Kleijn, J.M. Influence of the electric potential of the interface on the adsorption of proteins. Colloid. Surf. B 1994, 3, 91–100. [Google Scholar]
- Chong, A.S.M.; Zhao, X. Functionalization of SBA-15 with APTES and characterization of functionalized materials. J. Phys. Chem. B 2003, 107, 12650–12657. [Google Scholar]
- Zhao, X.S.; Lu, G.Q.; Whittaker, A.K.; Millar, G.J.; Zhu, H.Y. Comprehensive study of surface chemistry of MCM-41 using 29Si CP/MAS NMR, FTIR, Pyridine-TPD, and TGA. J. Phys. Chem. B 1997, 101, 6525–6531. [Google Scholar]
- Rosenholm, J.M.; Lindén, M. Wet-chemical analysis of surface concentration of accessible groups on different amino-functionalized mesoporous SBA-15 silicas. Chem. Mater 2007, 19, 5023–5034. [Google Scholar]
- Rimington, C. Spectral-absorption coefficients of some porphyrins in the Soret-band region. Biochem. J 1960, 75, 620. [Google Scholar]
- Smith, G.J.; Ghiggino, K.P.; Bennett, L.E.; Nero, T.L. The “Q-band” absorption spectra of hematoporphyrin monomer and aggregate in aqueous solution. Photochem. Photobiol 1989, 49, 49–52. [Google Scholar]
- Betancor, L.; Luckarift, H.R.; Seo, J.H.; Brand, O.; Spain, J.C. Three-dimensional immobilization of β-galactosidase on a silicon surface. Biotechnol. Bioeng 2008, 99, 261–267. [Google Scholar]
- Claiborne, A.; Fridovich, I. Chemical and enzymic intermediates in the peroxidation of o-dianisidine by horseradish peroxidase 1. Spectral properties of the products of dianisidine oxidation. Biochemistry 1979, 18, 2324–2329. [Google Scholar]
- Betancor, L.; Lopez-Gallego, F.; Hidalgo, A.; Alonso-Morales, N.; Mateo, G.D.O.C.; Fernandez-Lafuente, R.; Guisan, J.M. Different mechanisms of protein immobilization on glutaraldehyde activated supports: Effect of support activation and immobilization conditions. Enzyme Microb. Tech 2006, 39, 877–882. [Google Scholar]
- Sil, S.; Chakraborti, A.S. Hematoporphyrin interacts with myoglobin and alters its functions. Mol. Cell. Biochem 2002, 237, 103–110. [Google Scholar]
- Shugar, D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim. Biophys. Acta 1952, 8, 302–309. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar]


| Myoglobin | Lysozyme | |
|---|---|---|
| Protein loading (mg/g) | 88.5 mg/g | 122.4 mg/g |
| Enzyme activity (U/g) | 1398 U/g | 1.32 × 106 U/g |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dong, M.; Wu, Z.; Lu, M.; Wang, Z.; Li, Z. Combining the Physical Adsorption Approach and the Covalent Attachment Method to Prepare a Bifunctional Bioreactor. Int. J. Mol. Sci. 2012, 13, 11443-11454. https://doi.org/10.3390/ijms130911443
Dong M, Wu Z, Lu M, Wang Z, Li Z. Combining the Physical Adsorption Approach and the Covalent Attachment Method to Prepare a Bifunctional Bioreactor. International Journal of Molecular Sciences. 2012; 13(9):11443-11454. https://doi.org/10.3390/ijms130911443
Chicago/Turabian StyleDong, Mengxing, Zhuofu Wu, Ming Lu, Zhi Wang, and Zhengqiang Li. 2012. "Combining the Physical Adsorption Approach and the Covalent Attachment Method to Prepare a Bifunctional Bioreactor" International Journal of Molecular Sciences 13, no. 9: 11443-11454. https://doi.org/10.3390/ijms130911443




