Applications of Circular Dichroism for Structural Analysis of Gelatin and Antimicrobial Peptides
Abstract
:1. Introduction
2. Results and Discussion
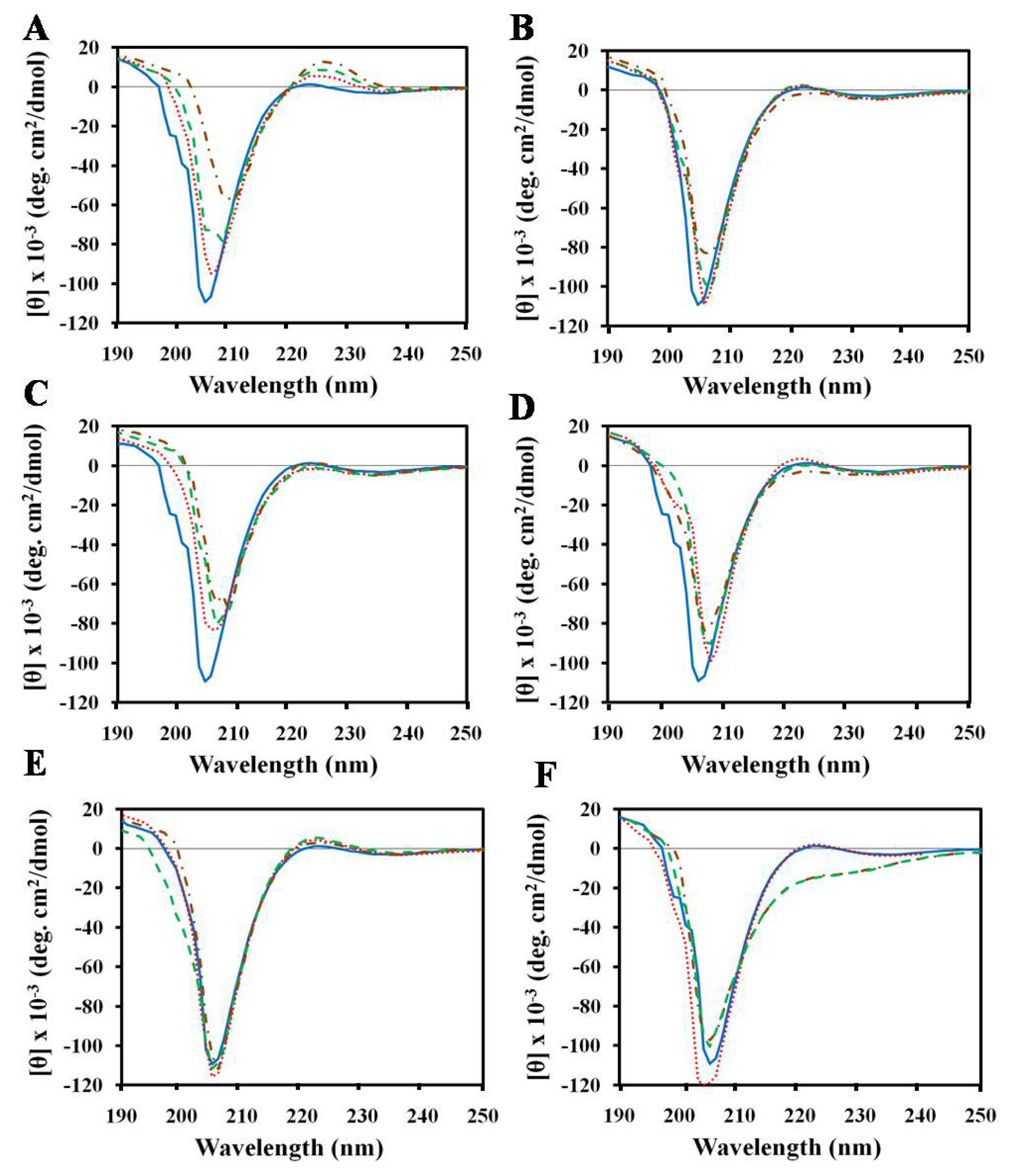
2.1. Effects of Antimicrobial Peptides on Gelatin Conformation
2.2. Effect of SDS on Gelatin Conformation
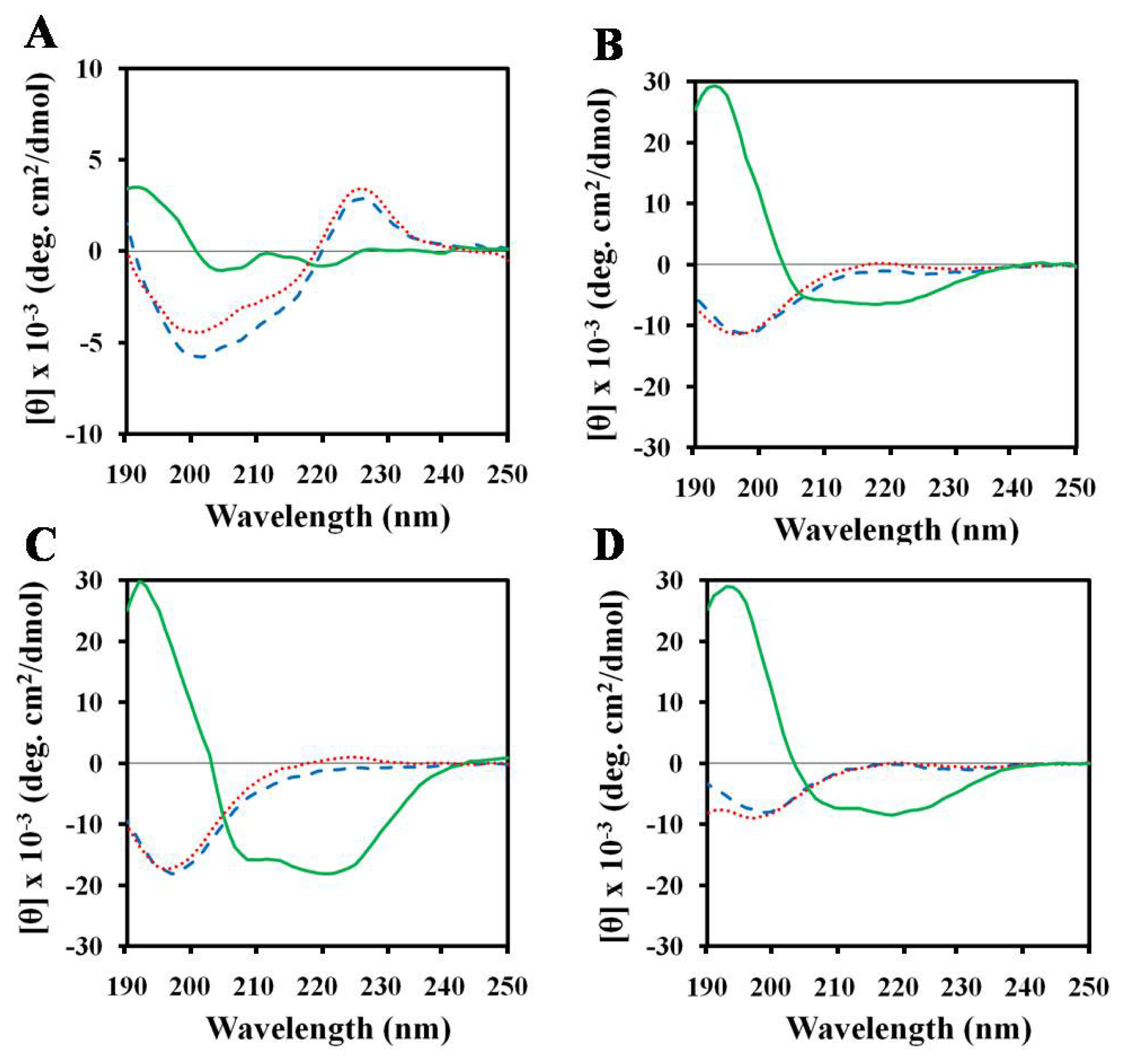
2.3. Structures of Peptides in Aqueous and SDS Solution
2.4. Structures of Peptides in Nonionic Surfactant
2.5. Structures of Peptides in Cell Wall Components
2.6. Characterization of the Trp Environment Using Fluorescence Spectroscopy
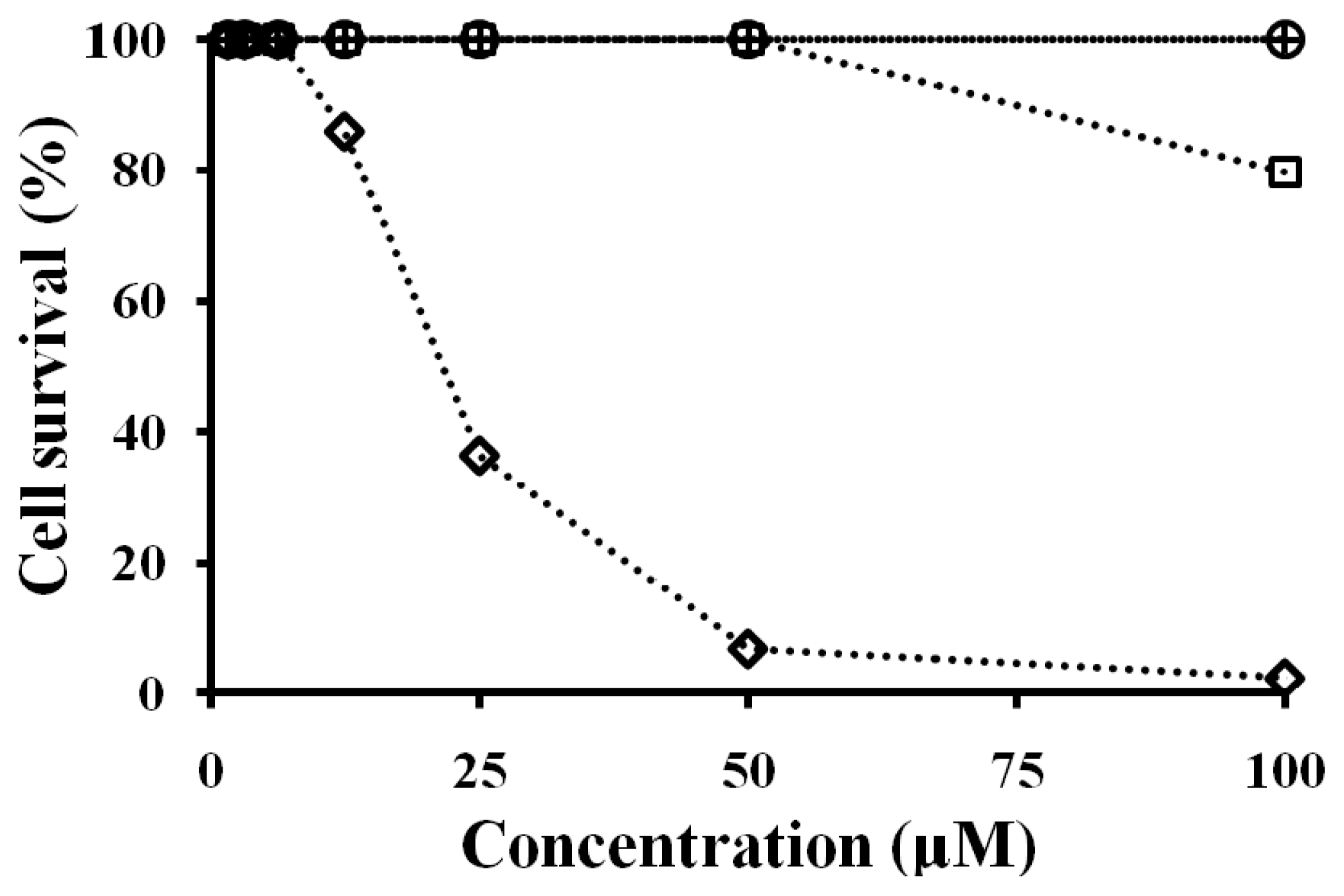
2.7. Antibacterial and Cytotoxicity Activities of Peptides
3. Experimental Section
3.1. Materials
3.2. Peptide Synthesis and Purification
3.3. Interaction of Peptides with Gelatin by CD Spectroscopy
3.4. Interaction of Peptides with Surfactants by CD Spectroscopy
3.5. Interaction of Peptides with Cell Wall Components by CD Spectroscopy
3.6. Trp Fluorescence Assay
3.7. Antibacterial Activity
3.8. Cell Culture and Cytotoxicity
4. Conclusion
Acknowledgements
References
- Greenfield, N.J. Methods to estimate the conformation of proteins and polypeptides from circular dichroism data. Anal. Biochem 1996, 235, 1–10. [Google Scholar]
- Johnson, W.C. Secondary structure of proteins through circular dichroism spectroscopy. Annu. Rev. Biophys. Biophys. Chem 1988, 17, 145–166. [Google Scholar]
- Johnson, W.C. Analyzing protein circular dichroism spectra for accurate secondary structures. Proteins 1999, 35, 307–312. [Google Scholar]
- Yeaman, M.R.; Yount, N.Y. Mechanism of antimicrobial peptide action and resistance. Pharmacol. Rev 2003, 55, 27–55. [Google Scholar]
- Hancock, R.E.; Chapple, D.S. Peptide antibiotics. Antimicrob. Agents Chemother 1999, 43, 1317–1323. [Google Scholar]
- Powers, J.P.; Hancock, R.E. The relationship between peptide structure and antibacterial activity. Peptides 2003, 24, 1681–1691. [Google Scholar]
- Greenfield, N.J. Applications of circular dichroism in protein and peptide analysis. Trend Anal. Chem 1999, 18, 236–244. [Google Scholar]
- Gopal, R.; Kim, Y.J.; Seo, C.H.; Hahm, K.S.; Park, Y. Reversed sequence enhances antimicrobial activity of a synthetic peptide. J. Peptide Sci 2011, 17, 329–334. [Google Scholar]
- Gopal, R.; Park, S.C.; Ha, K.J.; Cho, S.J.; Kim, S.W.; Song, P.I.; Nah, J.W.; Park, Y.; Hahm, K.S. Effect of leucine and lysine substitution on the antimicrobial activity and evaluation of the mechanism of the HPA3NT3 analog peptide. J. Peptide Sci 2009, 15, 589–594. [Google Scholar]
- Patrzykat, A.; Gallant, J.W.; Seo, J.K.; Pytyck, J.; Douglas, S.E. Novel antimicrobial peptides derived from flatfish genes. Antimicrob. Agents Chemother 2003, 47, 2464–2470. [Google Scholar]
- Zasloff, M. Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar]
- Kawanishi, N.; Christenson, H.K.; Ninham, B.W.J. Measurement of the interaction between adsorbed polyelectrolytes: Gelatin on mica surfaces. J. Phys. Chem 1990, 94, 4611–4617. [Google Scholar]
- Likos, C.N.; Vaynberg, K.A.; Lowen, H.; Wagner, N.J. Colloidal stabilization by adsorbed gelatin. Langmuir 2000, 16, 4100–4108. [Google Scholar]
- Tabata, Y.; Ikada, Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev 1998, 31, 287–301. [Google Scholar]
- Wetzel, R.; Buder, E.; Hermel, H.; Hütter, A. Conformations of different gelatins in solutions and in films an analysis of circular dichroism (CD) measurements. Colloid Polym. Sci 1987, 265, 1036–1045. [Google Scholar]
- Gardi, A.; Nischmann, H.S. Intracatenar cross-linking in gelatin with carbodiimide. Helv. Chim. Acta 1972, 55, 2468–2486. [Google Scholar]
- Wustneck, R.; Buder, E.; Wetzel, R.; Hermel, H. The modification of the triple helical structure of gelatin in aqueous solution 3. The influence of cationic surfactants. Colloid Polym. Sci 1989, 267, 429–433. [Google Scholar]
- Wustneck, R.; Buder, E.; Wetzel, R.; Hermel, H. The modification of the triple helical structure of gelatin in aqueous solution 1. The influence of anionic surfactants, pH-value, and temperature. Colloid Polym. Sci 1988, 266, 1061–1067. [Google Scholar]
- Guillen, M.C.G.; Turnay, J.; Fernandez-Diaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll 2002, 16, 25–34. [Google Scholar]
- Engel, J; Prockop, D.J. The zipper-like folding of collagen triple helices and the effects of mutations that disrupt the zipper. Annu. Rev. Biophys. Biophys. Chem 1991, 20, 137–152. [Google Scholar]
- Gayatri, R.; Sharma, A.K.; Rajaram, R.; Ramasami, T. Chromium (III)-induced structural changes and self-assembly of collagen. Biochem. Biophys. Res. Commun 2001, 283, 229–235. [Google Scholar]
- Leikina, E.; Mertts, M.V.; Kuznetsova, N.; Leikin, S. Type 1 collagen is thermally unstable at body temperature. Proc. Natl. Acad. Sci. USA 2002, 99, 1314–1318. [Google Scholar]
- Goo, H.C.; Hwang, Y.S.; Choi, Y.R.; Cho, H.N.; Suh, H. Development of collagenase-resistant collagen and its interaction with adult human dermal fibroblasts. Biomaterials 2003, 24, 5099–5113. [Google Scholar]
- Madhan, B.; Subramanian, V.; Rao, J.R.; Nair, B.U.; Ramasami, T. Stabilization of collagen using plant polyphenol: Role of catechin. Int. J. Biol. Macromol 2005, 37, 47–53. [Google Scholar]
- Fathima, N.N.; Devi, R.S.; Rekha, K.B.; Dhathathreyan, A. Collagen-curcumin interaction—A physico-chemical study. J. Chem. Sci 2009, 121, 509–514. [Google Scholar]
- Rosenblum, G.; Van den Steen, P.E.; Cohen, S.R.; Bitler, A.; Brand, D.D.; Opdenakker, G.; Sagi, I. Direct visualization of protease action on collagen triple helical structure. Plos One 2010, 5. [Google Scholar] [CrossRef]
- Mitra, T.; Sailakshmi, G.; Gnanamani, A.; Mandal, A.B. Di-carboxylic acid cross-linking interactions improves thermal stability and mechanical strength of reconstituted type I collagen. Part I. Oxalic acid. J. Therm. Anal. Calorim 2011, 105, 325–330. [Google Scholar]
- Ramachandran, G.N.; Kartha, G. Structure of collagen. Nature 1955, 176, 593–595. [Google Scholar]
- Rich, A.; Crick, F.H. The structure of collagen. Nature 1955, 176, 915–916. [Google Scholar]
- Cowan, P.M.; McGavin, S.; North, A.C. The polypeptide chain configuration of collagen. Nature 1955, 176, 1062–1064. [Google Scholar]
- Fathima, N.N.; Madhan, B.; Rao, J.R.; Nair, B.U. Effect of zirconium (IV) complexes on the thermal and enzymatic stability of type I collagen. J. Inorg. Biochem 2003, 95, 47–54. [Google Scholar]
- Fathima, N.N.; Bose, M.C.; Rao, J.R.; Nair, B.U. Stabilization of type I collagen against collagenases (type I) and thermal degradation using iron complex. J. Inorg. Biochem 2006, 100, 1774–1780. [Google Scholar]
- Fathima, N.N.; Suresh, R.; Rao, J.R.; Nair, B.U. Role of green tea polyphenols cross linking in alleviating UV radiation effect on collagen. J. Appl. Polym. Sci 2007, 104, 3642–3648. [Google Scholar]
- Franzblau, C.; Schmid, K.; Faris, B.; Beldekas, J.; Garvin, P.; Kagan, H.M.; Bruce, J.; Baum, B.J. The interaction of collagen with alpha1-acid glycoprotein. Biochim. Biophys. Acta 1976, 427, 302–314. [Google Scholar]
- Madhan, B.; Muralidharan, C.; Jayakumar, R. Study on the stabilisation of collagen with vegetable tannins in the presence of acrylic polymer. Biomaterials 2002, 23, 2841–2847. [Google Scholar]
- Fathima, N.N.; Madhan, B.; Rao, R.T.; Nair, B.U.; Ramasami, T. Interaction of aldehydes with collagen: Effect on thermal enzymatic and conformational stability. Int. J. Biol. Macromol 2004, 4, 241–247. [Google Scholar]
- Usha, R.; Rajaram, A.; Ramasami, T. Stability of collagen in the presence of 3,4-dihydroxyphenylalanine (DOPA). J. Photochem. Photobiol. B 2009, 97, 34–39. [Google Scholar]
- Ge, Y.; Macdonald, D.L.; Holroyd, K.J.; Thornsberry, C.; Wexler, H.; Zasloff, M. In vitro antibacterial properties of pexiganan, an analog of magainin. Antimicrob. Agents Chemother 1999, 43, 782–788. [Google Scholar]
- Gopinath, D.; Kumar, M.S.; Selvaraj, D.; Jayakumar, R. Pexiganan-incorported collagen matrices for infected wound-healing processes in rat. J. Biomed. Mater. Res. A 2005, 73, 320–331. [Google Scholar]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother 2003, 57, 145–155. [Google Scholar]
- Arul, V.; Gopinath, D.; Gomathi, K.; Jayakumar, R. Biotinylated GHK peptide incorporated collagenous matrix: A novel biomaterial for dermal wound healing in rats. J. Biomed. Mater. Res. B Appl. Biomater 2005, 73, 383–391. [Google Scholar]
- Schibli, D.J.; Epand, R.F.; Vogel, H.J.; Epand, R.M. Tryptophan-rich antimicrobial peptides: Comparative properties and membrane interactions. Biochem. Cell Biol 2002, 80, 667–677. [Google Scholar]
- Dathe, M.; Wieprecht, T. Structural features of helical antimicrobial peptides: Their potential to modulate activity on model membranes and biological cells. Biochim. Biophys. Acta 1999, 1462, 71–87. [Google Scholar]
- Tossi, A.; Sandri, L.; Giangaspero, A. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 2000, 55, 4–30. [Google Scholar]
- Powers, J.P.S.; Hancock, R.E.W. The relationship between peptide structure and antibacterial activity. Peptides 2003, 24, 1681–1691. [Google Scholar]
- Huang, Y.; Huang, J.; Chen, Y. Alpha-helical cationic antimicrobial peptides: Relationships of structure and function. Protein Cell 2010, 1, 143–152. [Google Scholar]
- Woody, R.W. Contributions of tryptophan side chains to the far-ultraviolet circular dichroism of proteins. Eur. Biophys. J 1994, 23, 253–262. [Google Scholar]
- Ladokhin, A.S.; Selsted, M.E.; White, S.H. Bilayer interactions of indolicidin, a small antimicrobial peptide rich in tryptophan, proline, and basic amino acids. Biophys. J 1997, 72, 794–805. [Google Scholar]
- Liu, Z.; Brady, A.; Young, A.; Rasimick, B.; Chen, K.; Zhou, C.; Kallenbach, N.R. Length effects in antimicrobial peptides of the (RW) n series. Antimicrob. Agents Chemother 2007, 597–603. [Google Scholar]
- Mollmann, S.H.; Elofsson, U.; Bukrinsky, J.T.; Frokjaer, S. Displacement of adsorbed insulin by tween 80 monitored using total internal reflection fluorescence and ellipsometry. Pharm. Res 2005, 22, 1931–1941. [Google Scholar]
- Torrent, M.; Navarro, S.; Moussaoui, M.; Nogues, M.V.; Boix, E. Eosinophil cationic protein high-affinity binding to bacteria-wall lipopolysaccharides and peptidoglycans. Biochemistry 2008, 47, 3544–3555. [Google Scholar]
- Ding, L.; Yang, L.; Weiss, T.M.; Waring, A.J.; Lehrer, R.I.; Huang, H.W. Interaction of antimicrobial peptides with lipopolysaccharides. Biochemistry 2003, 42, 12251–12259. [Google Scholar]
- Rosenfeld, Y.; Sahl, H.G.; Shai, Y. Parameters involved in antimicrobial and endotoxin detoxification activities of antimicrobial peptides. Biochemistry 2008, 47, 6488–6478. [Google Scholar]
- Van der Weerden, N.L.; Hancock, R.E.; Anderson, M.A. Permeabilization of fungal hyphae by the plant defension NAD1 occurs through a cell wall dependent process. J. Biol. Chem 2010, 285, 37513–37520. [Google Scholar]
- Lee, D.G.; Kim, D.H.; Park, Y.; Kim, H.K.; Shin, Y.K.; Choi, C.H.; Hahm, K.S. Fungicidal effect of antimicrobial peptides, PMAP-23, isolated from porcine myeloid against Candida albicans. Biochem. Biophys. Res. Commun 2001, 282, 570–574. [Google Scholar]
- Zhao, H.; Kinnunen, P.K.J. Binding of the antimicrobial peptide temporin L to liposomes assessed by Trp fluorescence. J. Biol. Chem 2002, 277, 25170–25177. [Google Scholar]
- Swamy, R.N.; Gnanamani, A.; Shanmugasamy, S.; Gopal, R.K.; Mandal, A.B. Bioinformatics in crosslinking chemistry of collagen with selective cross linkers. BMC Res. Notes 2011, 4. [Google Scholar] [CrossRef]
- Chan, D.I.; Prenner, E.J.; Vogel, H.J. Tryptophan- and arginine-rich antimicrobial peptides: Structure and mechanism of action. Biochim. Biophys. Acta 2006, 1758, 1184–1202. [Google Scholar]
- Chen, C.C.; Hsu, W.; Hwang, K.C.; Hwu, J.R.; Lin, C.C.; Horng, J.C. Contributions of cation-π interactions to the collagen triple helix stability. Arch. Biochem. Biophys 2011, 508, 46–53. [Google Scholar]
- Edwards, R.; Harding, K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis 2004, 17, 91–96. [Google Scholar]
- Kustos, T.; Kustos, I.; Kilar, F.; Rappai, G.; Kocsis, B. Effect of antibiotics on cell surface hydrophobicity of bacteria using orthopedic wound infections. Chemotherapy 2003, 9, 237–242. [Google Scholar]
- Gomathi, K.; Gopinath, D.; Rafiuddin Ahmed, M.; Jayakumar, R. Quercetin incorported collagen matrices for dermal wound healing processes in rat. Biomaterials 2003, 24, 2767–2772. [Google Scholar]
- Hima Bindu, T.V.L.; Vidyavathi, M.; Kavitha, K.; Sastry, T.P.; Suresh Kumar, R.V. Preparation and evaluation of chitosan-gelatin composite films for wound healing activity. Trends Biomater. Artif. Organs 2010, 24, 123–130. [Google Scholar]
- Hima Bindu, T.V.L.; Vidyavathi, M.; Kavitha, K.; Sastry, T.P. Preparation and evaluation of gentamicin loaded chitosan-gelatin composite films for wound healing activity. Int. J. Appl. Biol. Pharm. Technol 2011, 2, 453–463. [Google Scholar]
- Iwakura, A.; Tabata, Y.; Tamura, N.; Doi, K.; Nishimura, K.; Nakamura, T.; Shimizu, Y.; Fujita, M.; Komeda, M. Gelatin sheet incorporating basic fibroblast growth factor enhances healing of devascularized sternum in diabetic rats. Circulation 2001, 104, I325–I329. [Google Scholar]
- Hima Bindu, T.V.L.; Vidyavathi, M.; Kavitha, K.; Sastry, T.P.; Suresh Kumar, R.V. Preparation and evaluation of ciprofloxacin loaded chitosan-gelatin composite films for wound healing activity. Int. J. Drug. Deliv 2010, 2, 173–182. [Google Scholar]
- Thakur, G.; Mitra, A.; Rousseau, D.; Basak, A.; Sarkar, S.; Pal, K. Crosslinking of gelatin-based drug carriers by genipin induces changes in drug kinetic profiles in vitro. J. Mater. Sci. Mater. Med 2011, 22, 115–123. [Google Scholar]
- Gomez-Guillen, M.C.; Perez-Mateos, M.; Gomez-Estaca, J.; Lopez-Caballero, E.; Gimenez, B.; Montero, P. Fish gelatin: A renewable material for developing active biodegradable film. Trends Food Sci. Technol 2009, 20, 3–16. [Google Scholar]
- Akane, T.; Toshiaki, N.; Hiroshi, M. Acceleration of wound healing by gelatin film dressings with epidermal growth factor. J. Vet. Med. Sci 2005, 67, 909–913. [Google Scholar]
- Baker, M.A.; Maloy, W.L.; Zasloff, M.; Jacob, L.S. Anticancer efficacy of magainin 2 and analogue peptides. Cancer Res 1993, 53, 3052–3057. [Google Scholar]
- Johnstone, S.A.; Gelmon, K.; Mayer, L.D.; Hancock, R.E.W.; Bally, M.B. In vitro characterization of the anticancer activity of membrane-active cationic peptides. I. Peptide-mediated cytotoxicity and peptide-enhanced cytotoxic activity of doxorubicin against wild-type and p-glycoprotein over-expressing tumor cell lines. Anticancer Drug Des 2000, 15, 151–160. [Google Scholar]
- Gallo, R.L.; Ono, M.; Povsic, T.; Page, C.; Eriksson, E.; Klagsbrun, M.; Bernfield, M. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc. Natl. Acad. Sci. USA 1994, 91, 11035–11039. [Google Scholar]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol 2006, 24, 1551–1557. [Google Scholar]
- Atherton, E.; Sheppard, R.C. Solid Phase Peptide Synthesis: A Practical Approach; IRL Press: Oxford, UK, 1989. [Google Scholar]
- Chen, Y.H.; Yang, J.T.; Chau, K.H. Determination of the helix and beta from of proteins in aqueous solution by circular dichroism. Biochemistry 1974, 13, 3350–3359. [Google Scholar]

| Blue shift (nm) | |||||||
|---|---|---|---|---|---|---|---|
| Peptides | λmax buffer (nm) | Gelatin | SDS | Tween | Mannan | Laminarin | LPS |
| (KW)4 | 355 | 8 | 10 | 0 | 0 | 0 | 10 |
| NRC-16 | 356 | 8 | 25 | 0 | 0 | 0 | 17 |
| MIC (μM) | ||||
|---|---|---|---|---|
| Resistant strains | (KW)4 | HPA3NT3-analog | NRC-16 | Magainin-II |
| P. aeruginosa 3547 | 16 (32) | 8 (16) | 4 (8) | 32 (64) |
| P. aeruginosa 3592 | 32 (64) | 8 (16) | 8 (16) | 32 (64) |
| P. aeruginosa 4007 | 16 (32) | 4 (16) | 4 (8) | 32 (32) |
| P. aeruginosa 4891 | 8 (16) | 4 (8) | 4 (8) | 16 (32) |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gopal, R.; Park, J.S.; Seo, C.H.; Park, Y. Applications of Circular Dichroism for Structural Analysis of Gelatin and Antimicrobial Peptides. Int. J. Mol. Sci. 2012, 13, 3229-3244. https://doi.org/10.3390/ijms13033229
Gopal R, Park JS, Seo CH, Park Y. Applications of Circular Dichroism for Structural Analysis of Gelatin and Antimicrobial Peptides. International Journal of Molecular Sciences. 2012; 13(3):3229-3244. https://doi.org/10.3390/ijms13033229
Chicago/Turabian StyleGopal, Ramamourthy, Jin Soon Park, Chang Ho Seo, and Yoonkyung Park. 2012. "Applications of Circular Dichroism for Structural Analysis of Gelatin and Antimicrobial Peptides" International Journal of Molecular Sciences 13, no. 3: 3229-3244. https://doi.org/10.3390/ijms13033229
APA StyleGopal, R., Park, J. S., Seo, C. H., & Park, Y. (2012). Applications of Circular Dichroism for Structural Analysis of Gelatin and Antimicrobial Peptides. International Journal of Molecular Sciences, 13(3), 3229-3244. https://doi.org/10.3390/ijms13033229





