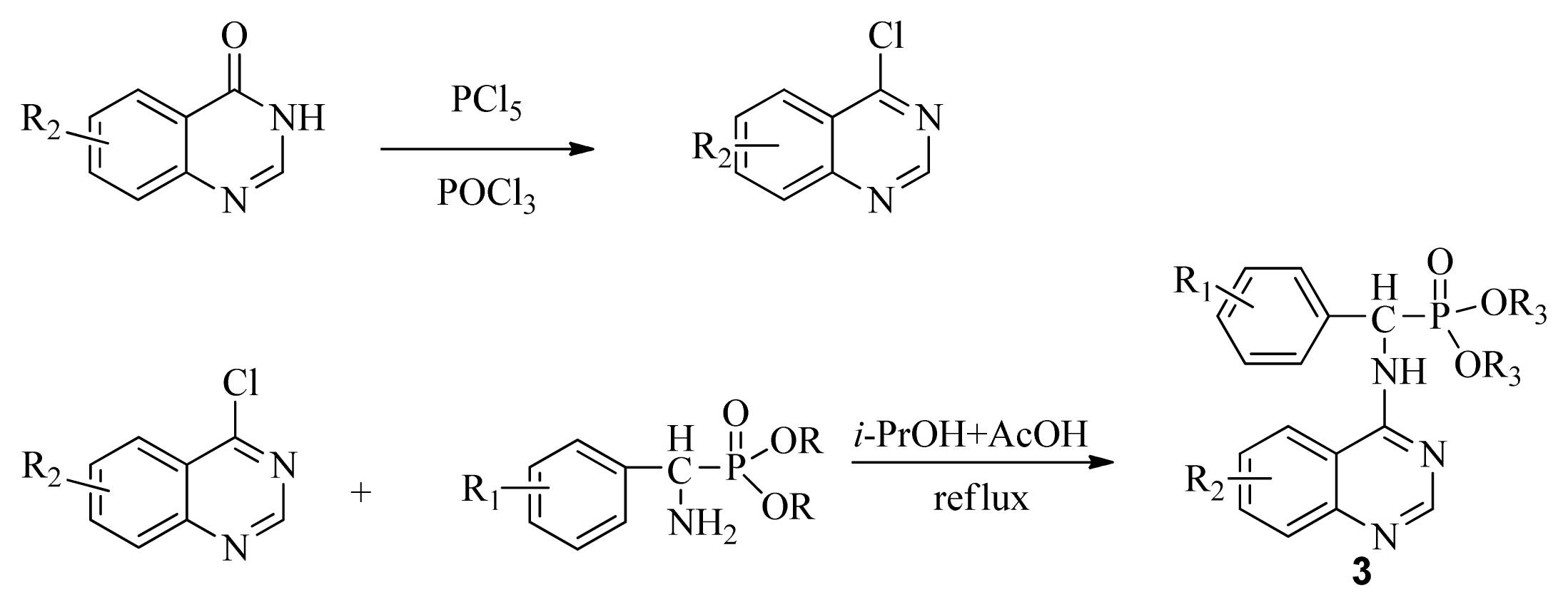
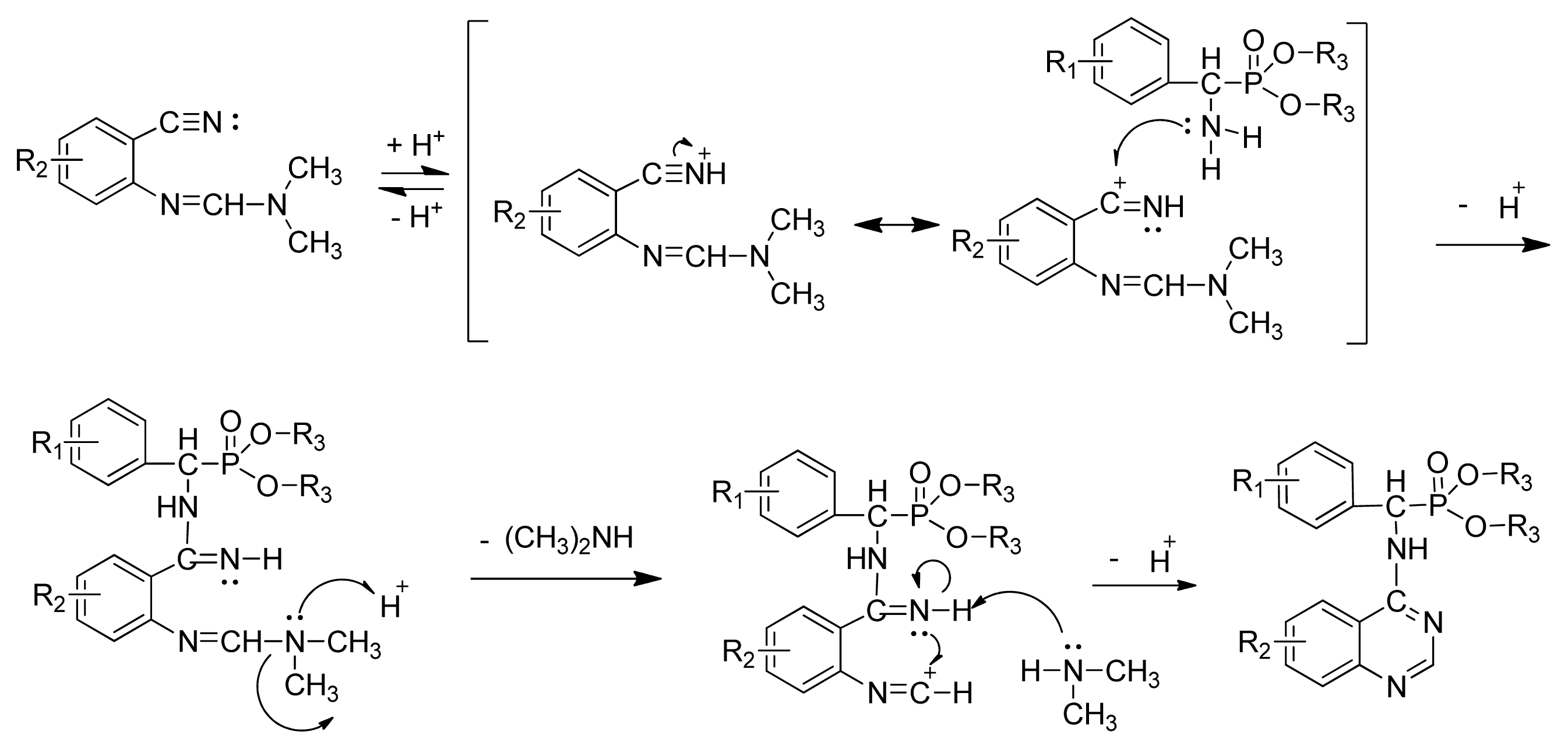
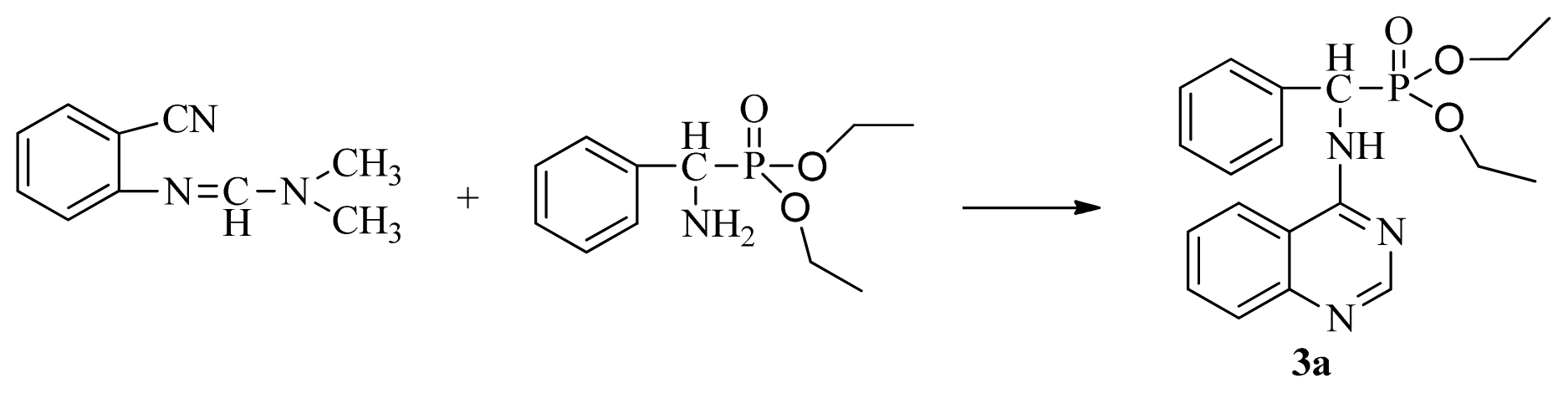
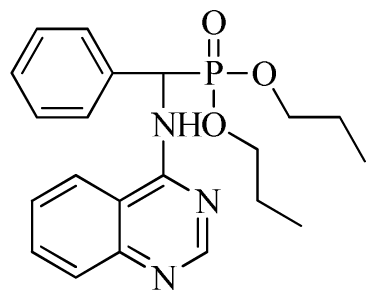
3.3. Preparation of Title Compounds 3a–3x
The intermediates N′-(2-cyanophenyl)-N,N-dimethylformamidine 1 (0.5 mmol) and diethyl amino(phenyl)methylphosphonate (0.5 mmol) were added to a 10 mL microwave reaction vial equipped with a magnetic stir bar. Glacial acetic acid [1 mL; 20% (v/v)] solution in 2-propanol (4 mL) was also added. The mixture was capped and irradiated in the microwave for 20 min at 150 °C then cooled to ambient temperature and purified in parallel using the following procedure. The contents of the vial were poured into single-mouth bottles (50 mL). The reaction vessel was washed with 2-propanol (3 mL × 5 mL), and then 2-propanol was poured into the single-mouth bottles. The solvent was concentrated under reduced pressure, the solid was obtained, and the crude product was purified then preparative TLC with a mixture of petroleum ether and ethyl acetate (v/v = 1:1) as the developing solvent to yield the title compounds 3a to 3x.
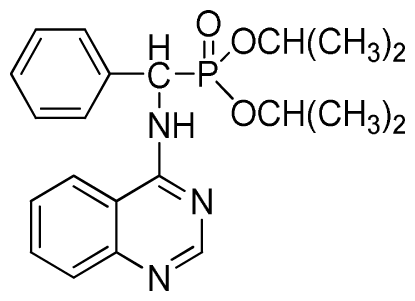
Diethyl phenyl(quinazolin-4-ylamino)methylphosphonate (3a): White solid, yield 78.5%; mp 201 °C to 203 °C; 1H NMR: (CDCl3, 500 MHz): δ 8.65 (s, 1H, quinazoline-2-H), 7.95 (d, J = 8.0 Hz, 1H, quinazoline-8-H), 7.86 (d, J = 8.6 Hz, 1H, quinazoline-5-H), 7.76 to 7.78 (m, 1H, Ar-4-H), 7.51 to 7.61 (m, 2H, quinazoline-6,7-H), 7.26 to 7.37 (m, 4H, Ar-2,3,5,6-H), 6.84 (s, 1H, N-H), 6.12 (d, J = 10.0 Hz, 1H, CH), 3.97 to 4.15 (m, 4H, 2CH2), 1.11 to 1.25 (m, 6H, 2CH3 ); 13C NMR (CDCl3, 125 MHz): δ 155.05, 133.04, 128.78, 128.65, 128.35, 128.28, 128.23, 126.46, 120.90, 77.36, 77.11, 76.86, 63.77, 63.71, 63.29, 52.01, 50.79, 16.48,16.28; 31P NMR(CDCl3, 500 MHz): δ 22.7; IR: ν 3296.76 (NH), 3070.14 (ArH), 1779.78 (CN), 1577.65 (Ar), 1206.79 (P=O), 987.02 (P-O-C) cm−1; Anal. Calcd for C19H22N3O3P: C 61.45%, H 5.97%, N 11.31%; Found. C 61.05%, H 5.93%, N 10.99%.
Di-n-propyl phenyl(quinazolin-4-ylamino)methylphosphonate (3b): White solid, yield 78.0%; mp 173 °C to 175 °C; 1H NMR (CDCl3, 500 MHz): δ 8.65 (s, 1H, quinazoline-2-H), 7.98 d, J = 8.0 Hz, 1H, quinazoline-8-H), 7.84 (d, J = 8.1 Hz, 1H, quinazoline-5-H), 7.74 to 7.77 (m, 1H, Ar-4-H), 7.61 to 7.63 (m, 2H, quinazoline-6,7-H), 7.26 to 7.34 (m, 4H, Ar-2,3,5,6-H), 6.79 (s, 1H, N-H), 6.18 (d, J = 20.0 Hz, 1H, CH), 3.63 to 4.07 (m, 4H, 2OCH2), 1.47 to 1.61 (m, 4H, 2CH2), 0.78 to 0.83 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 158.66, 158.59, 155.02, 149.69, 135.46, 132.84, 128.65, 128.61, 128.27, 128.22, 126.24, 120.95, 68.93, 68.60, 51.88, 50.65, 23.70, 23.66, 9.90, 9.82; 31P NMR(CDCl3, 500 MHz): δ 22.6; IR: ν 3263.64 (NH), 2968.56 (ArH), 1714.78 (CN), 1579.72 (Ar), 1238.36 (P=O), 1012.24 (P-O-C) cm−1; Anal. Calcd for C21H26N3O3P: C 63.15%, H 6.56%, N 10.52%; Found. C 63.19%, H 6.22%, N 10.63%.
Diisopropyl phenyl(quinazolin-4-ylamino)methylphosphonate (3c): White solid, yield 78.1%; mp 188 °C to 190 °C; 1H NMR (CDCl3, 500 MHz): δ 8.65 (s, 1H, quinazoline-2-H), 8.00 (d, J = 8.6 Hz, 1H, quinazoline-8-H), 7.85 (d, J = 8.0 Hz, 1H, quinazoline-5-H), 7.73 to 7.77 (m, 1H, Ar-4-H), 7.47 to 7.50 (m, 2H, quinazoline-6,7-H), 7.26 to 7.48 (m, 5H, Ar-2,3,5,6-H), 7.02 (s, 1H, N-H), 6.10 (d, J = 9.2 Hz, 1H, CH), 4.47 to 4.79 (m, 2H, 2CH), 0.89 to 1.31 (m, 12H, 4CH3); 13C NMR (CDCl3, 125 MHz): δ 158.77, 158.70, 155.10, 149.68, 132.78, 128.57, 128.52, 128.46, 128.41, 128.05, 126.21, 120.95, 71.99, 58.34, 52.48, 51.24, 24.28, 24.26, 24.16, 24.13; 31P NMR(CDCl3, 200 MHz): δ 20.9; IR: ν 3275.15 (NH), 2980.07 (ArH), 1620.24 (CN), 1577.85 (Ar), 1238.36 (P=O), 987.68 (P-O-C) cm−1; Anal. Calcd for C21H26N3O3P: C 63.15%, H 6.56%, N 10.52%; Found. C 63.04%, H 6.36%, N 10.62%.
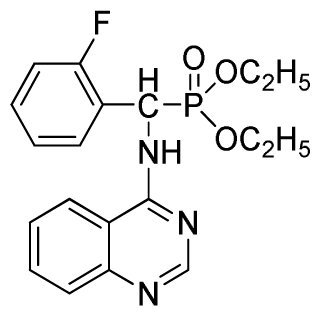
Diethyl(2-fluorophenyl)(quinazolin-4-ylamino)methylphosphonate (3d): White solid, yield 77.8%; mp 163 °C to 165 °C; 1H NMR (CDCl3, 500 MHz): δ 8.65 (s, 1H, quinazoline-2-H), 7.95 (d, J = 10.0 Hz, 1H, Ar-3-H), 7.85 (d, J = 15.0 Hz, 1H, quinazoline-8-H), 7.76 to 7.79 (m, 1H, quinazoline-5-H), 7.51 to 7.61 (m, 2H, quinazoline-6,7-H), 7.26 to 7.38 (m, 4H, Ar-4, 5, 6-H), 6.84 (s, 1H, NH), 6.16 (d, J = 10.0 Hz, 1H, CH), 4.15 to 4.17 (m, 2H, CH2), 3.98 to 4.14 (m, 2H, CH2), 1.11 to 1.27 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 161.92, 159.94, 158.67 (d, 1JCF = 246.5 Hz), 155.15, 149.78, 133.05, 129.57 (d, 2JCF = 23.8 Hz), 128.75 (d, 3JCF = 7.7 Hz), 126.46 (d, 3JCF = 7.6 Hz), 124.53, 121.15, 115.92 (d, 2JCF = 24.2 Hz), 115.13, 63.84, 63.77, 46.49, 45.14, 16.43, 16.26; 31P NMR (CDCl3, 200 MHz): δ 21.7; IR: ν 3304.06 (NH), 2987.74 (CH3), 1577.74, 1525.69, 1409.96 (C=C), 1361.74 (CH3), 1234.44 (P=O), 1228.2 (P-O-C), 804.32, 769.60 (=C–H) cm−1; Anal. Calcd for C19H21FN3O3P: C 58.61%; H, 5.44%, N 10.79%; Found. C 58.42%, H 5.33%, N 10.58%.
Di-n-propyl(2-fluorophenyl)(quinazolin-4-ylamino)methylphosphonate (3e): White solid, yield 77.5%; mp 111 °C to 113 °C; 1H NMR (DMSO-d6, 500 MHz): δ 9.05 (d, J = 6.0 Hz, 2H, quinazoline-2-H, Ar- 3-H), 7.85 to 8.10 (m, 4H, quinazoline-5,6,7,8-H), 7.35 (s, 1H, NH), 7.31 to 7.33 (m, 3H, Ar-4,5,6-H), 6.85 (d, J = 20.0 Hz, CH), 3.98 to 4.02 (m, 4H, 2-OCH2), 1.49 to 1.51 (m, 4H, 2-CH2), 1.46 to 1.51 (m, 4H, 2CH2), 0.74 to 0.78 (m, 3H, J = 7.5 Hz, CH3), 0.73 to 0.75 (m, H, 2CH3), 0.71 (t, 3H, J = 7.5 Hz, CH3); 13C NMR (DMSO-d6, 125 MHz): δ 161.30, 151.85 (d, 1JCF = 245.8 Hz), 136.64, 131.53, 131.15, 128.92 (d, 2JCF =24.2 Hz), 125.34 (d, 3JCF = 7.5 Hz), 125.15 (d, 3JCF = 7.5 Hz), 121.87, 121.76, 121.12, 115.91 (d, 2JCF =23.6 Hz), 115.57, 113.70, 68.71, 68.65, 45.74, 23.88, 23.84, 10.24, 10.19; 31P NMR(DMSO-d6, 200 MHz): δ 19.5; IR: ν 3294.05 (NH), 2977.74 (CH3), 1571.17, 1520.61, 1412.12 (C=C) 1360.12 (CH3), 1234.44 (P=O), 1228.2 (P-O-C) cm−1; Anal. Calcd for C21H25FN3O3P: C 60.43%, H 6.04%, N 10.07%; Found. C 60.14%, H 6.16%, N 10.42%.
Diisopropyl (2-fluorophenyl)(quinazolin-4-ylamino)methylphosphonate (3f): White solid, yield 77.5%; mp 111 °C to 113 °C; 1H NMR (DMSO-d6, 500 MHz): δ 9.05 (d, J = 6.0 Hz, 2H, quinazoline-2-H, Ar- 3-H), 7.85 to 8.10 (m, 4H, quinazoline-5,6,7,8-H), 7.35 (s, 1H, NH), 7.31 to 7.33 (m, 3H, Ar-4,5,6-H), 6.85 (d, J = 20.0 Hz, CH), 3.98 to 4.02 (m, 4H, 2-OCH2), 1.49 to 1.51 (m, 4H, 2-CH2), 1.46 to 1.51 (m, 4H, 2CH2), 0.74 to 0.78 (m, 3H, J = 7.5 Hz, CH3), 0.73 to 0.75 (m, H, 2CH3), 0.71 (t, 3H, J = 7.5 Hz, CH3); 13C NMR (DMSO-d6, 125 MHz): δ 161.30, 151.85 (d, 1JCF = 246.5 Hz), 136.64, 131.53, 131.15, 128.92 (d, 2JCF = 24.6 Hz), 125.34 (d, 3JCF = 7.5 Hz), 125.15 (d, 3JCF = 7.5 Hz), 121.87, 121.76, 121.12, 115.91 (d, 2JCF = 23.6 Hz), 115.57, 113.70, 68.71, 68.65, 45.74, 23.88, 23.84, 10.24, 10.19; 31P NMR(DMSO-d6, 200 MHz): δ 19.5; IR: ν 3294.05 (NH), 2977.74 (CH3), 1571.17, 1520.61, 1412.12 (C=C) 1360.12 (CH3), 1234.44 (P=O), 1228.2 (P-O-C) cm−1; Anal. Calcd for C21H25FN3O3P: C 60.43%, H 6.04%, N 10.07%; Found. C 60.14%, H 6.16%, N 10.42%.
Diethyl (6-nitroquinazolin-4-ylamino)(phenyl)methylphosphonate (3g): White solid, yield 85.9%; mp 132 °C to 134 °C; 1H NMR (CDCl3, 500 MHz): δ 8.62 (s, 1H, quinazoline-2-H), 8.30 (s, 1H, quinazoline-5-H), 8.05 (s, 1H, NH), 7.70 to 7.74 (m, 2H, quinazoline-7,8-H), 7.26~7.69 (m, 5H, Ar-2,3,4,5,6-H), 6.36 (d, J = 10 Hz, CH), 3.85 to 4.23 (m, 4H, 2-CH2), 1.16 to 1.24 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 158.46, 1H, 155.20, 135.24, 133.60, 131.47, 129.93, 128.66, 128.19, 121.55, 116.20, 119.13, 98.37, 63.72, 52.01, 50.81, 16.52, 16.47; 31P NMR(CDCl3, 200 MHz): δ 22.1; IR: ν 3435.28 (NH), 3134.83 (ArH), 1713.74 (CN), 1571.31 (Ar), 1227.79 (P=O), 1001.53 (P-O-C) cm−1; Anal. Calcd for C19H21N4O5P: C 54.81%, H 5.08%, N 13.46%; Found. C 54.62%, H 5.13%, N 13.66%.
Dipropyl (6-nitroquinazolin-4-ylamino)(phenyl)methylphosphonate (3h): Yellow solid, yield 85.7%; mp 196 °C to 198 °C; 1H NMR (CDCl3, 500 MHz): δ 8.62 (s, 1H, quinazoline-2-H), 8.73 (s, 1H, quinazoline-5-H), 8.13 (s, 1H, NH), 7.64 to 7.81 (m, 2H, quinazoline-7,8-H), 7.27 to 7.62 (m, 5H, Ar-2,3,4,5,6-H), 6.28 (d, 1H, J = 10 Hz, CH), 3.77 to 4.22 (m, 4H, 2-OCH2), 1.79 to 2.18 (m, 4H, 2CH2), 1.13 to 1.26 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 158.39, 158.33, 155.65, 145.42, 134.87, 133.22, 130.86, 128.68, 128.45, 128.40, 128.30, 120.09, 76.69, 63.81, 63.42, 63.36, 52.23, 50.90, 30.97, 16.44, 16.39; 31P NMR(CDCl3, 200 MHz): δ 21.6; IR: ν 3281.12 (NH), 2989.66 (–CH), 1630.35 (C=N), 1565.56, 1515.33, 1470.85, 1448.21 (Ar), 1240.13 (P=O) cm−1; Anal. Calcd for C21H25N4O5P: C 56.75%, H 5.67%, N 12.61%; Found. C 56.55%, H 5.58%, N 12.55%.
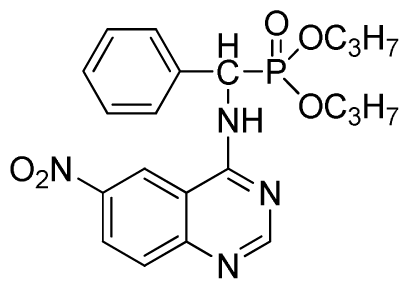
Dipropyl (6-nitroquinazolin-4-ylamino)(phenyl)methylphosphonate (3i): Yellow solid, yield 85.6%; mp 43 °C to 45 °C; 1H NMR (CDCl3, 500 MHz): δ 8.61 (s, 1H, quinazoline-2-H),7.92 (s, 1H, quinazoline-5-H),7.89 (s, 1H, NH), 7.67 to 7.68 (m, 2H, quinazoline-7,8-H), 7.25 to 7.64 (m, 5H, Ar-2,3,4,5,6-H), 6.22 (d, 1H, J = 10 Hz, CH), 4.58 to 4.84 (m, 4H, 2-OCH), 1.15 to 1.31 (m, 6H, 4CH3); 13C NMR (CDCl3, 125 MHz): δ 160.94, 158.93, 158.87, 154.56, 146.60, 135.73, 130.82, 128.77, 128.67, 128.49, 127.96, 122.35, 122.14, 106.34, 106.16, 72.52, 52.58, 51.36, 24.25, 24.18; 31P NMR(CDCl3, 200 MHz): δ 20.0; IR: ν 3277.06 (NH), 2980.02 (CH), 1614.42 (C=N), 1573.91, 1527.62, 1490.97, 1456.26(Ar), 1224.80 (P=O) cm−1; Anal. Calcd for C21H25N4O5P: C 56.75%, H 5.67%, N 12.61%; Found. C 56.61%, H 5.82%, N 12.83%.
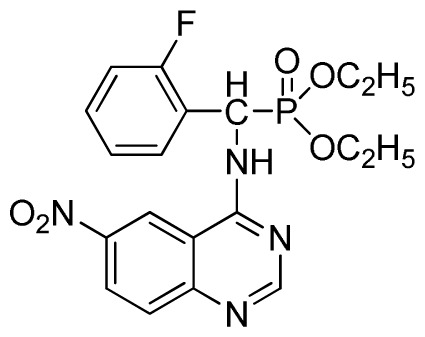
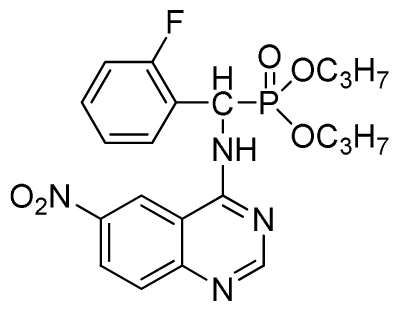
Diethyl (2-fluorophenyl)(6-nitroquinazolin-4-ylamino)methylphosphonate (3j): Yellow solid, yield 85.2%; mp 176 °C to 178 °C; 1H NMR (DMSO-d6, 500 MHz): δ 9.84 (s, 1H, quinazoline-2-H), 9.68 to 9.70 (d, J = 8.0 Hz, 1H, Ar-3-H), 8.67 (s, 1H, quinazoline-5-H), 8.04 to 8.50 (m, 2H, quinazoline-7, 8-H), 7.8 (s, 1H, NH), 7.20 to 7.35 (m, 3H, Ar-4,5,6-H), 6.70 (d, J = 8.0 Hz, 1H, CH), 3.90 to 4.04 (m, 4H, 2CH2), 1.01 to 1.07 (m, 6H, 2CH3); 13C NMR (DMSO-d6, 125 MHz): δ 160.57, 160.51, 158.12 (d, 1JCF = 246.4 Hz), 153.43, 145.03, 131.26, 129.89 (d, 2JCF = 24.8 Hz), 127.36 (d, 3JCF = 7.4 Hz), 125.06 (d, 3JCF = 7.5 Hz), 121.82, 115.78 (d, 2JCF = 23.5 Hz), 114.53, 63.48, 63.42, 44.73, 43.45, 39.98, 16.78, 16.45; 31P NMR(DMSO-d6, 200 MHz): δ 20.8; IR: ν 3259.70 (NH), 1575.84, 1525.69, 1490.97, 1456.26 (Ar), 2989.66 (–CH), 1622.13 (C=N), 1234.44 (P=O) cm−1; Anal. Calcd for C19H20FN4O5P: C 52.54%; H 4.64%; N 12.90%; Found. C 52.41%, H 4.59%, N 12.86%.
Dipropyl (2-fluorophenyl)(6-nitroquinazolin-4-ylamino)methylphosphonate (3k): Yellow solid, yield 85.0%; mp 168 °C to 170 °C; 1H NMR (CDCl3, 500 MHz): δ 9.30 (s, 1H, quinazoline-2-H), 8.73 to 8.75 (d, J = 10.0 Hz, 1H, Ar-3-H), 8.65 (s, 1H, quinazoline-5-H), 7.89 to 8.47 (m, 2H, quinazoline-7,8- H), 7.80 (s, 1H, NH), 7.09 to 7.27 (m, 3H, Ar-4,5,6-H), 6.81 (d, J = 15.0 Hz, 1H, CH), 4.14 to 4.19 (m, 4H, 2OCH2), 1.54 to 1.62 (m, 4H, 2CH2), 0.82 to 0.85 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 161.53, 160.16, 159.65 (d, 1JCF = 245.8 Hz), 158.03, 153.25, 144.74, 130.27, 129.91 (d, 2JCF = 24.8 Hz), 126.52 (d, 3JCF = 7.8 Hz), 124.56 (d, 3JCF = 7.5 Hz), 122.63, 119.92, 115.58 (d, 2JCF = 22.8 Hz), 114.69, 69.34, 45.37, 44.09, 23.72, 9.93; 31P NMR(CDCl3, 200 MHz): δ 20.2; IR: ν 3230.34 (NH), 1567.34, 1523.22, 1493.17, 1452.56 (Ar), 2990.05 (–CH), 1625.02 (C=N) 1230.48 (P=O) cm−1; Anal. Calcd for C21H24FN4O5P: C 54.55%, H 5.23%, N 12.12%; Found. C 54.43%, H 5.32%, N 12.20%.
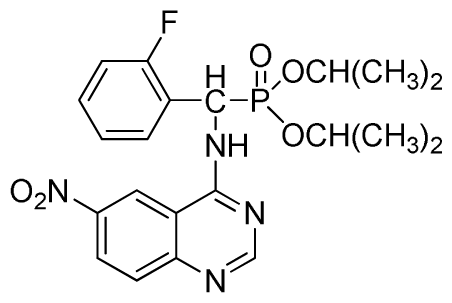
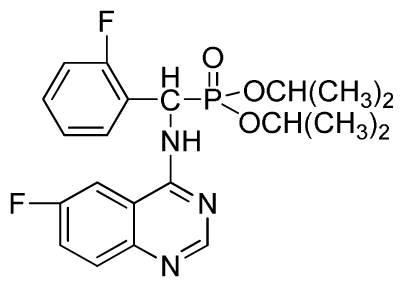
Diisopropyl(2-fluorophenyl)(6-nitroquinazolin-4-ylamino)methylphosphonate (3l): Yellow solid, yield 83.9%; mp 141 °C to 142 °C; 1H NMR (CDCl3, 500 MHz): δ 9.14 (s, 1H, quinazoline-2-H), 8.74 (d, J = 8.5 Hz, 1H, Ar-3-H), 8.50 (s, 1H, quinazoline-5-H), 7.91 to 7.92 (m, 2H, quinazoline-7, 8-H), 7.25 (s, 1H, NH), 7.07 to 7.12 (m, 3H, Ar-4,5,6-H), 6.56 (d, J = 10.0 Hz, 1H, CH), 4.58 to 4.83 (m, 4H, 2OCH), 0.92 to 1.29 (m, 12H, 4CH3); 13C NMR (CDCl3, 125 MHz): δ 161.80, 159.80, 158.14 (d, 1JCF = 246.2 Hz), 153.19, 144.87, 130.15, 129.88, 129.73 (d, 2JCF = 24.5 Hz), 126.61 (d, 3JCF = 7.9 Hz), 124.53 (d, 3JCF = 7.6 Hz), 122.96, 119.18, 115.58 (d, 2JCF = 23.5 Hz), 114.36, 73.15, 46.71, 45.43, 24.20, 24.11, 23.91, 23.17; 31P NMR(CDCl3, 200 MHz): δ 18.8; IR: ν 3255.84 (NH), 1577.77, 1527.62, 1490.97, 1456.26 (Ar), 2981.95 (–CH), 1622.13 (C=N), 1236.37 (P=O) cm−1; Anal. Calcd For C21H24FN4O5P: C 54.55%, H 5.23%, N 12.12%; Found. C 54.62%, H 5.18%, N 11.98%.
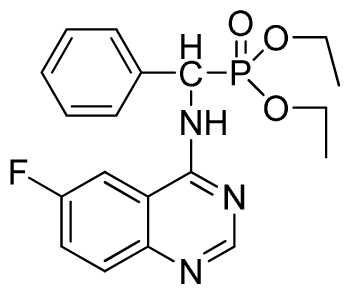
Diethyl (6-fluoroquinazolin-4-ylamino)(phenyl)methylphosphonate (3m): White solid, yield 86.1%; mp >300 °C; 1H NMR (CDCl3, 500 MHz): δ 8.62(s, 1H, quinazoline-2-H), 7.85 (s, 1H, quinazoline-5-H), 7.66 to 7.80 (m, 2H, quinazoline-7,8-H), 7.65 (s, 1H, N-H), 7.24 to 7.51 (m, 5H, Ar-2,3,4,5,6-H), 6.24 (d, J = 10.0 Hz, 1H, C-H), 4.03 to 4.22 (m, 4H, 2CH2), 1.14 to 1.26 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 160.97 (d, 1JCF = 243.8 Hz), 158.60, 154.41, 146.73, 135.24 (d, 3JCF = 8.0 Hz), 128.60, 128.35, 122.46 (d, 2JCF = 24.5 Hz), 122.27 (d, 3JCF = 7.9 Hz), 106.09 (d, 2JCF = 22.8 Hz), 77.27, 76.76, 63.74, 63.63, 63.31, 63.25, 52.00, 16.43, 16.10; 31P NMR(CDCl3, 200 MHz): δ 22.5; IR: ν 3267.4 (NH), 3068.8 (ArH), 1629.9 (CN), 1232.7 (P=O), 1022.2 (P-O-C) cm−1; Anal. Calcd for C19H21FN3O3P: C 58.61%, H 5.44%, N 10.97%; Found. C 58.77%, H 5.63%, N 10.92%.
Di-n-propyl(6-fluoroquinazolin-4-ylamino)(phenyl)methylphosphonate (3n): White solid, yield 86.1%; mp 186 °C to 188 °C; 1H NMR (CDCl3, 500 MHz): δ 8.62 (s, 1H, quinazoline-2-H), 8.20 (s, 1H, quinazoline-5-H N-H), 7.82 to 8.10 (m, 2H, quinazoline-7,8-H), 7.80 (s, 1H, N-H), 7.23 to 7.72 (m, 5H, Ar-2,3,4,5,6-H), 6.46 (d, J = 8.0 Hz, 1H, C-H), 3.99 to 4.14 (m, 4H, 2OCH2), 1.56 to 1.57 (m, 4H, 2CH2), 0.78~0.85 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 160.80 (d, 1JCF = 246.2 Hz), 159.05, 158.84, 154.40, 146.79, 135.38, 130.59 (d, 3JCF = 8.1 Hz), 130.52, 128.67, 128.62, 128.45, 127.96, 122.02 (d, 2JCF = 24.8 Hz), 106.96 (d, 3JCF = 7.9 Hz), 106.78 (d, 2JCF = 23.2 Hz), 68.89, 68.83, 51.86, 50.61, 23.86, 23.68; 31P NMR(CDCl3, 200 MHz): δ 22.1; IR: ν 3261.6 (NH), 2968.5 (ArH), 1714.7 (CN), 1236.4 (P=O), 1012.2 (P-O-C) cm−1; Anal. Calcd for C21H25FN3O3P: C 60.43%, H 6.04%, N 10.07%; Found. C 60.33%, H 5.95%, N 10.12%.
Diisopropyl (6-fluoroquinazolin-4-ylamino)(phenyl)methylphosphonate (3o): White solid, yield 85.3%; mp 204 °C to 206 °C; 1H NMR (CDCl3, 500 MHz): δ 8.63 (s, 1H, quinazoline-2-H), 8.13 (s, 1H, quinazoline-5-H), 7.77 to 8.12 (m, 2H, quinazoline-7,8-H), 7.80 (s, 1H, N-H), 7.27 to 7.75 (m, 5H, Ar-2,3,4,5,6-H), 6.17 (d, J = 10.0 Hz, 1H, C-H), 4.57 to 4.82 (m, 2H, 2CH), 1.16 to 1.31 (m, 12H, 4CH3); 13C NMR (CDCl3, 125 MHz): δ 160.92 (d, 1JCF = 245.4 Hz), 158.94, 158.76, 154.48, 146.76, 135.66, 130.8 (d, 3JCF = 8.0 Hz), 128.69, 128.64, 128.43, 127.95, 122.35 (d, 2JCF = 23.8 Hz), 122.15 (d, 3JCF = 8.0 Hz), 106.28, 106.09 (d, 2JCF = 23.4 Hz), 72.48, 52.55, 51.30, 24.20, 24.18, 24.15; 31P NMR(CDCl3, 200 MHz): δ 20.5; IR: ν 3288.6 (NH), 2985.8 (ArH), 1629.9 (CN), 1224.7 (P=O), 987(P-O-C) cm−1; Anal. Calcd for C21H25FN3O3P: C 60.43%, H 6.04%, N 10.07%; Found. C 60.21%, H 5.94%, N 9.85%.
Diethyl (2-fluorophenyl)(6-fluoroquinazolin-4-ylamino)methylphosphonate (3p): White solid, yield 85.3%; mp 142 °C to 144 °C; 1H NMR (CDCl3, 500 MHz): δ 8.61 (s, 1H, quinazoline-2-H), 8.03 (s, 1H, quinazoline-5-H), 7.79 to 7.82 (m, 1H, Ar-3-H), 7.68~7.70 (m, 2H, quinazoline-7,8-H), 7.46 (brs, 1H, N-H), 7.23 to 7.26 (m, 3H, Ar-4,5,6-H), 6.35 (d, J = 10.0 Hz, 1H, C-H), 3.83 to 4.22 (m, 4H, 2CH2), 1.16 to 1.23 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 160.94 (d, 1JCF = 244.5 Hz), 158.97 (d, 1JCF = 245.2 Hz), 154.48, 146.84, 135.32 (d, 3JCF = 8.0 Hz), 130.61(d, 2JCF = 24.8 Hz), 130.71(d, 2JCF = 24.5 Hz), 128.65 (d, 3JCF = 7.8 Hz), 128.12 (d, 3JCF = 7.6 Hz), 122.39 (d, 3JCF = 7.6 Hz), 106.77 (d, 2JCF = 23.2 Hz), 77.10, 63.47, 63.41, 51.98, 50.74, 16.50, 16.21, 16.17; 31P NMR(CDCl3, 200 MHz): δ 21.8; IR: ν 3265.5 (NH), 2985.8-3068.7 (ArH), 1714.7 (CN), 1244.9 (P=O), 1022.2 (P-O-C) cm−1; Anal. Calcd for C19H20F2N3O3P: C 56.02%, H 4.95%, N 10.32%; Found. C 56.26%, H 4.91%, N 9.89%.
Di-n-propyl (2-fluorophenyl)(6-fluoroquinazolin-4-ylamino)methylphosphonate (3q): White solid, yield 85.3%; mp 213 °C to 214 °C; 1H NMR (CDCl3, 500 MHz): δ 8.72 (s, 1H, quinazoline-2-H), 8.13 (s, 1H, quinazoline-5-H), 7.78 to 7.81 (m, 1H, Ar-3-H), 7.63 to 7.66 (m, 2H, quinazoline-7,8-H), 7.39 (s, 1H, N-H), 7.25 to 7.27 (m, 3H, Ar-4,5,6-H), 6.28 (d, J = 10.0 Hz, 1H, C-H), 3.92 to 4.10 (m, 4H, 2OCH2), 1.49 to 1.81 (m, 4H, 2CH2), 0.78 to 0.81 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 158.50 (d, 1JCF = 245.2 Hz), 155.71 (d, 1JCF = 244.5 Hz), 145.51, 133.29 (d, 3JCF = 7.8 Hz), 128.75 (d, 2JCF = 24.3 Hz), 128.55 (d, 3JCF = 7.6 Hz), 128.51 (d, 3JCF = 7.8 Hz), 128.34 (d, 2JCF = 23.8 Hz), 120.23 (d, 3JCF = 7.7 Hz), 116.85 (d, 2JCF = 22.5 Hz), 77.11, 69.24, 68.88, 52.26, 51.02, 23.93, 23.69, 9.99, 9.94; 31P NMR(CDCl3, 200 MHz): δ 21.7; IR: ν 3271.33 (NH), 2985.85 to 3070.72 (ArH), 1629.86 (CN), 1246.07 (P=O), 1020.34 (P-O-C) cm−1; Anal. Calcd for C21H24F2N3O3P: C 57.93%, H 5.56%, N 9.65%; Found. C 58.39%, H 5.27%, N 9.80%.
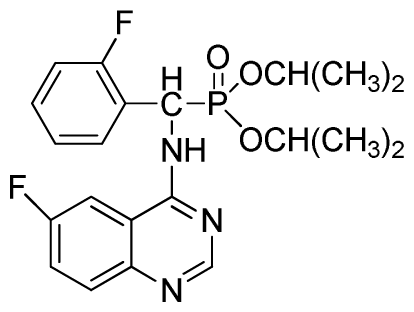
Diisopropyl(2-fluorophenyl)(6-fluoroquinazolin-4-ylamino)methylphosphonate (3r): White solid, yield 83.7%; mp 251 °C to 253 °C; 1H NMR (CDCl3, 500 MHz): δ 8.60 (s, 1H, quinazoline-2-H), 8.03(s, 1H, quinazoline-5-H), 7.80 to 7.82 (m, 1H, Ar-3-H), 7.65 to 7.69 (m, 2H, quinazoline-7,8-H), 7.43 (brs, 1H, N-H), 7.21 to 7.43 (m, 3H, Ar-4,5,6-H), 6.30 (d, J = 10.0 Hz, 1H, C-H), 4.60 to 4.86 (m, 2H, 2OCH), 1.15 to 1.32 (m, 12H, 4CH3); 13C NMR (CDCl3, 125 MHz): δ 160.90 (d, 1JCF = 245.6 Hz), 158.94 (d, 1JCF = 244.6 Hz), 154.57, 154.56, 146.84, 135.76 (d, 3JCF = 7.8 Hz), 130.74 (d, 2JCF = 25.8 Hz), 128.91 (d, 2JCF = 24.8 Hz), 128.87 (d, 3JCF = 7.5 Hz), 128.40 (d, 3JCF = 7.6 Hz), 127.95 (d, 2JCF = 23.8 Hz), 122.32 (d, 3JCF = 7.4 Hz), 106.83 (d, 2JCF = 22.5 Hz), 77.12, 72.54, 72.09, 52.55, 51.30, 24.24, 23.84, 23.23; 31P NMR(CDCl3, 200 MHz): δ 21.2; IR: ν 3270.21 (NH), 2981.93 to 3072.64 (ArH), 1629.58 (CN), 1238.43 (P=O), 993.32 (P-O-C) cm−1; Anal. Calcd for C21H24F2N3O3P: C 57.93%, H 5.56%, N 9.65%; Found. C 58.06%, H 5.86%, N9.66%.
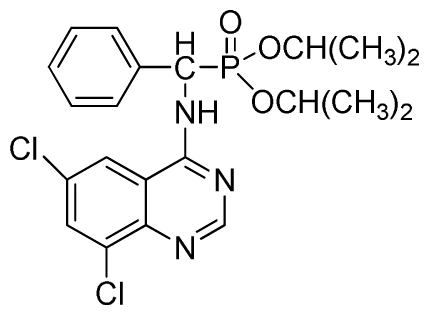
Diethyl(6,8-dichloroquinazolin-4-ylamino)(phenyl)methylphosphonate (3s): White solid, yield 86.0%; mp 178 °C to 180 °C; 1H NMR (CDCl3, 500 MHz): δ 8.73 (s, 1H, quinazoline-2-H), 8.13 (s, 1H, quinazoline-7-H), 7.81 (s, quinazoline-5-H), 7.74 (brs, 1H, N-H), 7.27 to 7.63 (m, 5H, Ar-2,3,4,5,6-H), 6.26 (d, J = 10.0 Hz, 1H, C-H), 3.76 to 4.26 (m, 4H, 2CH2), 1.13 to 1.26 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 158.39, 155.33, 155.65, 145.43, 134.87, 133.23, 130.86, 128.68, 128.45, 128.40, 128.29, 63.81, 63.42, 63.36, 52.23, 50.90, 30.96, 16.44, 16.40; 31P NMR(CDCl3, 200 MHz): δ 22.9; IR: ν 3275.18 (NH), 3072.64 (ArH), 1604.82 (CN), 1228.75 (P=O), 1022.27 (P-O-C) cm−1; Anal. Calcd for C19H20Cl2N3O3P: C 51.83%, H 4.58%, N 9.54%; Found. C 52.06%, H 4.71%, N 9.67%.
Dipropyl(6,8-dichloroquinazolin-4-ylamino)(phenyl)methylphosphonate (3t): White solid, yield 85.7%; mp 150 °C to 152 °C; 1H NMR (CDCl3, 500 MHz): δ 8.73 (s, 1H, quinazoline-2-H), 8.23 (s, 1H, quinazoline-7-H), 8.13 (brs, 1H, N-H), 7.79 (s, 1H, quinazoline-5-H), 7.25 to 7.68 (m, 5H, Ar-2,3,4,5,6-H), 6.35 (d, J = 10.0 Hz, 1H, C-H), 3.97 to 4.14 (m, 4H, 2OCH2), 1.52 to 1.61 (m, 4H, 2CH2), 0.81 to 0.82 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 158.55, 155.64, 145.47, 134.98, 133.16, 128.61, 128.57, 128.52, 128.19, 120.41, 116.89, 69.05, 68.91, 52.17, 50.93, 23.86, 23.62, 9.90, 19.88; 31P NMR(CDCl3, 200 MHz): δ 21.7; IR: ν 3275.18 (NH), 3072.66 (ArH), 1714.79 (CN), 1224.81 (P=O), 1031.26 (P-O-C) cm−1; Anal. Calcd for C21H24Cl2N3O3P: C 53.86%, H 5.17%, N 8.97%; Found. C 53.39%, H 5.27%, N 8.80%.
Diisopropyl (6,8-dichloroquinazolin-4-ylamino)(phenyl)methylphosphonate (3u): White solid, yield 85.2%; mp 187 °C to 190 °C; 1H NMR (CDCl3, 500 MHz): δ 8.73 (s, 1H, quinazoline-2-H), 8.05 (s, 1H, quinazoline-7-H), 7.82 (s, 1H, quinazoline-5-H), 7.64 (s, 1H, N-H), 7.27 to 7.43 (m, 5H, Ar-2,3,4,5,6-H), 6.16 (d, J = 10.0 Hz, 1H, C-H), 4.53 to 4.78 (m, 2H, 2OCH), 0.88 to 1.29 (m, 12H, 4CH3); 13C NMR (CDCl3, 125 MHz): δ 158.31, 158.24, 155.71, 145.42, 133.25, 128.59, 128.54, 128.21, 119.68, 116.64, 72.86, 60.40, 52.86, 51.61, 24.25, 24.23, 24.12, 23.87, 14.20; 31P NMR(CDCl3, 200 MHz): δ 20.2; IR: ν 3275.18 (NH), 3271.3 (NH), 3076.5 (ArH), 1730.2 (CN), 1238.7 (P=O), 1022.2 (P-O-C) cm−1; Anal. Calcd for C21H24Cl2N3O3P: C 53.86%, H 5.17%, N 8.97%; Found. C 53.92%, H 5.29%, N 8.33%.
Diethyl(6,8-dichloroquinazolin-4-ylamino)(2-fluorophenyl)methylphosphonate (3v): White solid, yield 84.5%; mp 128 °C to 130 °C; 1H NMR (CDCl3, 500 MHz): δ 8.61 (s, 1H, quinazoline-2-H), 7.96 (s, 1H, N-H), 7.79 to 7.84 (m, 2H, quinazoline-5,7-H), 7.23 to 7.27 (m, 4H, Ar-H), 6.35 (d, J = 10.0 Hz, 1H, C-H), 3.82 to 4.22 (m, 4H, 2CH2), 1.16 to 1.22 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 160.96 (d, 1JCF = 244.8 Hz), 158.99, 154.48, 146.83, 135.32, 130.82, 130.75(d, 2JCF = 24.8 Hz), 128.61 (d, 3JCF = 7.6 Hz), 128.15 (d, 3JCF = 7.4 Hz), 122.42 (d, 2JCF = 22.8 Hz), 106.69, 77.10, 63.59, 63.46, 51.99, 50.75, 16.50, 16.46, 16.22; 31P NMR(CDCl3, 200 MHz): δ 22.3; IR: ν 3267.45 (NH), 2947.43~3068.76 (ArH), 1629.82 (CN), 1246.19 (P=O), 1022.37 (P-O-C) cm−1; Anal. Calcd for C19H19Cl2FN3O3P: C 49.80%, H 4.18%, N 9.17%; Found. C 50.26%, H 4.61%, N 9.62%.
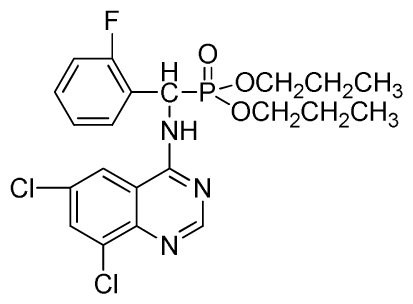
Dipropyl (6,8-dichloroquinazolin-4-ylamino)(2-fluorophenyl)methylphosphonate (3w): White solid, yield 83.4%; mp 178 °C to 180 °C; 1H NMR (CDCl3, 500 MHz): δ 8.72 (s, 1H, quinazoline-2-H), 8.12 (s, 1H, N-H), 7.80 (s, 1H, quinazoline-7-H), 7.63 (s, 1H, quinazoline-5-H), 7.26 to 7.29 (m, 4H, Ar-3,4,5,6-H), 6.27 (d, J = 8.5 Hz, 1H, C-H), 3.64 to 4.10 (m, 4H, 2OCH2), 1.48~1.80 (m, 4H, 2CH2), 0.79 to 0.81 (m, 6H, 2CH3); 13C NMR (CDCl3, 125 MHz): δ 158.46 (d, 1JCF = 246.2 Hz), 155.71, 145.50, 133.31, 130.95, 128.75 (d, 2JCF = 23.8 Hz), 128.54 (d, 3JCF = 7.4 Hz), 128.52, 128.49 (d, 3JCF = 7.5 Hz), 120.16 (d, 2JCF = 23.5 Hz), 116.83, 77.10, 69.25, 52.27, 51.03, 23.88, 23.74, 9.99, 9.94; 31P NMR(CDCl3, 200 MHz): δ 21.9; IR: ν 3275.1 (NH), 3072.6 (ArH), 1714.7 (CN), 1224.8 (P=O), 997.2 (P-O-C) cm−1; Anal. Calcd for C21H23Cl2FN3O3P: C 51.87%, H 4.77%, N 8.64%; Found. C 51.39%, H 5.21%, N 8.80%.
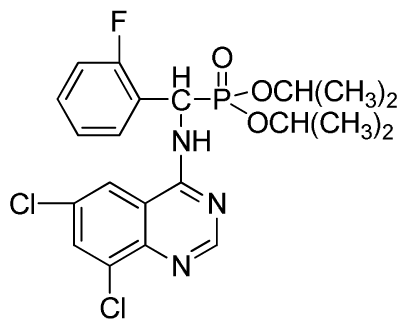
Diisopropyl (6,8-dichloroquinazolin-4-ylamino)(2-fluorophenyl)methylphosphonate (3x): White solid, yield 82.6%; mp 202 °C to 204 °C; 1H NMR (CDCl3, 500 MHz): δ 8.60 (s, 1H, quinazoline-2-H), 8.07 (s, 1H, N-H), 7.81 (s, 1H, N-H), 7.60 (s, 1H, quinazoline-7-H), 7.51 (s, 1H, quinazoline-5-H), 7.21 to 7.25 (m, 4H, Ar-3,4,5,6-H), 6.27 (d, J = 25.0Hz, 1H, CH), 4.60 to 4.86 (m, 2H, 2CH), 1.14 to 1.31 (m, 12H, 4CH3 ); 13C NMR (CDCl3, 125 MHz): δ 160.91, 159.06 (d, 1JCF = 246.2 Hz), 158.94, 154.56, 146.84, 135.75, 128.90 (d, 2JCF = 24.5 Hz), 128.45 (d, 3JCF = 7.4 Hz), 127.95(d, 3JCF = 7.6 Hz), 122.33, 122.14 (d, 2JCF = 23.6Hz), 116.02, 77.12, 72.50, 52.55, 24.24, 23.84, 23.27; 31P NMR(CDCl3, 200 MHz): δ 20.4; IR: ν 3269.36 (NH), 2981.91 (ArH), 1712.8 (CN), 1238.38 (P=O), 1014.66 (P-O-C) cm−1; Anal. Calcd for C21H23Cl2FN3O3P: C 51.87%, H 4.77%, N 8.64%; Found. C 51.46%, H 4.86%, N 8.66%.