Effect of β,β-Dimethylacrylshikonin on Inhibition of Human Colorectal Cancer Cell Growth in Vitro and in Vivo
Abstract
:1. Introduction
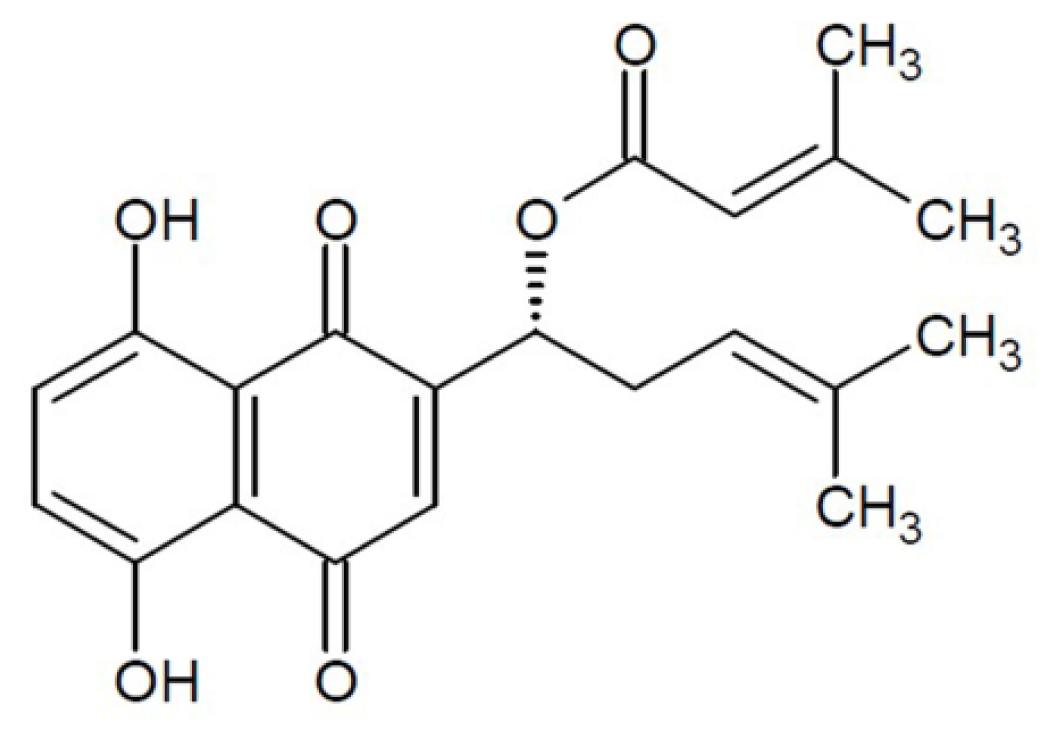
2. Results and Discussion
2.1. Effect of DA on Inhibition of Cell Growth in Vitro
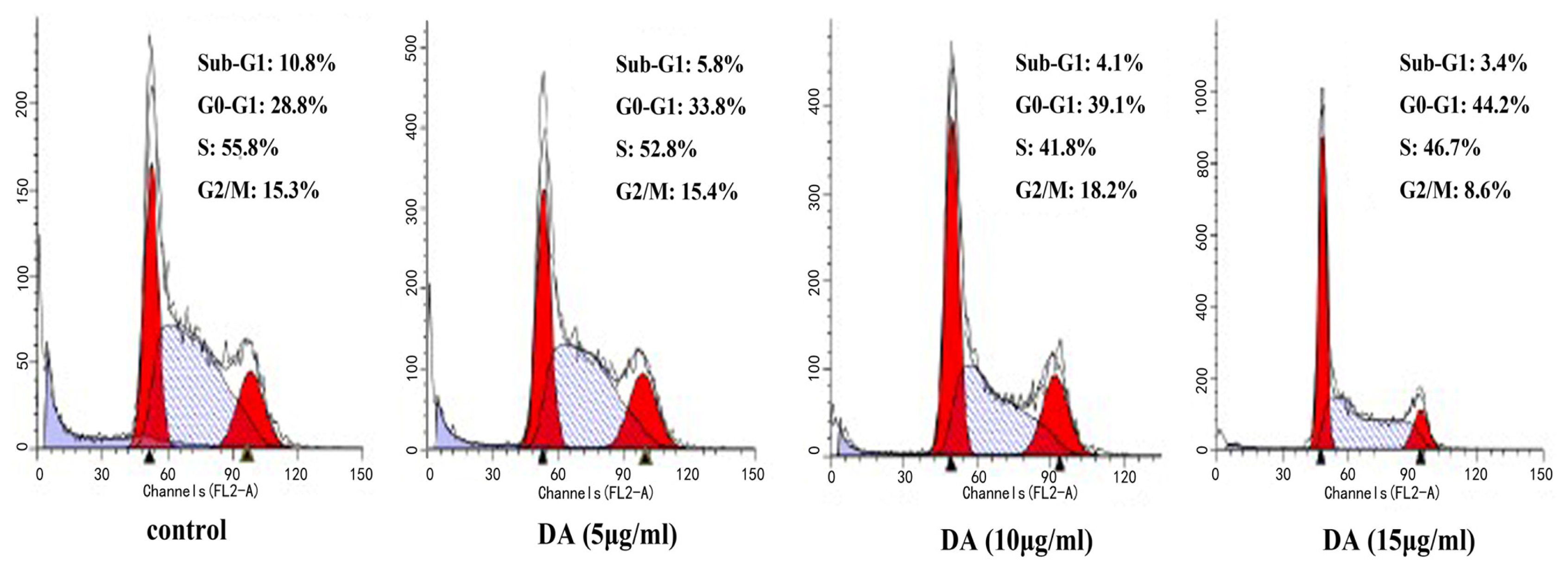
2.2. DA-Induced Cell Cycle Arrest in HCT-116 Cells
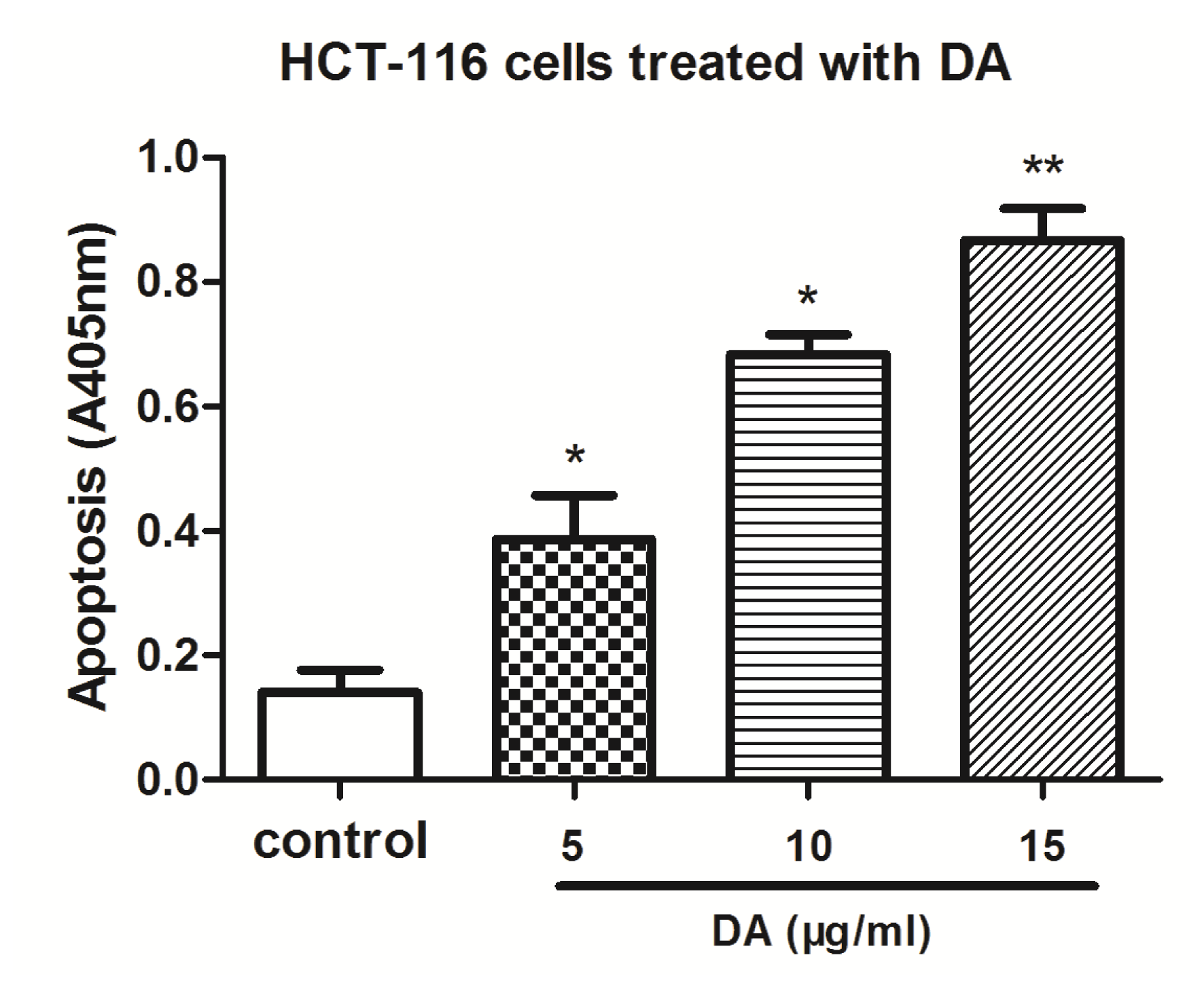
2.3. DA Induced Apoptosis in HCT-116 Cells
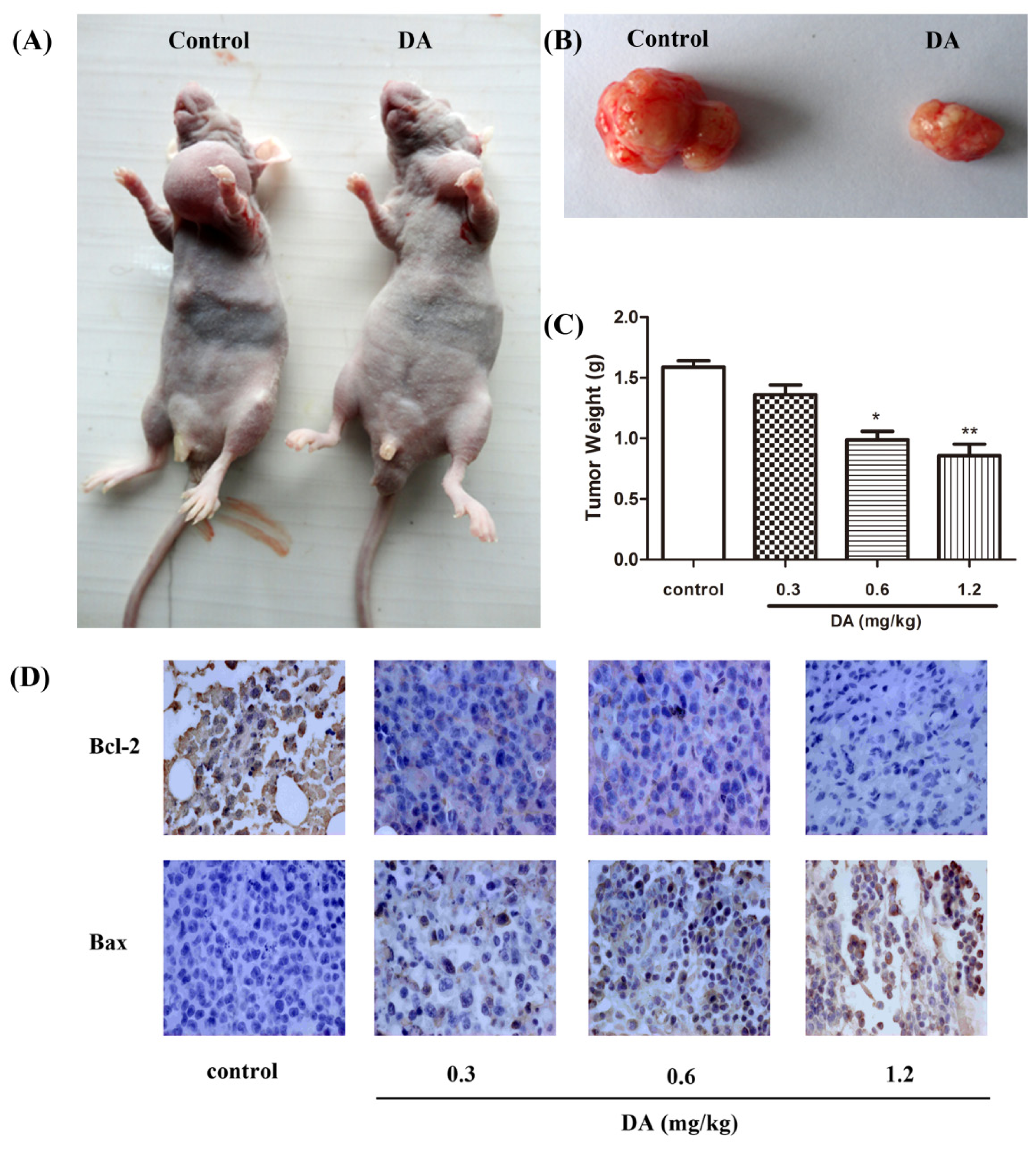
2.4. DA Regulates the Expression of Bcl-2 Family Proteins
2.5. Effect of DA on Inhibition of HCT-116 Xenografts in Nude Mice
3. Materials and Methods
3.1. Chemical Reagents
3.2. Cell Lines and Culture
3.3. Cell Proliferation Assay
3.4. Cell Cycle Analysis
3.5. Histone/DNA ELISA for Detection of Apoptosis
3.6. Western Blotting
3.7. Human Tumor Xenografts in Nude Mice
3.8. Immunohistochemical Staining
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Scripture, C.D.; Figg, W.D. Drug interactions in cancer therapy. Nat. Rev. Cancer 2006, 6, 546–558. [Google Scholar]
- Wang, C.C.; Li, J. An update on chemotherapy of colorectal liver metastases. World J. Gastroenterol 2012, 18, 25–33. [Google Scholar]
- Nagoor, N.H.; Muttiah, N.S.J.; Lim, C.S.; In, L.L.; Mohamad, K.; Awang, K. Regulation of apoptotic effects by erythrocarpine E, a cytotoxic limonoid from Chisocheton erythrocarpus in HSC-4 human oral cancer cells. PLoS One 2011, 6, e23661. [Google Scholar]
- Assimopoulou, A.N.; Sturm, S.; Stuppner, H.; Papageorgiou, V.P. Preparative isolation and purification of alkannin/shikonin derivatives from natural products by high-speed counter-current chromatography. Biomed. Chromatogr 2009, 23, 182–198. [Google Scholar]
- Zeng, Y.; Liu, G.; Zhou, L.M. Inhibitory effects of acetylshikonin on human gastric carcinoma cell line SGC-7901 in vitro and in vivo. World J. Gastroenterol 2009, 15, 1816–1820. [Google Scholar]
- Wu, Y.Y.; Wan, L.H.; Zheng, X.W.; Shao, Z.J.; Chen, J.; Liu, L.T.; Kuang, W.J.; Tan, X.S.; Zhou, L.M. Inhibitory effects of β,β-dimethylacrylshikonin on hepatocellular carcinoma in vitro and in vivo. Phytother. Res 2012, 26, 764–771. [Google Scholar]
- Staniforth, V.; Wang, S.Y.; Shyur, L.F.; Yang, N.S. Shikonins, phytocompounds from Lithospermum erythrorhizon, inhibit the transcriptional activation of human tumor necrosis factor alpha promoter in vivo. J. Biol. Chem 2004, 279, 5877–5885. [Google Scholar]
- Wang, Z.; Liu, T.; Gan, L.; Wang, T.; Yuan, X.; Zhang, B.; Chen, H.; Zheng, Q. Shikonin protects mouse brain against cerebral ischemia/reperfusion injury through its antioxidant activity. Eur. J. Pharmacol 2010, 643, 211–217. [Google Scholar]
- Ko, F.N.; Lee, Y.S.; Kuo, S.C.; Chang, Y.S.; Teng, C.M. Inhibition on platelet activation by shikonin derivatives isolated from Arnebia euchroma. BBA-Mol. Cell Res 1995, 1268, 329–334. [Google Scholar]
- An, S.; Park, Y.D.; Paik, Y.K.; Jeong, T.S.; Lee, Y.S. Human ACAT inhibitory effects of shikonin derivatives from Lithospermum erythrorhizon. Bioorg. Medicinal Chem. Lett 2007, 17, 1112–1116. [Google Scholar]
- Han, W.; Xie, J.; Li, L.; Liu, Z.; Hu, X. Necrostatin-1 reverts shikonin-induced necroptosis to apoptosis. Apoptosis 2009, 14, 674–686. [Google Scholar]
- Hou, Y.; Guo, T.; Wu, C.; He, X.; Zhao, M. Effect of shikonin on human breast cancer cells proliferation and apoptosis in vitro. Yakugaku Zasshi 2006, 126, 1383–1386. [Google Scholar]
- Min, R.; Tong, J.; Wenjun, Y.; Wenhu, D.; Xiaojian, Z.; Jiacai, H.; Jian, Z.; Wantao, C.; Chenping, Z. Growth inhibition and induction of apoptosis in human oral squamous cell carcinoma Tca-8113 cell lines by shikonin was partly through the inactivation of NF-kappa B pathway. Phytother. Res 2008, 22, 407–415. [Google Scholar]
- Xiong, W.; Luo, G.; Zhou, L.; Zeng, Y.; Yang, W. In vitro and in vivo antitumor effects of acetylshikonin isolated from Arnebia euchroma (Royle) Johnst (Ruanzicao) cell suspension cultures. Chin. Med 2009, 4, 14. [Google Scholar]
- Kim, S.H.; Kang, I.C.; Yoon, T.J.; Park, Y.M.; Kang, K.S.; Song, G.Y.; Ahn, B.Z. Antitumor activities of a newly synthesized shikonin derivative, 2-hyim-DMNQ-S-33. Cancer Lett 2001, 172, 171–175. [Google Scholar]
- Lee, H.J.; Lee, H.J.; Magesh, V.; Nam, D.; Lee, E.O.; Ahn, K.S.; Jung, M.H.; Ahn, K.S.; Kim, D.K.; Kim, J.Y.; Kim, S.H. Shikonin, acetylshikonin, and isobutyroylshikonin inhibit VEGF-induced angiogenesis and suppress tumor growth in lewis lung carcinoma-bearing mice. Yakugaku Zasshi 2008, 128, 1681–1688. [Google Scholar]
- Yano, H.; Mizoguchi, A.; Fukuda, K.; Haramaki, M.; Ogasawara, S.; Momosaki, S.; Kojiro, M. The herbal medicine sho-saiko-to inhibits proliferation of cancer cell lines by inducing apoptosis and arrest at the G0/G1 phase. Cancer Res 1994, 54, 448–454. [Google Scholar]
- Reed, J.C. Regulation of apoptosis by bcl-2 family proteins and its role in cancer and chemoresistance. Curr. Opin. Oncol 1995, 7, 541–546. [Google Scholar]
- McCurrach, M.E.; Connor, T.M.; Knudson, C.M.; Korsmeyer, S.J.; Lowe, S.W. Bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc. Nat. Acad. Sci. USA 1997, 94, 2345–2349. [Google Scholar]
- Yin, C.; Knudson, C.M.; Korsmeyer, S.J.; Van Dyke, T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature 1997, 385, 637–640. [Google Scholar]


© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fan, Y.; Jin, S.; He, J.; Shao, Z.; Yan, J.; Feng, T.; Li, H. Effect of β,β-Dimethylacrylshikonin on Inhibition of Human Colorectal Cancer Cell Growth in Vitro and in Vivo. Int. J. Mol. Sci. 2012, 13, 9184-9193. https://doi.org/10.3390/ijms13079184
Fan Y, Jin S, He J, Shao Z, Yan J, Feng T, Li H. Effect of β,β-Dimethylacrylshikonin on Inhibition of Human Colorectal Cancer Cell Growth in Vitro and in Vivo. International Journal of Molecular Sciences. 2012; 13(7):9184-9193. https://doi.org/10.3390/ijms13079184
Chicago/Turabian StyleFan, Yingying, Shaoju Jin, Jun He, Zhenjun Shao, Jiao Yan, Ting Feng, and Hong Li. 2012. "Effect of β,β-Dimethylacrylshikonin on Inhibition of Human Colorectal Cancer Cell Growth in Vitro and in Vivo" International Journal of Molecular Sciences 13, no. 7: 9184-9193. https://doi.org/10.3390/ijms13079184



