Comparative Studies on the Induction of Trichoderma harzianum Mutanase by α-(1→3)-Glucan-Rich Fruiting Bodies and Mycelia of Laetiporus sulphureus
Abstract
:1. Introduction
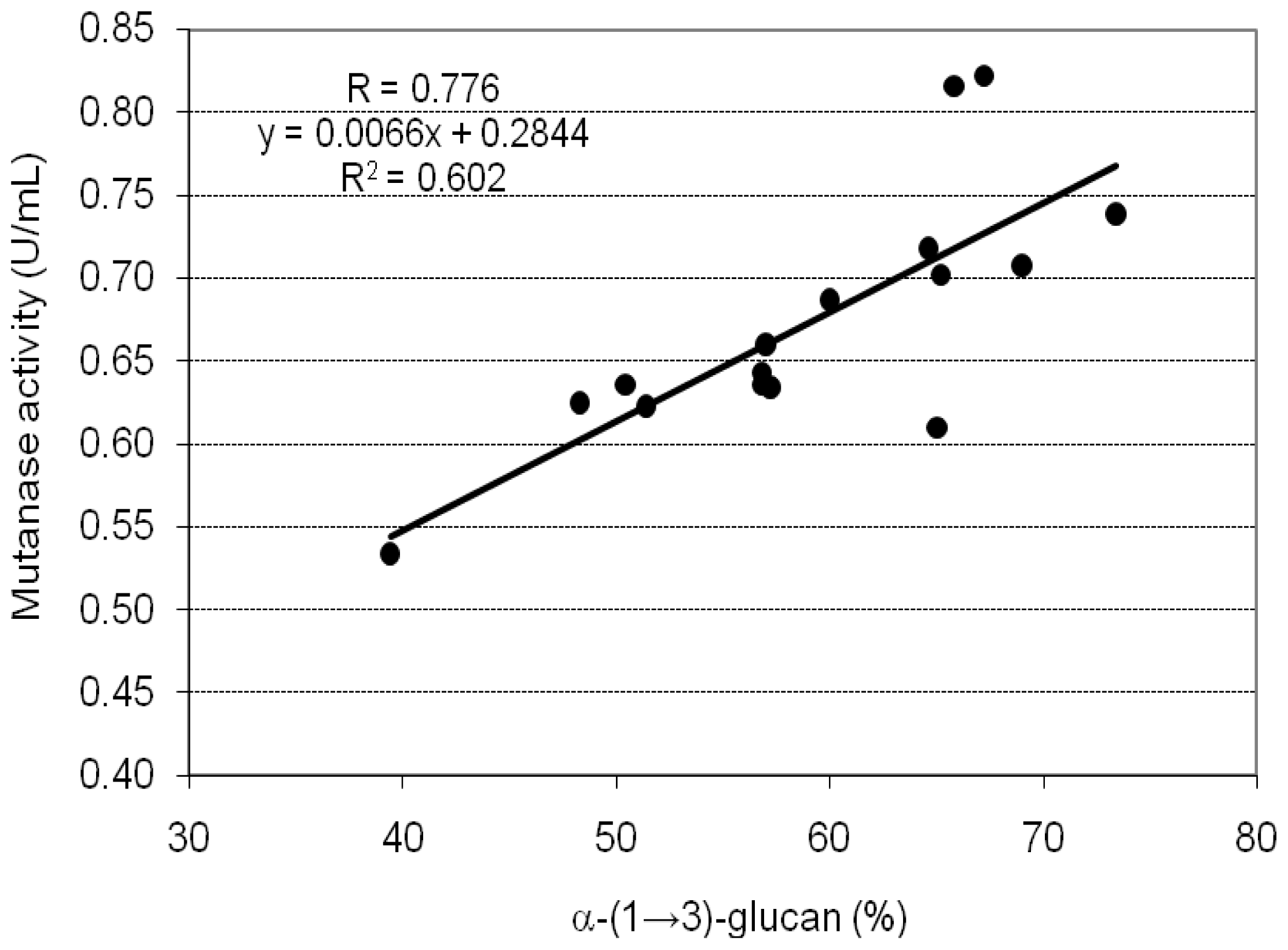
2. Results and Discussion
3. Experimental Section
3.1. Microorganisms
3.2. Laetiporus sulphureus Cultivation
3.3. Trichoderma harzianum Cultivation
3.4. Cell Wall and α-(1→3)-Glucan Preparation
3.5. Mutanase Assay
3.6. Preparation of Dextranase-Pretreated Mutan (DTM)
3.7. Genomic DNA Isolation, Amplification of ITS Sequences, and DNA Sequencing
3.8. Immunofluorescent Labeling of α-(1→3)-Glucan
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Budtz-Jörgensen, E.; Kelstrup, J. Enzymes as denture cleansers. Eur. J. Oral Sci 1977, 85, 209–215. [Google Scholar]
- Inoue, M.; Yakushiji, T.; Mizuno, J.; Yamamoto, Y.; Tanii, S. Inhibition of dental plaque formation by mouthwash containing an endo-a-1,3 glucanase. Clin. Prev. Dent 1990, 12, 10–14. [Google Scholar]
- Pleszczyńska, M.; Wiater, A.; Jodkowska, E.; Rusyan, E.; Dubielecka, M.; Małkiewicz, K.; Paduch, R.; Walasik, K.; Szczodrak, J. The effects of microbial mutanases on dental plaque aggregation and periodontal parameters. Pol. J. Environ. Stud 2011, 20, 91–96. [Google Scholar]
- Calo, L.; García, I.; Gotor, C.; Romero, L.C. Leaf hairs influence phytopathogenic fungus infection and confer an increased resistance when expressing a Trichoderma α-1,3-glucanase. J. Exp. Bot 2006, 57, 3911–3920. [Google Scholar]
- Balasubramanian, N.; Juliet, G.A.; Srikalaivani, P.; Lalithakumari, D. Release and regeneration of protoplasts from the fungus Trichothecium roseum. Can. J. Microbiol 2003, 49, 263–268. [Google Scholar]
- Villettaz, J.C. Enzymatic Treatment of Wine and Must. In U.S. Patent; 4,439,455, 27 March 1984. [Google Scholar]
- Gozard, J.P.; Jarry, A.; Luccioni, A. Enzymatic Treatment of Solutions of Polysaccharide Biopolymers. In U.S. Patent; 4,775,632, 4 October 1988. [Google Scholar]
- Wiater, A.; Szczodrak, J.; Pleszczyńska, M. Mutanase induction in Trichoderma harzianum by cell wall of Laetiporus sulphureus and its application for mutan removal from oral biofilms. J. Microbiol. Biotechnol 2008, 18, 1335–1341. [Google Scholar]
- Vasaitis, R.; Menkis, A.; Lim, Y.W.; Seok, S.; Tomsovsky, M.; Jankovsky, L.; Lygis, V.; Slippers, B.; Stenlid, J. Genetic variation and relationships in Laetiporus sulphureus s. lat., as determined by ITS rDNA sequences and in vitro growth rate. Mycol. Res 2009, 113, 326–336. [Google Scholar]
- Wu, S.; Zorn, H.; Krings, U.; Berger, R.G. Characteristic volatiles from young and aged fruiting bodies of wild Polyporus sulfureus (Bull.:Fr.) Fr. J. Agric. Food Chem 2005, 53, 4524–4528. [Google Scholar]
- Gąsiorowski, K.; Brokos, B.; Lamer-Zarawska, E.; Trocha-Grimshaw, J. Polysaccharides from Laetiporus sulphureus (Basidiomycetes) II. Evaluation of immunostimulative and antitumour activity. Bull. Pol. Acad. Sci. Biol. Sci 1993, 41, 347–352. [Google Scholar]
- Mlinarič, A.; Kac, J.; Pohleven, F. Screening of selected wood-damaging fungi for the HIV-1 reverse transcriptase inhibitors. Acta Pharm 2005, 55, 69–79. [Google Scholar]
- Turkoglu, A.; Duru, M.E.; Mercan, N.; Kivrak, I.; Gezer, K. Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem 2007, 101, 267–273. [Google Scholar]
- Davoli, P.; Mucci, A.; Schenetti, L.; Weber, R.W.S. Laetiporic acids, a family of non-carotenoid polyene pigments from fruitbodies and liquid cultures of Laetiporus sulphureus (Polyporales, Fungi). Phytochemistry 2005, 66, 817–823. [Google Scholar]
- Ershova, E.; Tikhonova, O.V.; Lur’e, L.M.; Efremenkova, O.V.; Kamzolkina, O.V.; Dudnik, V. Antimicrobial activity of Laetiporus sulphureus strains in submurged culture. Antibiot. Khimioter 2003, 48, 18–22. [Google Scholar]
- Jelsma, J.; Kreger, D.R. Observations the cell-wall compositions of the bracket fungi Laetiporus sulphureus and Piptoporus betulinus. Arch. Microbiol 1978, 119, 249–255. [Google Scholar]
- Wiater, A.; Szczodrak, J.; Pleszczyńska, M.; Próchniak, K. Production and use of mutanase from Trichoderma harzianum for effective degradation of streptococcal mutans. Braz. J. Microbiol 2005, 36, 137–146. [Google Scholar]
- Wiater, A.; Pleszczyńska, M.; Szczodrak, J.; Próchniak, K. α-(1→3)-Glucans from cell wall of Laetiporus sulphureus (Bull.:Fr.) Murrill-isolation, characteristics and application for induction of mutanase synthesis. Biotechnologia 2008, 81, 174–189. [Google Scholar]
- Guggenheim, B.; Haller, R. Purification and properties of an a-(1-3)-glucanohydrolase from Trichoderma harzianum. J. Dent. Res 1972, 51, 394–402. [Google Scholar]
- Quivey, R.G.; Kriger, P.S. Raffinose-induced mutanase production from Trichoderma harzianum. FEMS Microbiol. Lett 1993, 112, 307–312. [Google Scholar]
- Inoue, M.; Egami, T.; Yokogawa, K.; Kotani, H.; Morioka, T. Isolation, identification and some cultural conditions of Streptomyces species that produce water-insoluble polyglucan hydrolase. Agric. Biol. Chem 1975, 39, 1391–1400. [Google Scholar]
- Pleszczyńska, M.; Wiater, A.; Szczodrak, J. Mutanase from Paenibacillus sp. MP-1 produced inductively by fungal α-1,3-glucan and its potential for the degradation of mutan and Streptococcus mutans biofilm. Biotechnol. Lett 2010, 32, 1699–1704. [Google Scholar]
- Meyer, M.T.; Phaff, H.J. Purification and properties of (1→3)-α-glucanases from Bacillus circulans WL-12. J. Gen. Microbiol 1980, 118, 197–208. [Google Scholar]
- Imai, K.; Kobayashi, M.; Matsuda, K. Properties of an α-1,3-glucanase from Streptomyces sp. KI-8. Agric. Biol. Chem 1977, 41, 1889–1895. [Google Scholar]
- Alfonso, C.; Santamaria, F.; Nuero, O.M.; Prleto, A.; Leal, J.A.; Reyes, F. Biochemical studies on the cell wall degradation of Fusarium oxysporum f. sp. lycopersici race 2 by its own lytic enzymes for its biocontrol. Lett. Appl. Microbiol 1995, 20, 105–109. [Google Scholar]
- Santamaria, F.; Nuero, O.M.; Alfonso, C.; Prieto, A.; Leal, J.A.; Reyes, F. Cell wall degradation of Fusarium oxysporum f. sp. lycopersici race 2 by lytic enzymes from different Fusarium species for its biocontrol. Lett. Appl. Microbiol 1995, 20, 385–390. [Google Scholar]
- Jaroszuk-Œciseł, J.; Kurek, E.; Słomka, A.; Janczarek, M.; Rodzik, B. Activities of cell wall degrading enzymes in autolyzing cultures of three Fusarium culmorum isolates: Growth promoting, deleterious and pathogenic to rye (Secale cereale). Mycologia 2011, 103, 929–945. [Google Scholar]
- Grün, C.H. Structure and Biosynthesis of Fungal α-Glucans. Ph.D. Dissertation, Utrecht University, Rome, Italy, March 2003. [Google Scholar]
- Lamer-Zarawska, E.; Trocha-Grimshaw, J.; Gąsiorowki, K.; Królicki, Z.A.; Krzyżanowska, J. Polysaccharides from Laetiporus sulphureus (Basidomycetes) I. Isolation Procedure and Preliminary Chemical Characterization. Bull. Pol. Acad. Sci. Biol. Sci 1993, 41, 339–346. [Google Scholar]
- Kartal, S.N.; Munir, E.; Kakitani, T.; Imamura, Y. Bioremediation of CCA-treated wood by brow-rot fungi Fomitopsis palustris, Coniophora puteana, and Laetiorus sulphureus. J. Wood Sci 2004, 50, 182–188. [Google Scholar]
- Jaouani, A.; Sayadi, S.; Vanthournhout, M.; Penninckx, M.J. Potent fungi for decolourisation of oil mill wastewaters. Enzym. Microb. Technol 2003, 33, 802–809. [Google Scholar]
- Sani, R.K.; Banerjee, U.C. Decolorization of triphenylmethane dyes and textile and dye-stuff effluent by Kurthi sp. Enzyme Microb. Technol 1999, 24, 433–437. [Google Scholar]
- Mandels, M.; Parrish, F.W.; Reese, E.T. Sophorose as an inducer of cellulase in Trichoderma viride. J. Bacteriol 1962, 83, 400–408. [Google Scholar]
- Somogyi, M. A new reagent for the determination of sugars. J. Biol. Chem 1945, 160, 61–68. [Google Scholar]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem 1944, 153, 375–380. [Google Scholar]
- Wiater, A.; Szczodrak, J.; Pleszczyńska, M. Optimization of conditions for the efficient production of mutan in streptococcal cultures and post-culture liquids. Acta Biol. Hung 2005, 56, 137–150. [Google Scholar]
- Borges, M.I.; Azevedo, M.O.; Bonatelli, J.R.; Felipe, M.S.S.; Astolfi-Filho, S. A practical method for the preparation of total DNA from filamentous fungi. Fungal Genet. Newsl 1990, 37, 2–41. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, L. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Fujikawa, T.; Kuga, Y.; Yano, S.; Yoshimi, A.; Tachiki, T.; Abe, K.; Nishimura, M. Dynamics of cell wall components of Magnaporthe grisea during infectious structure development. Mol. Microbiol 2009, 73, 553–570. [Google Scholar]



| Specimen | GenBankaccession NO. | Geographicorigin | Host tree | Fruiting body sizeª | Fruiting body maturityb | α-(1→3)-Glucan(%)c |
|---|---|---|---|---|---|---|
| A1 | HM245764 | Lublin(51°13′N, 22°33′E) | Prunus cerasus | + | + | 17.3 ± 0.8 |
| A2 | HM245765 | Piaseczno(52°03′N, 21°00′E) | Salix alba | +++ | + | 26.9 ± 1.3 |
| A3 | HM245763 | Łomża(53°10′N, 22°03′E) | S. alba | ++ | + | 28.0 ± 1.2 |
| A4 | HM245772 | Warszawa(52°13′N, 21°00′E) | Salix caprea | +++ | + | 36.2 ± 0.9 |
| A5 | HM245770 | Lublin(51°13′N, 22°33′E) | S. alba | + | + | 37.6 ± 1.1 |
| B1 | HM245771 | Węgorzewo(54°12′N, 21°44′E) | Quercus robur | ++ | ++ | 36.5 ± 0.5 |
| B2 | HM245766 | Lublin(51°13′N, 22°33′E) | S. alba | + | ++ | 37.0 ± 1.4 |
| B3 | HM245768 | Gęś(51°42′N, 23°00′E) | Prunus avium | +++ | ++ | 41.0 ± 1.5 |
| C1 | HM245761 | Lublin(51°13′N, 22°33′E) | Fraxinus excelsior | ++ | +++ | 42.8 ± 1.3 |
| C2 | HM245762 | Drobin(52°44′N, 19°59′E) | Salix chrysocoma | ++ | +++ | 44.7 ± 1.9 |
| C3 | HM245759 | Lublin(51°13′N, 22°33′E) | S. chrysocoma | ++ | +++ | 46.4 ± 1.8 |
| C4 | HM245760 | Frampol(50°40′N, 22°40′E) | S. alba | +++ | +++ | 46.7 ± 2.1 |
| C5 | HM245758 | Kurów(51°23′N, 22°11′E) | S. caprea | +++ | +++ | 46.9 ± 0.8 |
| C6 | HM245774 | Bytów(54°10′N, 17°29′E) | Q. robur | ++ | +++ | 46.9 ± 1.7 |
| C7 | HM245767 | Zielona(50°59′N, 22°40′E) | P. cerasus | + | +++ | 47.0 ± 1.1 |
| C8 | HM245773 | Legionowo(52°24′N, 20°55′E) | Robinia pseudoacacia | ++ | +++ | 47.8 ± 0.7 |
| CWP | Mutanase activity | ||
|---|---|---|---|
| Specimen | α-(1→3)-Glucan (%) | U/mL | U/g α-(1→3) Glucan |
| A1 | 39.4 ± 0.8 | 0.534 ± 0.025 | 3.388 ± 0.112 |
| A2 | 50.4 ± 1.9 | 0.636 ± 0.028 | 3.155 ± 0.098 |
| A3 | 48.3 ± 1.3 | 0.625 ± 0.013 | 3.235 ± 0.052 |
| A4 | 56.8 ± 2.1 | 0.643 ± 0.009 | 2.830 ± 0.121 |
| A5 | 57.2 ± 1.6 | 0.634 ± 0.030 | 2.771 ± 0.046 |
| B1 | 56.8 ± 1.9 | 0.636 ± 0.007 | 2.799 ± 0.077 |
| B2 | 57.0 ± 1.7 | 0.660 ± 0.015 | 2.895 ± 0.133 |
| B3 | 60.0 ± 1.1 | 0.687 ± 0.025 | 2.863 ± 0.135 |
| C1 | 64.6 ± 2.2 | 0.718 ± 0.015 | 2.779 ± 0.127 |
| C2 | 65.8 ± 1.5 | 0.816 ± 0.019 | 3.100 ± 0.037 |
| C3 | 67.2 ± 1.7 | 0.822 ± 0.023 | 3.058 ± 0.121 |
| C4 | 65.2 ± 1.2 | 0.702 ± 0.005 | 2.692 ± 0.099 |
| C5 | 73.4 ± 2.2 | 0.739 ± 0.029 | 2.517 ± 0.078 |
| C6 | 69.0 ± 1.7 | 0.708 ± 0.013 | 2.565 ± 0.067 |
| C7 | 65.0 ± 1.3 | 0.610 ± 0.017 | 2.346 ± 0.111 |
| C8 | 51.4 ± 0.9 | 0.623 ± 0.015 | 3.030 ± 0.135 |
| Mycelium age | ||||||
|---|---|---|---|---|---|---|
| 1-Week-Old | 3-Week-old | 5-Week-old | ||||
| Medium | Biomassyield (g/L) | α-(1→3)-Glucan (%)a | Biomassyield (g/L) | α-(1→3)-Glucan (%)a | Biomassyield (g/L) | α-(1→3)-Glucan (%)a |
| I | 1.26 ± 0.054 | 4.7 ± 0.17 | 2.82 ± 0.097 | 11.0 ± 0.36 | 3.40 ± 0.096 | 12.4 ± 0.19 |
| II | 0.08 ± 0.004 | 2.3 ± 0.05 | 0.58 ± 0.020 | 5.8 ± 0.16 | 1.01 ± 0.032 | 15.1 ± 0.35 |
| III | 1.35 ± 0.037 | 7.4 ± 0.15 | 3.52 ± 0.081 | 8.2 ± 0.24 | 5.26 ± 0.156 | 13.1 ± 0.42 |
| IV | 1.44 ± 0.034 | 4.0 ± 0.09 | 3.75 ± 0.135 | 19.7 ± 0.55 | 5.53 ± 0.138 | 17.9 ± 0.54 |
| V | 0.02 ± 0.001 | n.d. | 0.05 ± 0.002 | n.d. | 0.05 ± 0.002 | n.d. |
| VI | 0.04 ± 0.002 | n.d. | 0.05 ± 0.002 | n.d. | 0.05 ± 0.002 | n.d. |
| VII | 0.01 ± 0.001 | n.d. | 0.02 ± 0.001 | n.d. | 0.03 ± 0.001 | n.d. |
| VIII | 1.68 ± 0.055 | 7.3 ± 0.23 | 2.39 ± 0.051 | 10.5 ± 0.21 | 2.85 ± 0.130 | 7.6 ± 0.21 |
| IX | 0.07 ± 0.002 | n.d. | 0.18 ± 0.005 | n.d. | 0.21 ± 0.011 | n.d. |
| CWP | Mutanase activity | ||
|---|---|---|---|
| Source | α-(1→3)-Glucan (%) | U/mL | U/g α-(1→3)-Glucan |
| 1 week-old mycelium on medium: | |||
| I | 8.0 ± 0.23 | 0.063 ± 0.003 | 1.969 ± 0.058 |
| II | 4.3 ± 0.11 | 0.034 ± 0.001 | 1.976 ± 0.034 |
| III | 13.2 ± 0.32 | 0.030 ± 0.001 | 0.568 ± 0.020 |
| IV | 7.0 ± 0.21 | 0.063 ± 0.002 | 2.250 ± 0.087 |
| VIII | 12.0 ± 0.34 | 0.067 ± 0.003 | 1.396 ± 0.060 |
| 3 week-old mycelium on medium: | |||
| I | 16.2 ± 0.45 | 0.109 ± 0.004 | 1.682 ± 0.048 |
| II | 12.6 ± 0.24 | 0.074 ± 0.002 | 1.468 ± 0.066 |
| III | 15.6 ± 0.55 | 0.106 ± 0.003 | 1.699 ± 0.080 |
| IV | 27.8 ± 0.51 | 0.341 ± 0.011 | 3.067 ± 0.092 |
| VIII | 15.4 ± 0.33 | 0.119 ± 0.004 | 1.932 ± 0.056 |
| 5 week-old mycelium on medium: | |||
| I | 17.4 ± 0.53 | 0.115 ± 0.003 | 1.652 ± 0.052 |
| II | 22.0 ± 0.81 | 0.186 ± 0.006 | 2.114 ± 0.085 |
| III | 16.8 ± 0.47 | 0.129 ± 0.005 | 1.920 ± 0.086 |
| IV | 25.8 ± 0.88 | 0.293 ± 0.009 | 2.839 ± 0.125 |
| VIII | 12.0 ± 0.48 | 0.114 ± 0.003 | 2.375 ± 0.052 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wiater, A.; Pleszczyńska, M.; Szczodrak, J.; Janusz, G. Comparative Studies on the Induction of Trichoderma harzianum Mutanase by α-(1→3)-Glucan-Rich Fruiting Bodies and Mycelia of Laetiporus sulphureus. Int. J. Mol. Sci. 2012, 13, 9584-9598. https://doi.org/10.3390/ijms13089584
Wiater A, Pleszczyńska M, Szczodrak J, Janusz G. Comparative Studies on the Induction of Trichoderma harzianum Mutanase by α-(1→3)-Glucan-Rich Fruiting Bodies and Mycelia of Laetiporus sulphureus. International Journal of Molecular Sciences. 2012; 13(8):9584-9598. https://doi.org/10.3390/ijms13089584
Chicago/Turabian StyleWiater, Adrian, Małgorzata Pleszczyńska, Janusz Szczodrak, and Grzegorz Janusz. 2012. "Comparative Studies on the Induction of Trichoderma harzianum Mutanase by α-(1→3)-Glucan-Rich Fruiting Bodies and Mycelia of Laetiporus sulphureus" International Journal of Molecular Sciences 13, no. 8: 9584-9598. https://doi.org/10.3390/ijms13089584





