Carotenoids, Fatty Acid Composition and Heat Stability of Supercritical Carbon Dioxide-Extracted-Oleoresins
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Standards
2.2. Plant Material, Matrices and Lycopene Extraction
2.2.1. S-CO2 Extractions
2.2.2. Solvent Extraction
2.3. Treatments of Supercritical CO2-Extracted Oleoresins
2.4. Qualitative and Quantitative Carotenoids Analysis
2.5. Lipid Determination
2.6. In Vitro Antioxidant Activity Analysis
2.7. Analytical Quality Control
2.8. Statistical Analysis
3. Results and Discussion
3.1. Tomato Oleoresins
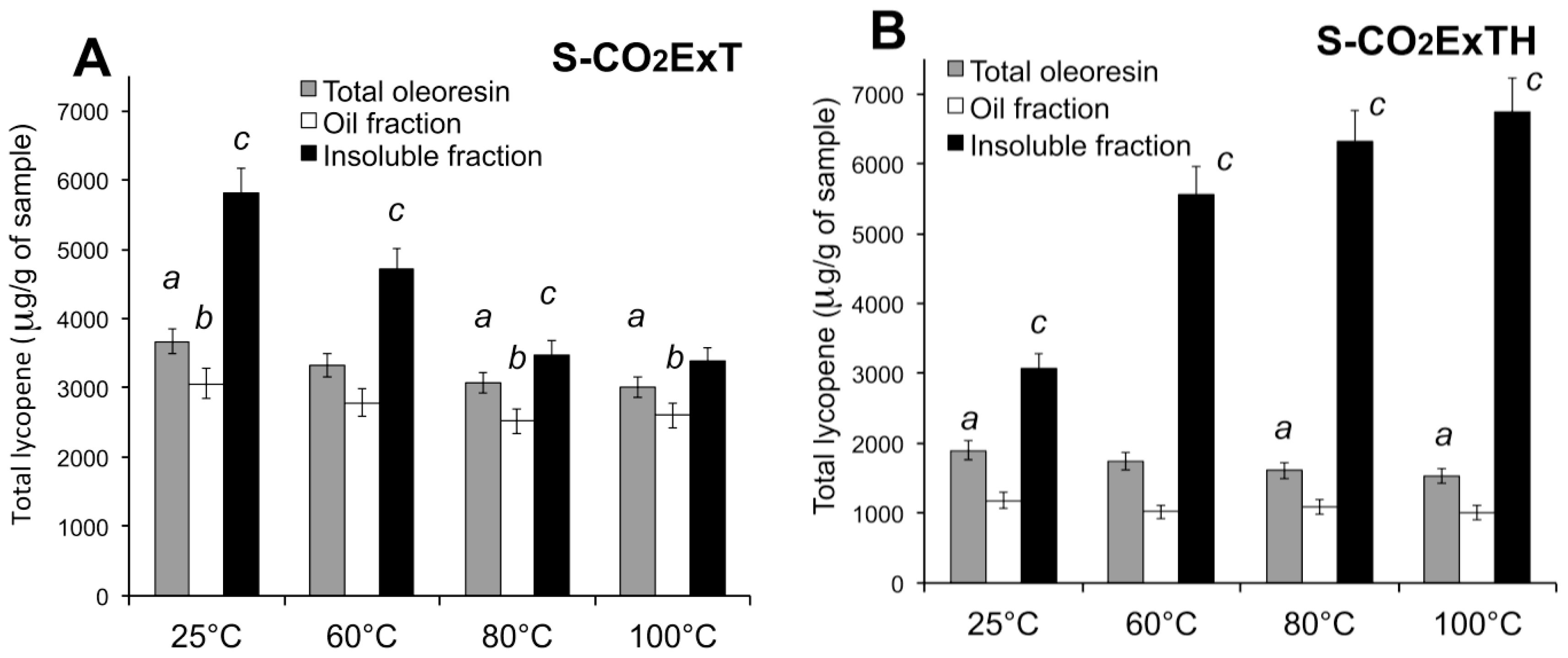
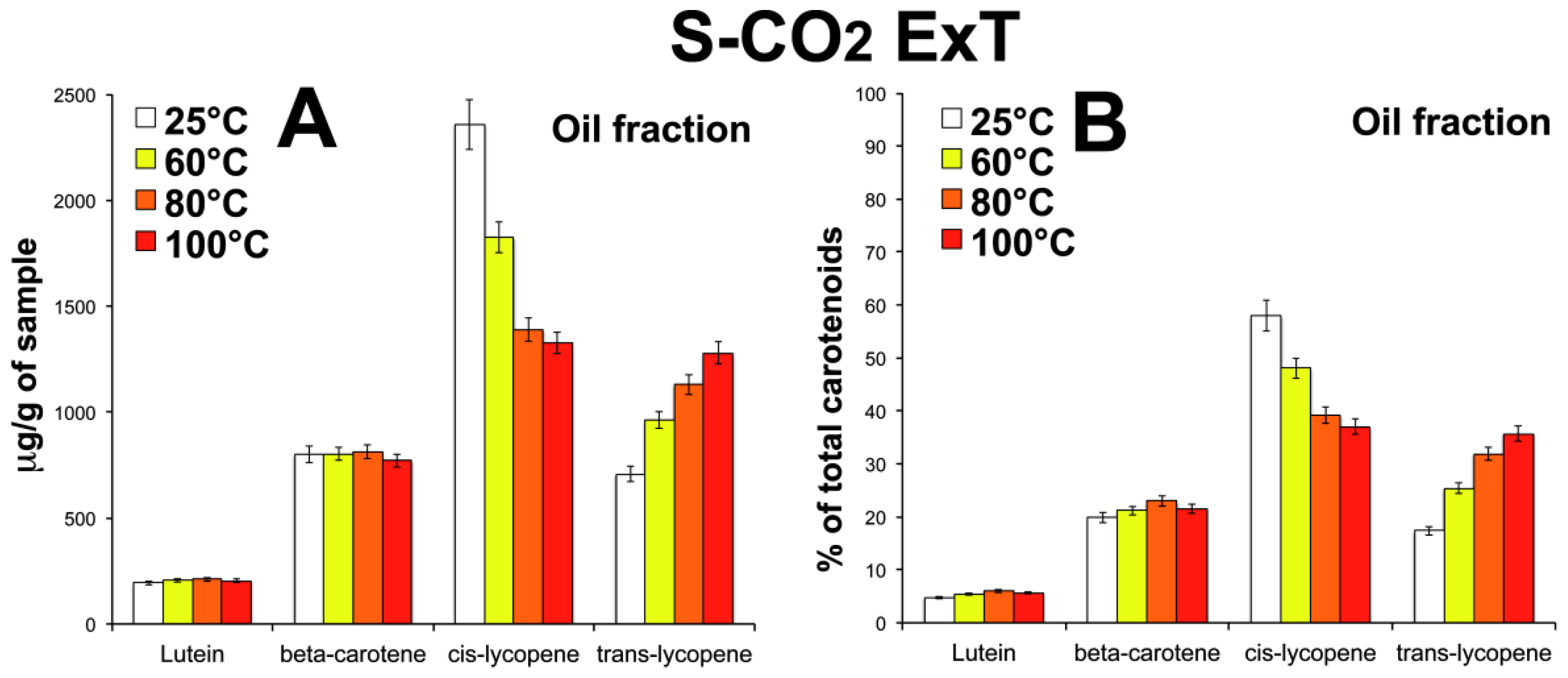
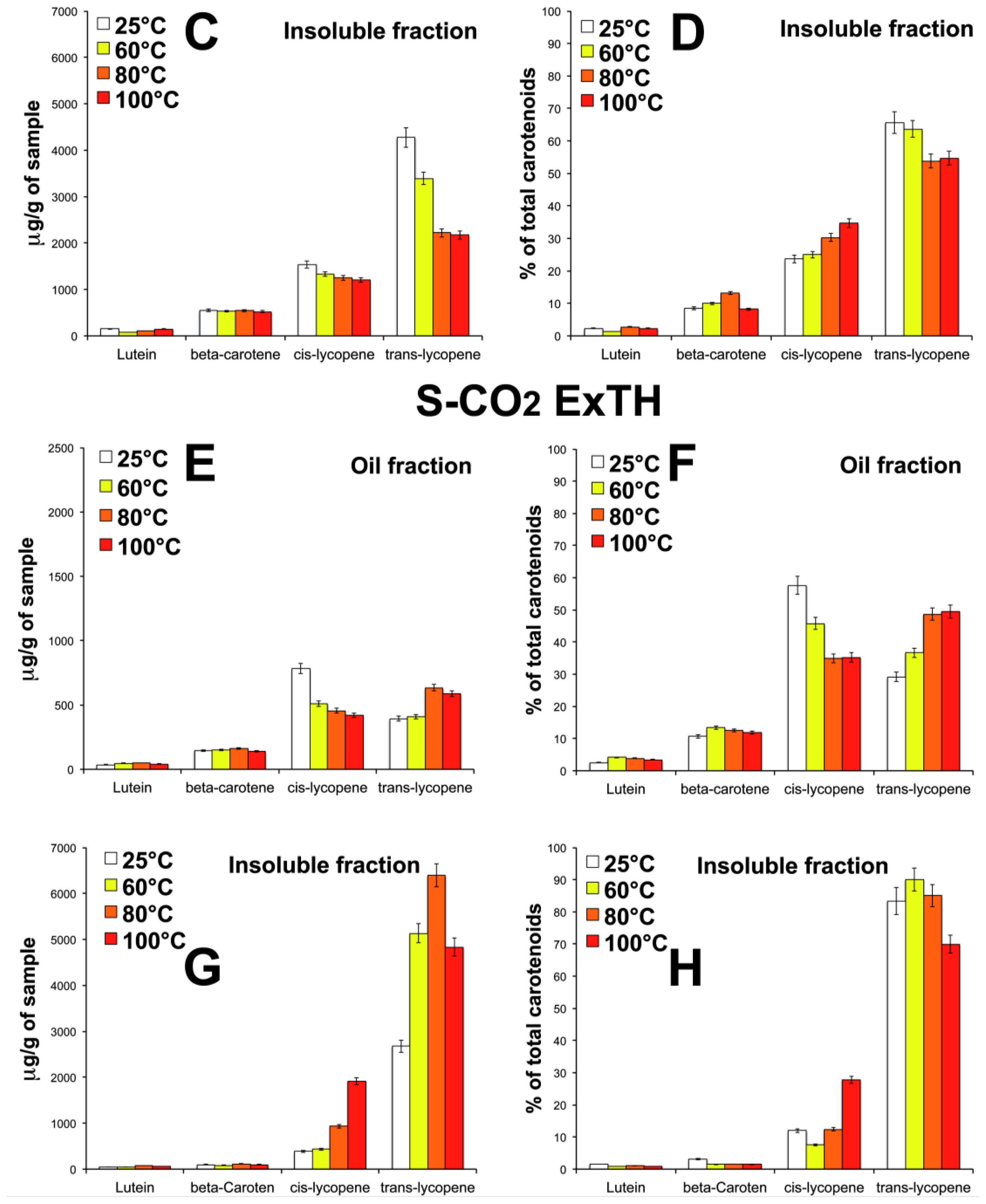
3.2. Lycopene Content and Carotenoid Composition in Tomato Oleoresins
3.3. Fatty Acid Composition in Tomato Oleoresins
3.4. Antioxidant Activity of Total and of Oil- and Insoluble-Fractions
3.5. Effect of Temperature on Carotenoid Composition of Oleoresins
3.6. Effect of Temperature on Antioxidant Activity of Oleoresins
4. Conclusions
Acknowledgments
Abbreviations
| S-CO2 | supercritical carbon dioxide |
| S-CO2ExT | oleoresin extracted from pure tomato powder |
| S-CO2ExTH | oleoresin extracted from tomato powder added with an equal amount of dry hazelnut powder |
| TE | Trolox equivalents |
| FA | fatty acid |
| PUFA | polyunsaturated fatty acid |
- Conflict of InterestThe authors declare no conflict of interest.
- Financial SupportThis work was financially supported by “Ministero dell’Istruzione, dell’Università e della Ricerca” (MIUR) by Project 7885: “Lycopene production from tomato berries by innovative systems”.
References
- Rao, A.V.; Agarwal, S. Role of antioxidant lycopene in cancer and heart disease. J. Am. Coll. Nutr 2000, 19, 563–569. [Google Scholar]
- Van Breemen, R.B.; Pajkovic, N. Multitargeted therapy of cancer by lycopene. Cancer Lett 2008, 269, 339–351. [Google Scholar]
- Agarwal, S.; Rao, A.V. Tomato lycopene and its role in human health and chronic diseases. Can. Med. Assoc. J 2000, 163, 739–744. [Google Scholar]
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.W., Jr. The tomato as a functional food. J. Nutr. 2005, 135, 1226–1230. [Google Scholar]
- Leone, A.; Zefferino, R.; Longo, C.; Leo, L.; Zacheo, G. Supercritical CO2-extracted tomato oleoresins enhance gap junction intercellular communications and recover from mercury chloride inhibition in keratinocytes. J. Agric. Food Chem 2010, 58, 4769–4778. [Google Scholar]
- Livny, O.; Kaplan, I.; Reifen, R.; Polak-Charcon, S.; Madar, Z.; Schwartz, B. Lycopene inhibits proliferation and enhances gap-junctional communication of KB-1 human oral tumor cells. J. Nutr 2002, 132, 3754–3759. [Google Scholar]
- Zefferino, R.; Leone, A.; Piccaluga, S.; Cincione, R.; Ambrosi, L. Mercury modulates interplay between IL-1β, TNF-α, and gap junctional intercellular communication in keratinocytes: mitigation by lycopene. J. Immunotoxicol 2008, 5, 353–360. [Google Scholar]
- Palozza, P.; Parrone, N.; Catalano, A.; Simone, R. Tomato lycopene and inflammatory cascade: basic interactions and clinical implications. Curr. Med. Chem 2010, 17, 2547–2563. [Google Scholar]
- Ried, K.; Fakler, P. Protective effect of lycopene on serum cholesterol and blood pressure: Meta-analyses of intervention trials. Maturitas 2011, 68, 299–310. [Google Scholar]
- Singh, P.; Goyal, G.K. Dietary lycopene: Its properties and anticarcinogenic effects. Compr. Rev. Food Sci. Food Saf 2008, 7, 255–270. [Google Scholar]
- Boileau, T.W.M.; Boileau, A.C.; Erdman, J.W. Bioavailability of all-trans and cis-isomers of lycopene. Exp. Biol. Med 2002, 227, 914–919. [Google Scholar]
- Unlu, N.Z.; Bohn, T.; Francis, D.M.; Nagaraja, H.N.; Clinton, S.K.; Schwartz, S.J. Lycopene from heat-induced cis-isomer-rich tomato sauce is more bioavailable than from all-trans-rich tomato sauce in human subjects. Br. J. Nutr 2007, 98, 140–146. [Google Scholar]
- Teodoro, A.J.; Perrone, D.; Martucci, R.B.; Borojevic, R. Lycopene isomerisation and storage in an in vitro model of murine hepatic stellate cells. Eur. J. Nutr 2009, 48, 261–268. [Google Scholar]
- Lambelet, P.; Richelle, M.; Bortlik, K.; Franceschi, F.; Giori, A.M. Improving the stability of lycopene Z-isomers in isomerised tomato extracts. Food Chem 2009, 112, 156–161. [Google Scholar]
- Xianquan, S.; Shi, J.; Kakuda, Y.; Yueming, J. Stability of lycopene during food processing and storage. J. Med. Food 2005, 8, 413–422. [Google Scholar]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar]
- Shi, L.-E.; Zhang, Z.-L.; Xing, L.-Y.; Yang, D.-D.; Guo, Y.-P.; Guo, X.-F.; Zhao, L.-M.; Tang, Z.-X. Antioxidants extraction by supercritical CO2. J. Med. Plants Res 2011, 5, 300–308. [Google Scholar]
- Ciurlia, L.; Bleve, M.; Rescio, L. Supercritical carbon dioxide co-extraction of tomatoes (Lycopersicum esculentum L.) and hazelnuts (Corylus avellana L.): A new procedure in obtaining a source of natural lycopene. J. Superscrit. Fluids 2009, 49, 338–344. [Google Scholar]
- Topal, U.; Sasaki, M.; Goto, M.; Hayakawa, K. Extraction of lycopene from tomato skin with supercritical carbon dioxide: Effect of operating conditions and solubility analysis. J. Agric. Food Chem 2006, 54, 5604–5610. [Google Scholar]
- Yi, C.; Shi, J.; Xue, S.J.; Jiang, Y.; Li, D. Effects of supercritical fluid extraction parameters on lycopene yield and antioxidant activity. Food Chem 2009, 113, 1088–1094. [Google Scholar]
- Leone, A.; Conti, A. National Research Council, Institute of Food Production (CNR-ISPA), Lecce, Italy. Supercritical CO2 extraction from a tomato/hazelnut matrix results in production of oleoresin containing allergenic proteins from hazelnut. 2010. (Unpublished work). [Google Scholar]
- Gòmez-Prieto, M.S.; Caja, M.M.; Herraiz, M.; Santa-Marìa, G. Supercritical fluid extraction of all-trans-lycopene from tomato. J. Agric. Food Chem 2003, 51, 3–7. [Google Scholar]
- Van Breemen, R.B.; Xu, X.; Viana, M.A.; Chen, L.; Stacewicz-Sapuntzakis, M.; Duncan, C.; Bowen, P.E.; Sharifi, R. Liquid chromatography–mass spectrometry of cis- and all-trans-lycopene in human serum and prostate tissue after dietary supplementation with tomato sauce. J. Agric. Food Chem 2002, 50, 2214–2219. [Google Scholar]
- Boileau, A.C.; Merchen, N.R.; Wasson, K.; Atkinson, C.A.; Erdman, J.W., Jr. Cis-lycopene is more bioavailable than trans-lycopene in vitro and in vivo in lymph cannulated ferrets. J. Nutr. 1999, 129, 1176–1181. [Google Scholar]
- Wu, K.; Schwartz, S.J.; Platz, E.A.; Clinton, S.K.; Erdman, J.W., Jr; Ferruzzi, M.G.; Willett, W.C.; Giovannucci, E.L. Variations in plasma lycopene and specific isomers over time in a cohort of US men. J. Nutr. 2003, 133, 1930–1936. [Google Scholar]
- Colle, I.J.P.; Lemmens, L.; Tolesa, G.N.; van Buggenhout, S.; de Vleeschouwer, K.; van Loey, A.M.; Hendrickx, M.E. Lycopene degradation and isomerization kinetics during thermal processing of an olive oil/tomato emulsion. J. Agric. Food Chem 2010, 58, 12784–12789. [Google Scholar]
- Parcerisa, J.; Richardson, D.G.; Rafecas, M.; Codony, R.; Boatella, J. Fatty acids, tocopherol and sterol content of some hazelnut varieties (Corylus avellana L.) harvested in Oregon (USA). J. Chromatogr. A 1998, 805, 259–268. [Google Scholar]
- Anguelova, T.; Warthesen, J. Lycopene stability in tomato powders. J. Food Sci 2000, 65, 67–70. [Google Scholar]
- Hackett, M.M.; Lee, J.H.; Francis, D.; Schwartz, S.J. Thermal stability and isomerization of lycopene in tomato oleoresins from different varieties. J. Food Sci 2004, 69, 536–541. [Google Scholar]
- Ax, K.; Mayer-Miebach, E.; Link, B.; Schuchmann, H.; Schubert, H. Stability of lycopene in oil-in-water emulsions. Eng. Life Sci 2003, 3. [Google Scholar]
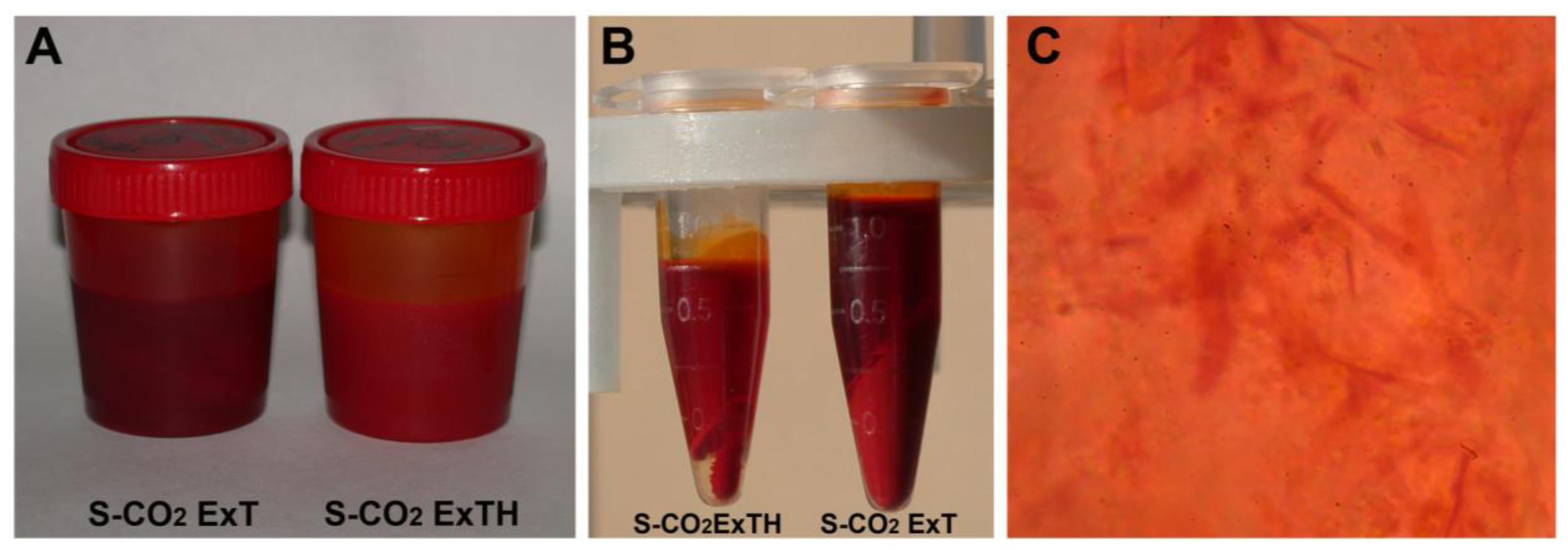
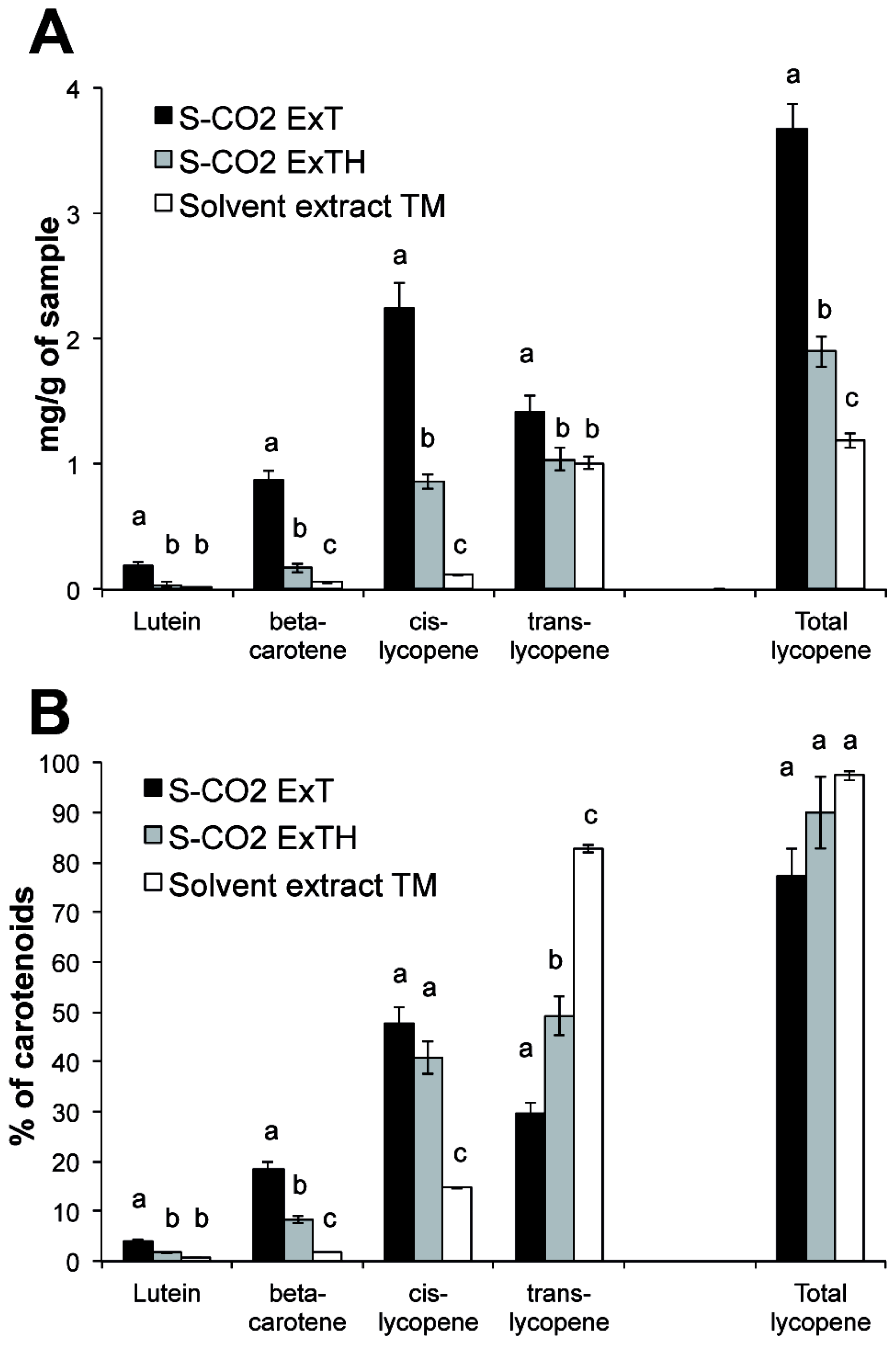

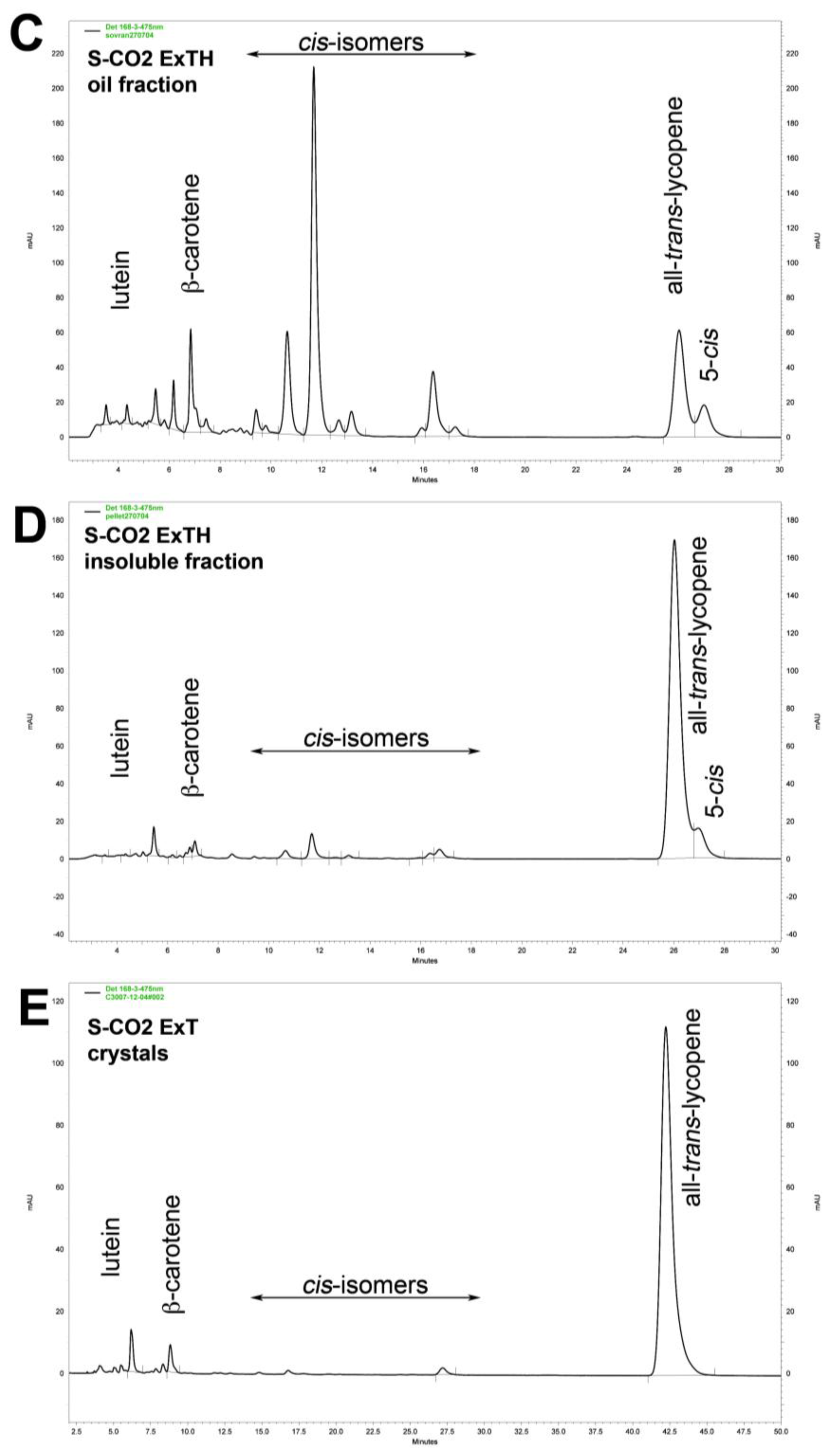
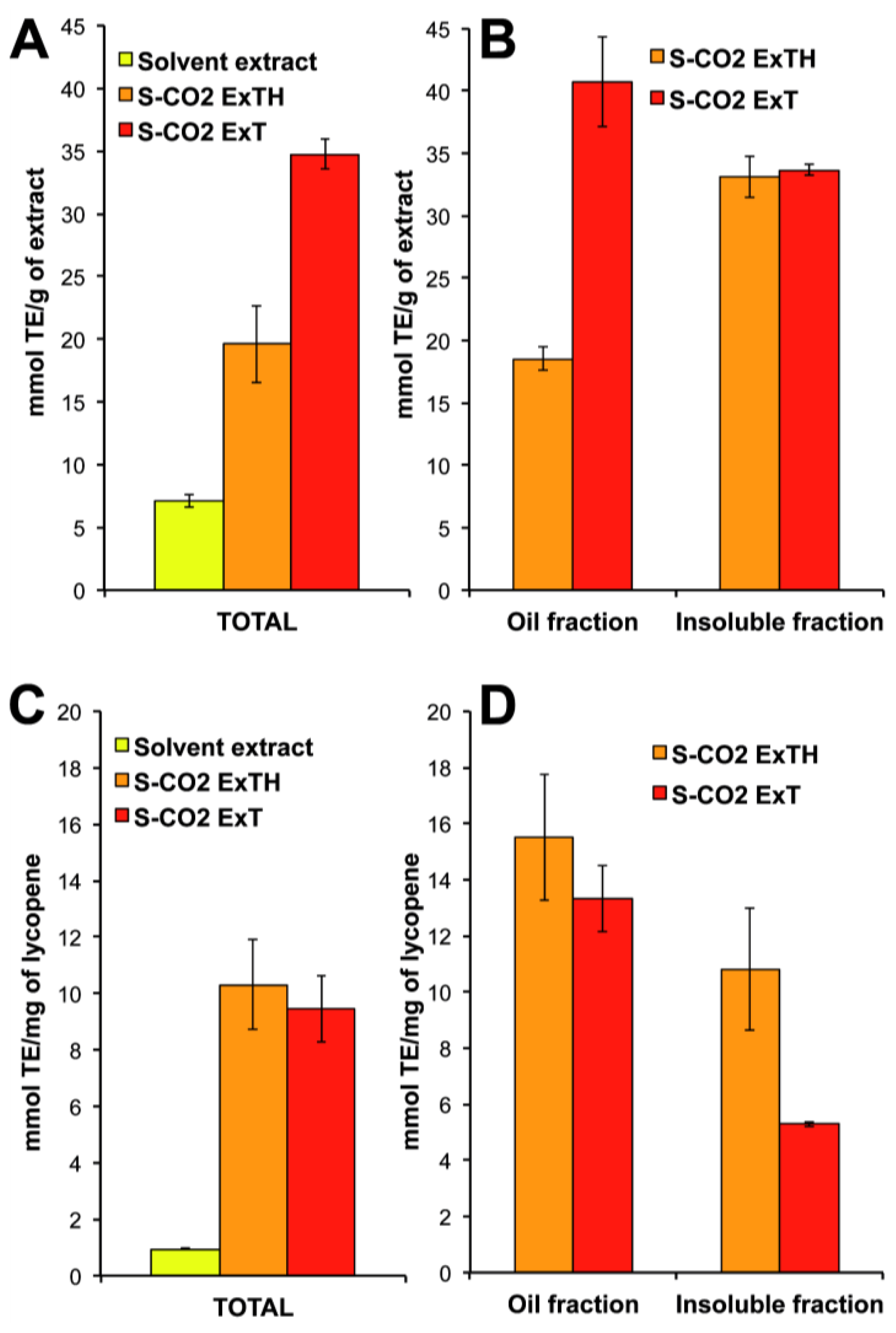

| Carotenoids | S-CO2 Oleoresins | Solvent extract | |||||||
|---|---|---|---|---|---|---|---|---|---|
| S-CO2 ExT a | S-CO2 ExTH b | Tomato matrix | |||||||
| Oil fraction | Insoluble fraction | Oil fraction | Insoluble fraction | ||||||
| μg/g of oleoresin | % of carotenoids | μg/g of oleoresin | % of carotenoids | μg/g of oleoresin | % of carotenoids | μg/g of oleoresin | % of carotenoids | % of carotenoids | |
| Lutein | 193 (±12) | 4.8 | 149 (±9) | 2.3 | 34 (±7) | 2.53 | 50 (±5) | 1.5 | 0.9 |
| β-carotene | 801 (±22) | 19.8 | 549 (±19) | 8.4 | 145 (±17) | 10.7 | 99 (±7) | 3.1 | 4.7 |
| cis-lycopene | 2353 (±88) | 58 | 1539 (±78) | 23.6 | 782 (±34) | 57.77 | 385 (±10) | 12 | 9.9 |
| trans-lycopene | 706 (±18) | 17.4 | 4277 (±120) | 65.7 | 392 (±10) | 28.99 | 2677 (±71) | 83.4 | 84.5 |
| Total lycopene | 3060 | 75.5 | 5816 | 89.3 | 1175 | 86.77 | 3062 | 95.4 | 94.4 |
| Total carotenoids | 4056 | 100 | 6515 | 100 | 1354 | 100 | 3211 | 100 | 100 |
| Lycopene (mg/100 g of oleoresin) | 306 | 581.6 | 117.5 | 306.2 | 119 | ||||
| Fatty acids | Tomato powder | S-CO2-Extracted-Oleoresins | |
|---|---|---|---|
| S-CO2 ExT a | S-CO2 ExTH b | ||
| Percentage (%)
| |||
| Palmitic acid (16:0) | 23.77 (1.88) | 5.59 (0.32) | 5.24 (0.22) |
| Palmitoleic acid (16:1) | n.d. c | n.d. c | 0.12 (0.01) |
| Stearic acid (18:0) | 2.98 (0.05) | 2.61 (0.05) | 2.55 (0.04) |
| Oleic acid (18:1) | 4.22 (0.98) | 80.46 (2.59) | 83.04 (3.03) |
| Linoleic acid (18:2) | 52.59 (1.78) | 9.33 (0.77) | 8.9 (0.81) |
| Linolenic acid (18:3) | 7.38 (0.57) | 0.71 (0.03) | 0.15 (0.01) |
| Others d | 9.06 (0.2) | 1.3 (0.01) | n.d. c |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Longo, C.; Leo, L.; Leone, A. Carotenoids, Fatty Acid Composition and Heat Stability of Supercritical Carbon Dioxide-Extracted-Oleoresins. Int. J. Mol. Sci. 2012, 13, 4233-4254. https://doi.org/10.3390/ijms13044233
Longo C, Leo L, Leone A. Carotenoids, Fatty Acid Composition and Heat Stability of Supercritical Carbon Dioxide-Extracted-Oleoresins. International Journal of Molecular Sciences. 2012; 13(4):4233-4254. https://doi.org/10.3390/ijms13044233
Chicago/Turabian StyleLongo, Cristiano, Lucia Leo, and Antonella Leone. 2012. "Carotenoids, Fatty Acid Composition and Heat Stability of Supercritical Carbon Dioxide-Extracted-Oleoresins" International Journal of Molecular Sciences 13, no. 4: 4233-4254. https://doi.org/10.3390/ijms13044233
APA StyleLongo, C., Leo, L., & Leone, A. (2012). Carotenoids, Fatty Acid Composition and Heat Stability of Supercritical Carbon Dioxide-Extracted-Oleoresins. International Journal of Molecular Sciences, 13(4), 4233-4254. https://doi.org/10.3390/ijms13044233





