2.1. Peanut Skin Color
The effects of gamma radiation and storage on color are shown in
Table 1. Peanut skin color varies from light brown to deep red, and most color pigments in plants, especially red, purple and blue, belong to the flavonoid class of anthocyanins, with other flavonoid compounds acting as co-pigments [
20]. Color can be affected by gamma radiation [
11–
13]. In the present study no color change due to irradiation was found in in-shell and blanched samples at time zero. In-shell peanuts had their
L * (lightness) reduced at5.2 kGy (twelfth month), and at 7.2 kGy and 10.0 kGy (ninth month). The same was observed in blanched samples at 7.2 kGy (third month). An increase in
L * in blanched samples was observed at 10.0 kGy (third and sixth months). No gamma radiation effect was observed in Chroma of in-shell samples during the whole storage and in blanched samples an increase was observed only at 10.0 kGy at the sixth month. °Hue decreased in in-shell and blanched samples at 7.2 kGy and 10.0 kGy (twelfth month).
According to previous work [
21], the color of IAC-Tatu ST peanut cultivar was not affected by the short-term effects of gamma radiation at doses of up to 15.0 kGy. However, IAC-Runner 886 cultivar was affected with respect to
L * and Croma, which was indicated by moderate darkening of this cultivar. Mexis and Kontominas [
11], reported no short-term effect of gamma radiation on the color of raw peanuts (up to 7 kGy), which is in good agreement with the present work.
The lower the
L * value of a sample is, the darker the product becomes. In the present study, at the end of the storage time all samples, with or without irradiation, presented lower
L * values than those at time zero. Also it is worth noting that control and gamma-irradiated at 5.2 kGy in-shell samples presented lower
L * only at the twelfth month of storage while the same behavior was observed from the ninth and third months of storage in samples irradiated with 7.2 kGy and 10.0 kGy, respectively. In view of this it is possible to suggest that gamma irradiation accelerated the decrease in
L * value in in-shell samples. A negative correlation was found between storage time and
L * in in-shell (
r = −0.8147,
p < 0.01) and blanched samples (
r = −0.4401,
p < 0.05). The colors of in-shell samples were the most affected by storage time (
Table 2). Few changes were observed in Chroma and °Hue during storage time, but no correlation was found between storage time and Chroma or °Hue. Although peanut shells could have played a role in protecting the kernel, the results from the present work suggest that the shells were not efficient in preventing the changes to the color of the skins.
The blanching process involves heating, through which means Maillard compounds can be generated. According to Davis
et al. [
22], Maillard compounds present antioxidant properties. Several works [
23,
24] demonstrate that phenolic and antioxidant compounds are involved in redox reactions leading to color changes. In this way, browning reactions due to the oxidation of the kernels can be retarded by the antioxidant properties of Maillard compounds in blanched peanuts, which could explain the lowest correlation between storage time and
L * in blanched samples. Free radical production due to water radiolysis is produced by gamma radiation. Thus oxidation changes, which cause color changes, can be influenced by moisture content and water activity. The present study demonstrated that the water activity of the samples was not the same (
Table 3). Also the moisture content of the control in-shell sample (5.93%) was higher than that of the blanched control samples (3.38%), which along with the presence of Maillard compounds is helpful in explaining the lowest correlation between storage time and
L *.
The results of the present study agree with the findings of Golge and Ova [
25], who reported that three months storage had no effect on the
L * (up to 5.0 kGy) of pine nuts. In the present study, the
L * values were the most affected by storage, and °Hue was the least affected. If time zero is taken into account, no color effect was observed due to irradiation (
Table 1), which in fact agrees with the study of Mexis
et al. [
26] which reported no short-time effect on the
L * value of gamma irradiated (up to 3.0 kGy) raw unpeeled almond kernels. However, during long-term storage, the
L * values decreased leading to a gradual darkening of the almonds especially in irradiated samples. According to the authors this effect was noticed even for samples packaged under N
2. In the present study few of the differences observed occurred immediately after the third month of storage. Although significant differences were detected by Tukey’s test (
p < 0.05), no correlation was found between irradiation and
L *, Chroma or °Hue (
Table 2); thus, it is possible to suggest that the differences could be due to the storage effect.
2.2. Water Activity
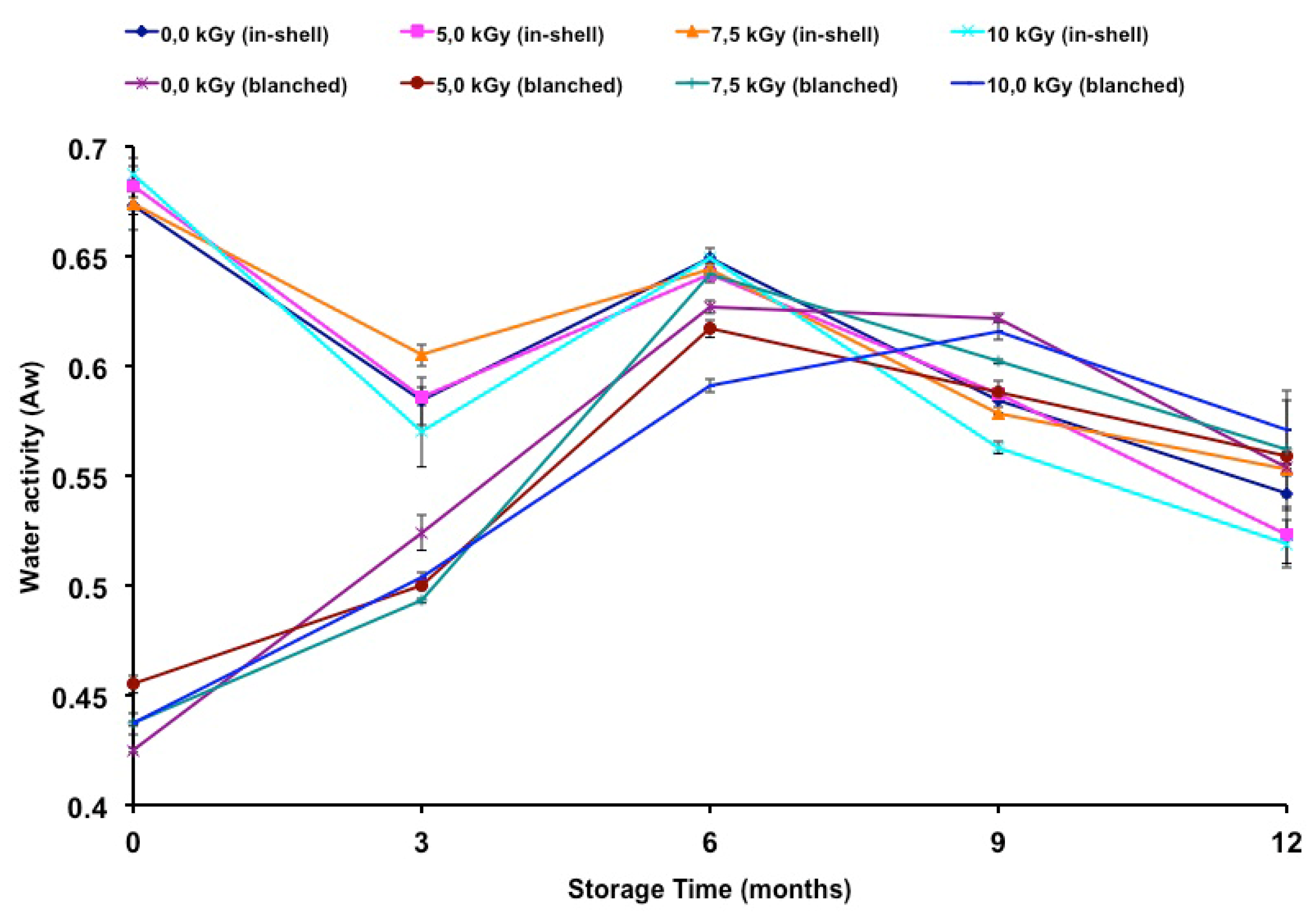
The water activities of gamma-irradiated and stored peanuts are presented in
Table 3. The water activity of in-shell peanuts was lower at 10.0 kGy (ninth and twelfth months). Higher water activity was observed at 7.2 kGy (third month). The highest water activity in blanched samples was observed at radiation doses of 5.2 kGy (time zero) and 7.2 kGy (sixth month), while the lowest was observed at doses of 5.2 kGy (time zero, sixth and ninth months), 7.2 kGy (time zero, third and ninth months) and 10.0 kGy (time zero, third and sixth months). The water activity was measured as a possible helpful parameter to explain fungal infection as well as changes in fatty acid composition due to an oxidation process. The difference in the initial water activity of the in-shell and blanched samples can be related to the humidity of the samples. The control sample of the in-shell and blanched samples presented moisture contents of 5.93% and 3.38%, respectively. According to Nakai
et al. [
8] the water activity can be influenced by the drying process. Although some changes were noticed in the water activity of the samples these changes could not be explained by the irradiation process and may have been due to industrial drying of the samples. Furthermore the blanched samples were treated by heat in order to remove the skins. As well as in the drying process, the uniformity of the temperature on the samples could have caused the differences in the blanched samples.
Although peanut samples demonstrated significant differences according to Tukey’s multiple test (
p < 0.05), no correlation was found between the gamma radiation and water activity of in-shell (
r = −0.1890) or blanched samples (
r = −0.0911).
Figure 1 shows the change in water activity during storage time. Relative to t time zero, there was a decrease in the water activity of in-shell samples, gamma-irradiated or not. In contrast, an increase in water activity was observed in blanched samples, irradiated or not, throughout the entire storage period. A negative correlation was found between storage time and the water activity of in-shell samples (
r = −0.7946;
p < 0.01). A positive correlation was found between the storage time and the water activity of blanched samples (
r = 0.7008;
p < 0.01).
The storage time influenced the water activity of both in-shell and blanched peanuts. Nakai
et al. [
8] reported water activity that ranged from 0.38 to 0.62 (in-shell peanuts) and from 0.44 to 0.63 (peeled peanuts) for samples stored for twelve months. According to Reed
et al. [
9], oxidation and sensory changes in peanuts were higher at lower (0.19) than at higher water activity (0.60). In the present study, the in-shell samples presented higher water activity than those of the blanched samples until the third month of storage. Also it is worth noting that on the sixth, ninth and twelfth months of storage the water activities became similar between in-shell and blanched samples (
Figure 1). Since water activity plays an important role in fungal growth as well as on the oxidation stability of food products it is correct to state that both kinds of samples became equally susceptible to both of these effects. In previous work [
19], in-shell samples presented the lowest concentration of secondary oxidation compounds when compared to blanched and peeled samples. Thus, water activity could have played an important role in preventing the formation of secondary compounds, volatile compounds such as aldehydes and ketones, causing off flavors that are responsible for rejection by consumers. Water activity should be taken into account regarding oxidation stability and microbiological safety; however, other factors such as packaging, light exposure, the presence of antioxidants, moisture content, gas concentrations, pH, temperature and overall storage conditions should not be underestimated.
2.3. Mycotoxic Fungi
Table 4 presents the frequency of mycotoxic fungi in gamma-irradiated and stored peanuts. In-shell peanuts, irradiated or not, did not present mycotoxic fungi throughout the entire storage period. Positive results were obtained for non-irradiated blanched samples at time zero and at the third and sixth months of storage. Gamma-irradiated blanched peanuts (5.2–10.0 kGy) did not present potentially aflatoxigenic fungi. This result is in good agreement with the findings of Chiou
et al. [
3], whose studies demonstrated 5.2 kGy to be a suitable dose for fungi disinfestation in peanuts. In a previous study [
19], we suggested that, regarding oxidation compounds and tocopherol content, in-shell gamma-irradiated peanuts were the best feedstock when compared to gamma-irradiated peeled and blanched samples. In the present study, in-shell samples were the best feedstock because no mycotoxic fungi were found during this kind of storage. Hilmy
et al. [
27] recontaminated gamma-irradiated (5.2 kGy) peanuts (a growth medium) to study the effects of atmospheric humidity on fungal growth and its toxin production by
Aspergillus flavus after irradiation. According to the authors, although the lowest relative humidity of 91% (compared with 97%) and irradiation doses (0.5 kGy and 1 kGy) could not inhibit the aflatoxin B1 production of
Aspergillus flavus in peanuts, the doses could delay the production by up to five and 14 days, respectively.
Gamma radiation cannot prevent recontamination; therefore, it is always necessary to protect gamma-irradiated products from recontamination sources. Insects are known to be vectors of mycotoxin-producing fungi. According to Nesci
et al. [
28], a strongly positive correlation was observed between peanut samples contaminated with insects and insects contaminated with
Aspergillus section
Flavi. Low doses of gamma radiation (0.2–0.8 kGy) are also efficient for killing and sterilizing insects (disinfestation of food) [
29]. Nesci
et al. [
28] state that any action undertaken to reduce insect infestation during storage could help to reduce aflatoxin-producing fungi.
In addition to the recontamination issue, temperature and water activity play important roles in fungi growth. According to Horn [
30], at temperatures of 15, 22, 30, 37 and 45 °C,
Aspergillus flavus presented the highest growth at 22 °C–37 °C. In the present study, the storage temperature ranged from 23.06 °C to 25.01 °C (from time zero to the third month), 24.04 °C to 28.98 °C (from the fourth to the sixth month), 20.83 °C to 22.67 °C (from the seventh to the ninth month) and 20.95 °C to 23.35 °C (from the tenth to the twelfth month), temperatures that favored fungi growth. According to Nakai
et al. [
8], the growth of
Aspergillus flavus was mainly influenced by temperature and relative humidity. The authors detected
Aspergillus flavus growing at a water activity of 0.38 to 0.65, which is similar to the water activity observed in the present study (
Table 3) but lower than that reported by Goncalez
et al. [
31], whose studies demonstrated higher
Aspergillus flavus growth with water activity of 0.97.
As mentioned before [
19], regarding tocopherol content and oxidation stability, gamma-irradiated in-shell samples were shown to be the best feedstock. Based on the data presented in
Table 4, we also suggest that in-shell peanuts are less susceptible to mycotoxic fungi contamination. Furthermore, in the present study 5.2 kGy was demonstrated be the suitable dose to prevent mycotoxic fungi growth.
2.4. Fatty Acid Composition
Table 5 reports the fatty acid compositions of lipid extracts from gamma-irradiated and stored peanut samples. The oleic-to-linoleic acid (O/L) ratio is a quality index employed to determine the genetic characteristics of peanuts classified as normal, mid-, and high-oleic types, ranging from 1 to 1.5; 1.5 to 9.0, and above 9.0, respectively [
32]. The present study was carried out with normal oleic peanuts. Palmitic acid, oleic acid, and linoleic acid were the major fatty acids. According to Andersen and Gorbet [
33], the remaining fatty acids, stearic, arachidic, eicosenoic, behenic, and lignoceric acids, normally occur in weight percentages between 0.02% and 4.0%, which in fact agrees with values observed in the present study.
In a recent study, Shin
et al. [
32] analyzed 151 samples from two-year crops and noticed that there was a large variation with respect to the fatty acid content in samples classified as normal, mid-, or high-oleic. The authors reported that palmitic acid (C16:0) ranged from 5.31% to 11.49%; stearic acid (C18:0), 1.46% to 4.76%; oleic acid (C18:1, ω9), 44.78% to 82.17%; linoleic acid (C18:2, ω6), 2.85% to 33.92%; arachidic acid (C20:0), 0.87% to 2.18%; gondoic acid (C20:1, ω9), 1.09% to 3.13%; behenic acid (C22:0), 0.73% to 4.37%; and lignoceric acid (C24:0), 0.41% to 2.12%. These data are in agreement with those obtained in the current work.
Mexis and Kontominas [
11,
13] reported an increase in the saturated fatty acid content and a decrease in the mono- and polyunsaturated fatty acid content due to the irradiation of peanuts, pistachio and hazelnuts at doses of up to 7.0 kGy. However, according to another study by Mexis and Kontominas [
12], monounsaturated fatty acids, as opposed to polysaturated fatty acids, were preferentially attacked by oxygen to produce primary and secondary oxidation products in gamma-irradiated cashew nuts.
Regarding the effects of gamma radiation on the major fatty acids, an increase in palmitic acid was observed at doses of 7.2 kGy (ninth month) in in-shell samples and 10.0 kGy (third month) in blanched samples (
Table 5). The oleic acid content of in-shell samples was reduced by gamma radiation (7.2 kGy) at the third month of storage and increased at doses of 5.2, 7.2 or 10.0 kGy at the twelfth month. The oleic acid content of blanched samples increased at doses of 7.2 kGy or 10.0 kGy at the ninth month. The linoleic acid content of the in-shell samples decreased at the ninth month of storage (5.2 kGy) and at the sixth and ninth months of storage in blanched samples (7.2 kGy and 10.0 kGy). The saturated fatty acid content increased at the ninth month of storage in in-shell samples (5.2 kGy and 7.2 kGy) and at the third month of storage in blanched samples (10.0 kGy). The polyunsaturated fatty acid content decreased at the ninth month of storage in the in-shell samples (5.2 kGy) and at the sixth and ninth months in the blanched samples (7.2 kGy and 10.0 kGy). The monounsaturated fatty acid content decreased at time zero (10.0 kGy) and at the third month of storage (7.2 kGy) in in-shell samples and at time zero (5.2 kGy and 10.0 kGy) and at the third month of storage (7.2 kGy); the same decrease was observed in blanched samples. There was an increase at time zero (7.2 kGy) and at the sixth and ninth months of storage (7.2 kGy and 10.0 kGy) in blanched samples.
Regarding storage effects, a decrease in the palmitic acid content of in-shell samples was observed in samples submitted to 10.0 kGy (ninth month) of radiation. The oleic and monounsaturated fatty acid contents increased or remained unaffected during storage. Moreover, the linoleic and polyunsaturated fatty acid contents decreased or remained unaffected during storage. A decrease in the saturated fatty acid content of in-shell samples was observed at the ninth month of storage (0.0 kGy), and an increase was observed at the twelfth month (0.0 kGy) in blanched samples. The decrease in the polyunsaturated fatty acid content and the concomitant increase in the monounsaturated fatty acid content are explained by O’Keefe
et al. [
34]. The authors stated that the ratio of the oxidation rates of stearic, oleic, linoleic and linolenic acids was 1:10:100:200.
Table 6 shows Pearson’s correlation between gamma radiation and fatty acids as well as between storage time and fatty acids.
Only modest correlation existed between the stearic acid content of in-shell samples and gamma-radiation doses (
r = 0.4935,
p < 0.05). However, only a small concentration of stearic acid was found in the samples (
Table 5). In turn, this correlation does not have a practical influence on the fatty acid profile. No correlation was found between gamma radiation and the remaining fatty acids of the samples. In-shell peanuts presented a positive correlation between storage time and oleic (
r = 0.7239,
p < 0.01), arachidic (
r = 0.5927,
p < 0.01), behenic (
r = 0.6304,
p < 0.01), lignoceric (
r = 0.5760,
p < 0.01) and monounsaturated fatty acids (0.7452,
p < 0.01). The same was observed for oleic (
r = 0.6181,
p < 0.01), arachidic (
r = 0.6167,
p < 0.01), behenic (
r = 0.6505,
p < 0.01) and monounsaturated fatty acids (0.6397,
p < 0.01) in blanched samples. A negative correlation was found between storage time and linoleic acids in in-shell (
r = −0.7870,
p < 0.01) and blanched (
r = −0.7267,
p < 0.01) samples. Linoleic acids are the only polyunsaturated fatty acids found in peanuts. In turn, a negative correlation was also found between storage time and polyunsaturated fatty acid content.
According to Finley and Shahidi [
35], highly unsaturated fatty acids have been documented as having positive effects in reducing the risk of certain forms of cardiovascular disease, inflammatory diseases, as well as maternal and infant nutrition. If gamma radiation does not correlate negatively with the presence of polyunsaturated and monounsaturated fatty acids in peanuts, it can be inferred that there are no negative effects on the benefits offered to individuals who consume gamma-irradiated peanuts.
2.5. Total Phenolic Contents
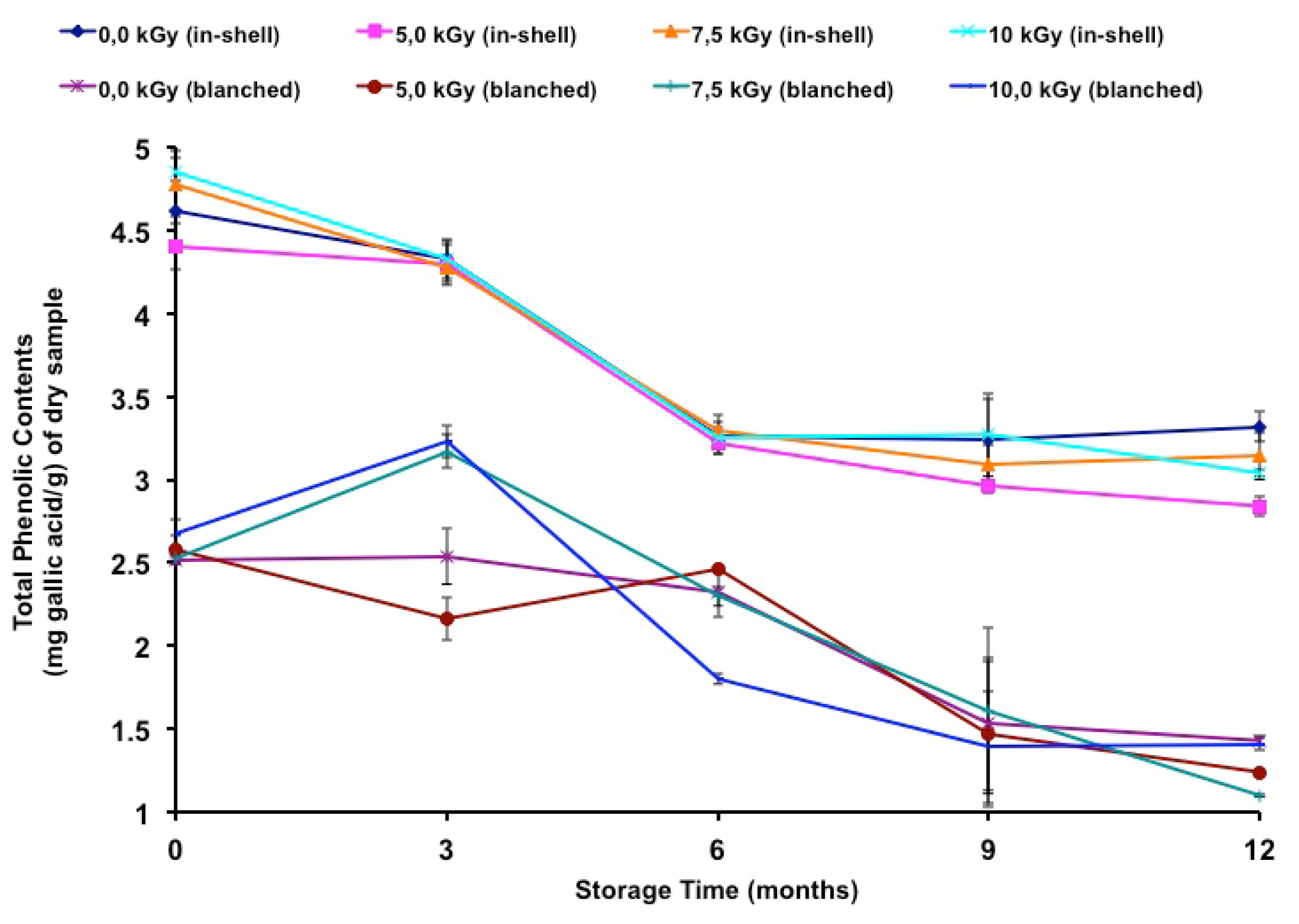
The total phenolic contents of gamma-irradiated and stored peanuts are shown in
Table 7. It has been noted in the literature that polyphenol contents vary between different peanut products. In gallic acid equivalents, peanut skins presented a total phenolic content ranging from 97.0 [
36] to 143.6 [
37], while peanuts, peanut butter [
38] and peanut oil [
39] presented contents of 3.96 mg/g, 5.36 mg/g, and 0.08 mg/g, respectively. The total phenolic contents of the non-irradiated in-shell samples are in good agreement with those reported by Wu
et al. [
38], while blanched samples presented lower concentrations than those in in-shell samples. The blanching process consists of removing the peanut skins; thus, as expected, the process changed the total phenolic content of the oilseed. Although peanut skins represent a small fraction of the kernel, on average 2.6% by weight [
40], its removal represented a 45.4% loss in total phenolic content.
In the present study, few samples presented moderate differences due to irradiation. In-shell samples were negatively affected at 5.2 kGy and 10.0 kGy at the twelfth month of storage, while blanched samples were negatively affected at 5.2 kGy (third and twelfth months), 7.2 kGy (twelfth month) and 10.0 kGy (sixth month). A positive effect was also observed at 7.2 kGy and 10.0 kGy (third month) in blanched samples. In a previous study [
21], no short-term effect was observed with respect to the total phenolic contents of peeled cv. IAC-Runner 886 peanut at radiation doses of up to 15.0 kGy. However, a positive effect was observed in cv. IAC-Tatu ST at doses of 7.2 kGy and 10.0 kGy. A random effect was reported by Toledo
et al. [
41] in soybean submitted to 2.0, 4.0 and 8.0 kGy of radiation. Štajner
et al. [
42] reported an increase in the total phenolic content of gamma-irradiated soybeans at doses up to 10.0 kGy. According to Harrison and Were [
43], the increase in the amount of phenolic compounds due to gamma radiation could be attributed to their release from glycosidic components and the degradation of larger phenolic compounds into smaller ones by gamma radiation. Villavicencio
et al. [
44] reported no effect in gamma-irradiated Brazilian beans (var. Carioca and var. Macaçar) at doses of 0.5, 1.0, 2.5 and 5.0 kGy; however, a dose of 10.0 kGy decreased the total phenolic contents of var. Macaçar. Though Tukey’s test (
p < 0.05) indicated significant differences due to irradiation, no correlation was found between irradiation and the total phenolic contents of in-shell (
r = −0.0214) or blanched samples (
r = 0.0326).
In the present study, there was a decrease (Tukey’s test
p < 0.05) in the total phenolic contents during the storage period (
Table 7) and a negative correlation was found between storage time and the total phenolic contents of in-shell (
r = −0.9101,
p < 0.01) and blanched samples (
r = −0.8591,
p < 0.01).
Figure 2 shows the total phenolic contents during long-term storage.
Nasar-Abbas
et al. [
45] reported that light exposure and temperature played an important role in the decrease in the total phenolic contents of stored faba beans. Additionally, Abramovič
et al. [
46] reported that there was a temperature effect on the polar phenolic content of stored Camelina sativa oil. Although some studies have reported gamma-radiation effects on the total phenolic contents of food products, there is still a lack of data that can explain the changes caused by gamma radiation. Thus, new studies should focus on this area of radiation chemistry.
2.6. ABTS Free Radical Scavenging Activity
Table 8 shows the results from an ABTS free radical scavenging activity assay. As mentioned before, the in-shell samples presented higher total phenolic contents than the blanched samples. Moreover, the ABTS free radical scavenging activity results of the in-shell samples were higher than those of the blanched samples. Removing peanut skin represented a 39.0% loss in ABTS free radical scavenging activity, which is in good agreement with the loss in the total phenolic content, which was approximately 45.4% (
Table 7).
A decrease in the ABTS free radical scavenging activity was observed in in-shell samples at 5.2 kGy (sixth and twelfth months) and at 7.2 kGy (sixth month). In an earlier study [
47], random effects due to gamma radiation (up to 10.0 kGy) were observed on the ABTS free radical scavenging activity of peanut skins. However, when ethanolic peanut skin extracts were applied in a model system of soybean oil (heated at 110 °C with an airflow of 9 L/h), the Oil Stability Index Rancimat method demonstrated that gamma radiation did not affect the antioxidant properties of the peanut skins. On the other hand, higher ABTS free radical scavenging activity was observed in gamma-irradiated blanched samples relative to that of non-irradiated samples at time zero (
Table 8). At the third month of storage and later, no ABTS free radical scavenging activity was observed in blanched samples, irradiated or not.
Similar results were reported by Dixit
et al. [
48]. According to the authors, gamma-irradiated soybean genotypes showed an increase in antioxidant constituents and antioxidant properties at low doses of 0.5 kGy and 2.0 kGy, while the antioxidant effects of soy seeds either decreased or remained constant at a higher dose of 5.0 kGy. These findings are in good agreement with those of the present study with respect to the short-term effects (time zero) of gamma radiation on ABTS free radical scavenging activity. Although significant differences have been observed in in-shell and blanched samples (Tukey’s test,
p < 0.05), no correlation was found between gamma radiation dose and ABTS free radical scavenging activity in in-shell samples (
r = −0.1005). On the other hand, positive correlation was found between irradiation and the ABTS free radical scavenging activity of the blanched samples (
r = 0.9355,
p < 0.01).
There was a decrease in ABTS free radical scavenging activity during the storage time (
Table 8). Only modest negative correlation was found between storage time and the ABTS free radical scavenging activity of in-shell samples (
r = −0.5779,
p < 0.01). Talcott
et al. [
49] reported a significant decrease in ORAC (oxygen radical absorbance capacity) in normal, mid-, and high-oleic acid runner peanut cultivars stored for four months at different temperatures (20 °C and 35 °C). According to the authors, free
p-coumaric acid, three esterified derivatives of
p-coumaric, and two esterified derivatives of hydroxybenzoic acid were the predominant polyphenolics present and their rates of change were similar among cultivars and independent of storage time or temperature. However, only modest correlations existed between total phenolics and antioxidant capacity. The authors stated that decreases during storage were a good indication that soluble (80% methanol) phytochemicals with antioxidant capacity, most likely polyphenolics and/or protein-polyphenolic complexes, had altered during storage. In the present work, though phenolic compounds were present in blanched samples during the whole storage (
Table 7), the decrease in their concentration along with molecular alterations could have influenced their ABTS free radical scavenging activity.
Phenolic and polyphenolic compounds constitute an important class of secondary plant metabolites that act as free radical scavengers and inhibitors of LDL cholesterol oxidation and DNA breakage, among other roles they play. Indeed, the role of food phenolics and polyphenolics in the prevention of cardiovascular disease and certain types of cancer is well recognized [
14].
As mentioned before, no correlation was found between gamma radiation and polyunsaturated or monounsaturated fatty acids. Furthermore, no negative correlation was found between gamma radiation and total phenolic contents or ABTS free radical scavenging activity. Thus, after one year of analysis, it can be concluded that irradiation protected against mycotoxic fungi growth (microbiological safety) and retained the nutraceutical components of the samples during long-term storage.