Generation and Characterization of a Novel Recombinant Antibody Against 15-Ketocholestane Isolated by Phage-Display
Abstract
:1. Introduction
2. Results and Discussion
2.1. Panning the Fab-phage Library with 15-KA
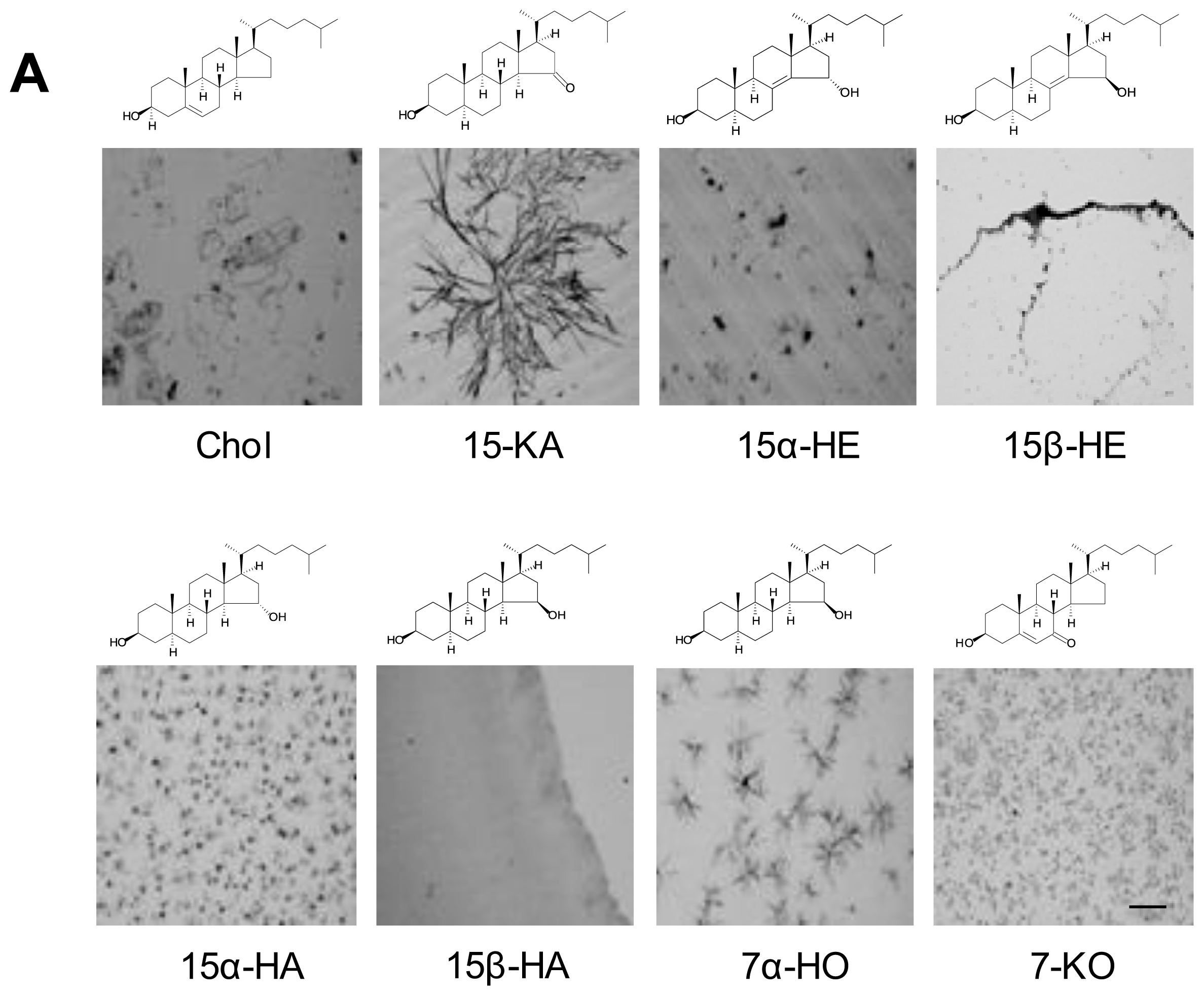
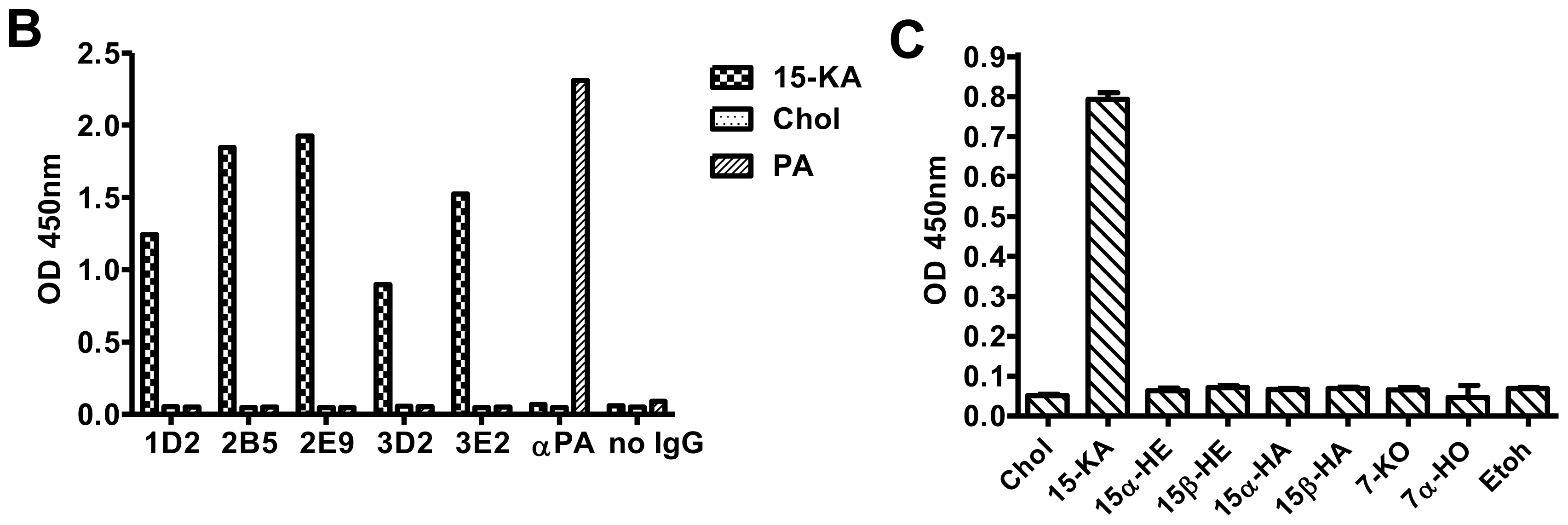
2.2. Specificity of Anti-15-KA Human IgG for Crystalline 15-KA
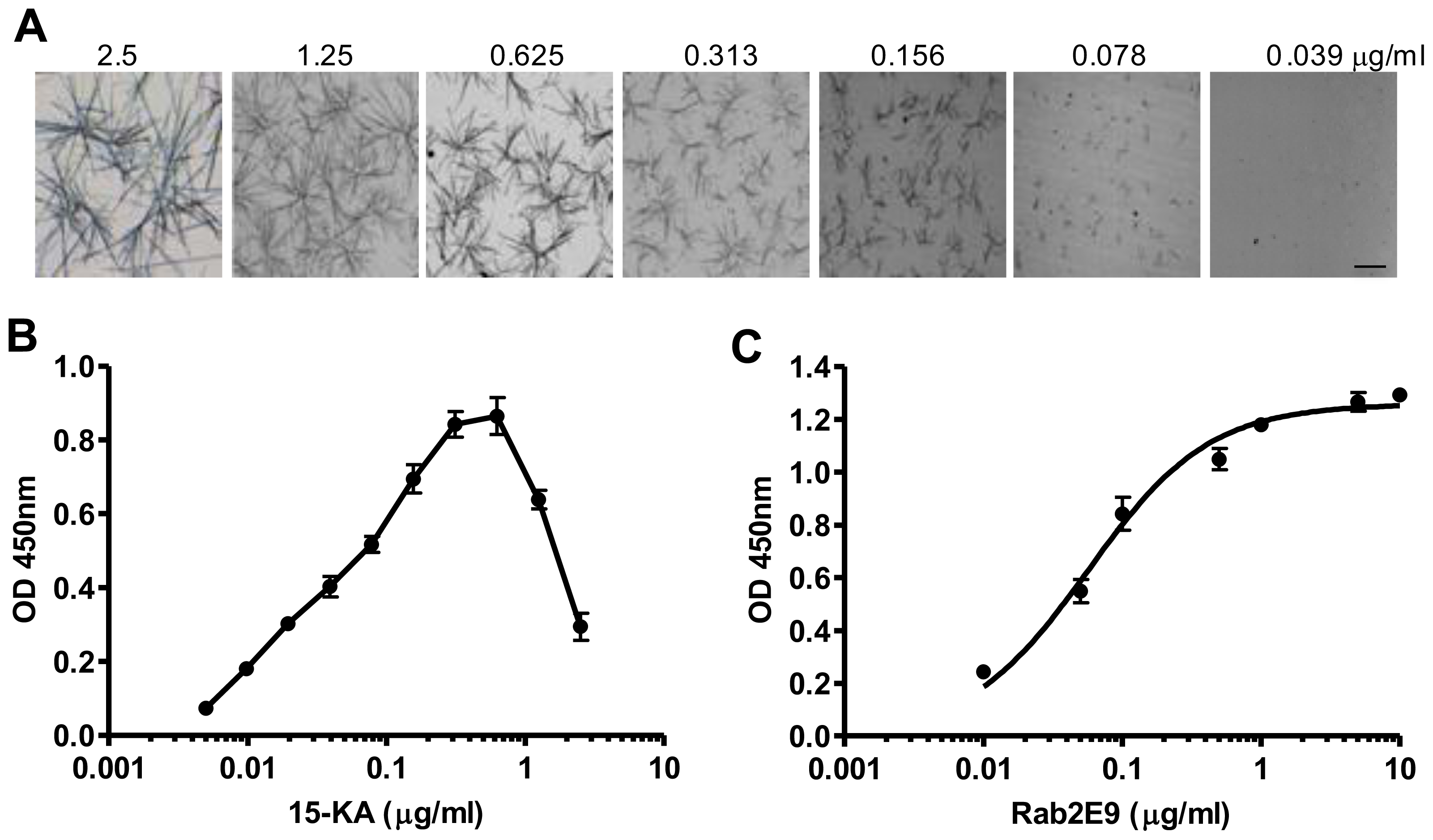
2.3. Sensitivity of Human Anti-15-KA IgG
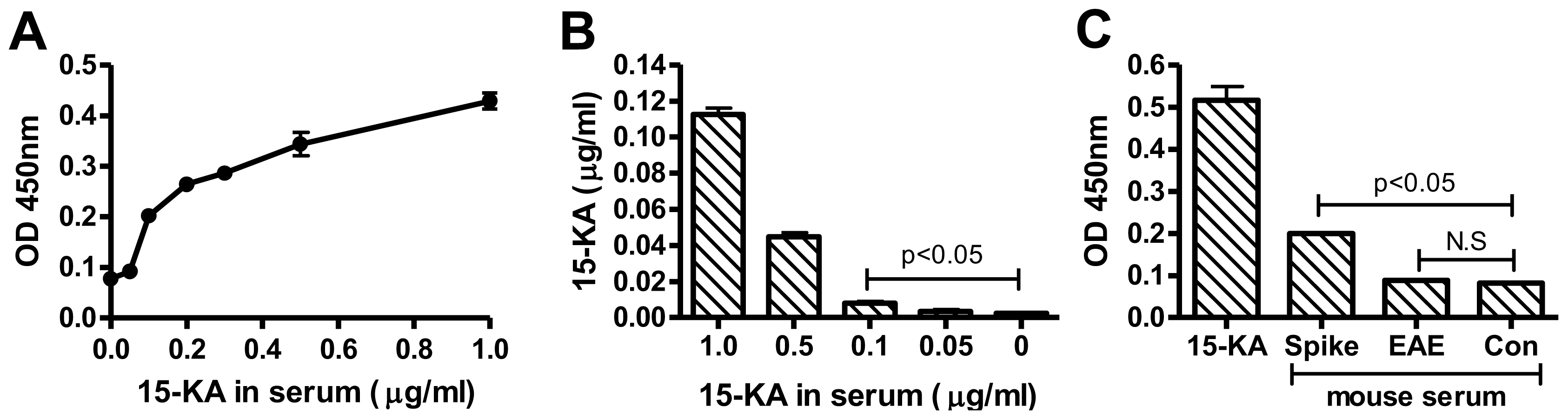
2.4. Detection of 15-KA in EAE Mouse Serum with RAb2E9
2.5. Discussion
3. Experimental Section
3.1. Materials
3.2. Fab-phage Library Screening
3.3. Conversion of Fab-phage to Human IgG1
3.4. Oxysterol Crystal Imaging and ELISA
3.5. Lipid Extraction from 15-KA Spiked and EAE Mouse Serum
3.6. Analysis of 15-KA Content Using High Performance LC-MS
4. Conclusions
Acknowledgment
References
- Waldmann, T.A. Monoclonal antibodies in diagnosis and therapy. Science 1991, 252, 1657–1662. [Google Scholar]
- McNeil, H.P.; Chesterman, C.N.; Krilis, S.A. Immunology and clinical importance of antiphospholipid antibodies. Adv. Immunol 1991, 49, 193–280. [Google Scholar]
- Leoni, V. Oxysterols as markers of neurological disease—A review. Scand. J. Clin. Lab Invest 2009, 69, 22–25. [Google Scholar]
- Gilardi, F.; Viviani, B.; Galmozzi, A.; Boraso, M.; Bartesaghi, S.; Torri, A.; Caruso, D.; Crestani, M.; Marinovich, M.; de Fabiani, E. Expression of sterol 27-hydroxylase in glial cells and its regulation by liver X receptor signaling. Neuroscience 2009, 164, 530–540. [Google Scholar]
- Jeitner, T.M.; Voloshyna, I.; Reiss, A.B. Oxysterol derivatives of cholesterol in neurodegenerative disorders. Curr. Med. Chem 2011, 18, 1515–1525. [Google Scholar]
- Bjorkhem, I.; Lovgren-Sandblom, A.; Piehl, F.; Khademi, M.; Pettersson, H.; Leoni, V.; Olsson, T.; Diczfalusy, U. High levels of 15-oxygenated steroids in circulation of patients with multiple sclerosis: fact or fiction? J. Lipid Res 2011, 52, 170–174. [Google Scholar]
- Kanter, J.L.; Narayana, S.; Ho, P.P.; Catz, I.; Warren, K.G.; Sobel, R.A.; Steinman, L.; Robinson, W.H. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat. Med 2006, 12, 138–143. [Google Scholar]
- Quintana, F.J.; Farez, M.F.; Viglietta, V.; Iglesias, A.H.; Merbl, Y.; Izquierdo, G.; Lucas, M.; Basso, A.S.; Khoury, S.J.; Lucchinetti, C.F.; Cohen, I.R.; Weiner, H.L. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2008, 105, 18889–18894. [Google Scholar]
- Farez, M.F.; Quintana, F.J.; Gandhi, R.; Izquierdo, G.; Lucas, M.; Weiner, H.L. Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat. Immunol 2009, 10, 958–964. [Google Scholar]
- Bjorkhem, I.; Diczfalusy, U. Oxysterols: Friends, foes, or just fellow passengers? Arterioscler. Thromb. Vasc. Biol 2002, 22, 734–742. [Google Scholar]
- Dzeletovic, S.; Breuer, O.; Lund, E.; Diczfalusy, U. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal. Biochem 1995, 225, 73–80. [Google Scholar]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid. Res 2010, 51, 3299–3305. [Google Scholar]
- Griffiths, W.J.; Wang, Y. Are 15-oxygenated sterols present in the human circulation? J. Lipid Res 2011, 52, 4–5. [Google Scholar]
- Boullerne, A.; Petry, K.G.; Geffard, M. Circulating antibodies directed against conjugated fatty acids in sera of patients with multiple sclerosis. J. Neuroimmunol 1996, 65, 75–81. [Google Scholar]
- Singh, K.V.; Kaur, J.; Varshney, G.C.; Raje, M.; Suri, C.R. Synthesis and characterization of hapten-protein conjugates for antibody production against small molecules. Bioconjug. Chem 2004, 15, 168–173. [Google Scholar]
- Biro, A.; Cervenak, L.; Balogh, A.; Lorincz, A.; Uray, K.; Horvath, A.; Romics, L.; Matko, J.; Fust, G.; Laszlo, G. Novel anti-cholesterol monoclonal immunoglobulin G antibodies as probes and potential modulators of membrane raft-dependent immune functions. J. Lipid Res 2007, 48, 19–29. [Google Scholar]
- Swartz, G.M., Jr; Gentry, M.K.; Amende, L.M.; Blanchette-Mackie, E.J.; Alving, C.R. Antibodies to cholesterol. Proc. Natl. Acad. Sci. USA 1988, 85, 1902–1906. [Google Scholar]
- Perl-Treves, D.; Kessler, N.; Izhaky, D.; Addadi, L. Monoclonal antibody recognition of cholesterol monohydrate crystal faces. Chem. Biol 1996, 3, 567–577. [Google Scholar]
- De Haard, H.J.; van Neer, N.; Reurs, A.; Hufton, S.E.; Roovers, R.C.; Henderikx, P.; de Bruine, A.P.; Arends, J.W.; Hoogenboom, H.R. A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J. Biol. Chem 1999, 274, 18218–18230. [Google Scholar]
- Chan, C.E.; Chan, A.H.; Lim, A.P.; Hanson, B.J. Comparison of the efficiency of antibody selection from semi-synthetic scFv and non-immune Fab phage display libraries against protein targets for rapid development of diagnostic immunoassays. J. Immunol. Methods 2011, 373, 79–88. [Google Scholar]
- Lim, A.P.; Chan, C.E.; Wong, S.K.; Chan, A.H.; Ooi, E.E.; Hanson, B.J. Neutralizing human monoclonal antibody against H5N1 influenza HA selected from a Fab-phage display library. Virol. J 2008, 5. [Google Scholar] [CrossRef]
- Croxford, J.L.; Miyake, S.; Huang, Y.Y.; Shimamura, M.; Yamamura, T. Invariant V(alpha)19i T cells regulate autoimmune inflammation. Nat. Immunol 2006, 7, 987–994. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol 1959, 37, 911–917. [Google Scholar]
- Shui, G.; Guan, X.L.; Low, C.P.; Chua, G.H.; Goh, J.S.; Yang, H.; Wenk, M.R. Toward one step analysis of cellular lipidomes using liquid chromatography coupled with mass spectrometry: application to Saccharomyces cerevisiae and Schizosaccharomyces pombe lipidomics. Mol. Biosyst 2010, 6, 1008–1017. [Google Scholar]
| Clone | VL CDR3 | VL gene | VH CDR3 | VH gene |
|---|---|---|---|---|
| 1D2 | GTHWPYTFGQG | KV2D-28 | GVVGSMDV | HV1-18 |
| 2B5 | ALQSPFTFGPG | KV2D-28 | PPDNRGFYFDF | HV1-3 |
| 2E9 | GTHWPYTFGQG | KV2D-28 | ERAVTNYYYYYGMDV | HV1-3 |
| 3D2 | ALQTPSFGGG | KV2D-28 | ETYSSSWYAPKYFDY | HV1-3 |
| 3E2 | ALRSPSFGPG | KV2D-28 | TPRWLQNIPNDY | HV3-21 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Islam, M.O.; Lim, Y.T.; Chan, C.E.Z.; Cazenave-Gassiot, A.; Croxford, J.L.; Wenk, M.R.; Macary, P.A.; Hanson, B.J. Generation and Characterization of a Novel Recombinant Antibody Against 15-Ketocholestane Isolated by Phage-Display. Int. J. Mol. Sci. 2012, 13, 4937-4948. https://doi.org/10.3390/ijms13044937
Islam MO, Lim YT, Chan CEZ, Cazenave-Gassiot A, Croxford JL, Wenk MR, Macary PA, Hanson BJ. Generation and Characterization of a Novel Recombinant Antibody Against 15-Ketocholestane Isolated by Phage-Display. International Journal of Molecular Sciences. 2012; 13(4):4937-4948. https://doi.org/10.3390/ijms13044937
Chicago/Turabian StyleIslam, Md. Omedul, Yan Ting Lim, Conrad En Zuo Chan, Amaury Cazenave-Gassiot, J. Ludovic Croxford, Markus R. Wenk, Paul A. Macary, and Brendon J. Hanson. 2012. "Generation and Characterization of a Novel Recombinant Antibody Against 15-Ketocholestane Isolated by Phage-Display" International Journal of Molecular Sciences 13, no. 4: 4937-4948. https://doi.org/10.3390/ijms13044937




